“How one walks through the world, the endless small adjustments of balance, is affected by the shifting weights of beautiful things.”
Elaine Scarry
“Along the way you got distracted, lured, invoked, or tripped on a rock in some split moment you blinked.”
L.V. Hall
Given the aging population, and the personal and socioeconomic toll attributed to accidental falls1, it is appropriate that a huge volume of research has been devoted to the problem over the last 30 years. Additionally, evidence demonstrating the value of walking in terms of quality of life, cognitive function, and mortality is robust, confirming that optimal health care promotes walking in older patients despite the fall risk.2 However, this prolonged and vigorous research effort has given rise to an excessively broad array of often self-evident fall “risk factors”. For example, a recent review offers 19 categories of intrinsic fall risk factors,3 and respected sources list the circular variables “balance and gait impairments” and “previous falls” as leading fall risk factors.3,4
Are “risk factors” such as “balance/gait impairments” and “history of previous falls” useful, or merely re-statements of the problem, begging the question: “Why is the patient’s balance poor?” Would our society be satisfied if it asked why certain ships frequently sank and the engineers’ answers, following years of research, were that these boats had poor buoyancy, erratic navigational control, and had commonly sunk in the prior year, without saying why? No, the taxpayer’s money would have been misused. The same is true for much of falls research in its present state. We need more insightful, fundamental, and clinically applicable answers as to why some patients fall than “poor balance”. The physician needs to know why balance is poor. We are challenged to identify the attributes essential for balance and assemble them into a coherent conceptual model that will encompass the excessive and confusing number of fall risk factors and simplify the situation for the time-constrained clinician.
The flaw inherent to the “impaired balance as a risk factor for falls” approach is that it treats “balance” as a discrete physiologic entity, such as high frequency hearing or elbow flexor strength. In reality good balance is likely a rapid synergistic interaction between various physiologic and cognitive attributes that allow rapid and precise response to a perturbation. Although there have been efforts to identify the physiologic factors responsible for balance, including in particular the seminal contributions of Stephen Lord and colleagues,5 and the need for a model has been recognized,6 there is no clinically accessible conceptual model that integrates the various discrete attributes required for optimal balance and makes them available to the practitioner.
In prior work my colleagues and I studied a cohort of older subjects, about 2/3 with diabetic peripheral neuropathy (DPN) and 1/3 without, a group that represented a spectrum of peripheral neurologic function. We evaluated lower limb neuromuscular status by measuring hip strength and ankle proprioceptive precision in the frontal plane (abduction/adduction and inversion/eversion, respectively). We found that the ratio of normalized frontal plane hip strength and ankle proprioceptive precision in degrees (HipSTR:AnkPRO) powerfully predicted unipedal stance time (UST),7 falls, and fall-related injuries.8
However, we also evaluated gait on smooth and uneven surfaces, and HipSTR:AnkPRO did not correlate with frontal plane step control on either surface despite lower limb evaluations being frontal plane measures. This represented a critical limitation given the biomechanical importance of lateral foot placement to stability during gait9 and the increased injury potential of lateral falls.10 Moreover, HipSTR:AnkPRO did not identify the few subjects who sustained major, life-changing injuries during the year of follow-up. Searching for answers I turned attention to two novel evaluations of neurocognitive function performed at baseline. These were Simple Reaction Time Latency (Simple RTclin Latency) and Complex Reaction Time Accuracy (Complex RTclin Accuracy) as described in the companion article.11 Briefly, the former is determined by the time in msec a vertically oriented rod falls before it is caught by subject hand closure, while the latter is measured by the percentage of trials in which the subject correctly catches the device when lights on it illuminate upon descent, and correctly withholds catching it when the lights do not illuminate. The outcome is msec for the former, and percentage of accurate responses for the latter. The challenging component for Complex RTclin Accuracy is to withhold catching the device when the lights do not illuminate, and to make that decision within the 420 msec prior to the device striking the ground.
As reported in the companion article,11 baseline assessments of Complex RTclin Accuracy and Simple RTclin Latency, and their ratio, demonstrated potent associations with UST and frontal plane gait variability on an uneven surface in the subjects with DPN but not in the subjects with normal peripheral neuromuscular function. Remarkably, the ratio of Complex RTclin Accuracy:Simple RTclin Latency was the sole predictors of frontal plane gait variability and explained 60% of the variability in extreme frontal plane steps for the DPN subjects on the uneven surface. (Figure 3, companion paper) In addition, the few subjects with major injuries showed a decreased Complex RTclin Accuracy:Simple RTclin Latency, consistent with computer models finding that cognitive slowing leading to a 300 msec delay in implementing an avoidance strategy while falling markedly increases the likelihood of experiencing fracture level forces at impact.12
Figure 3.
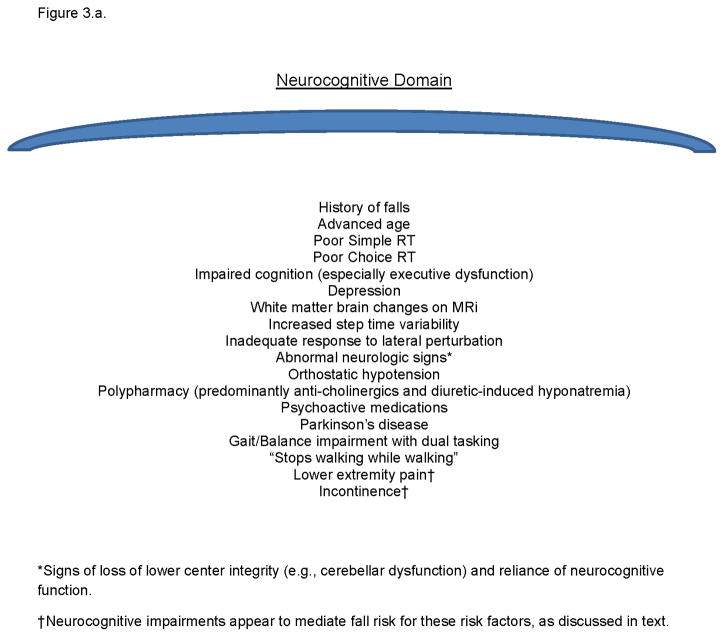
Figure 3.a. A list of identified fall risk factors which are associated with impairments in neurocognitive processing speed.
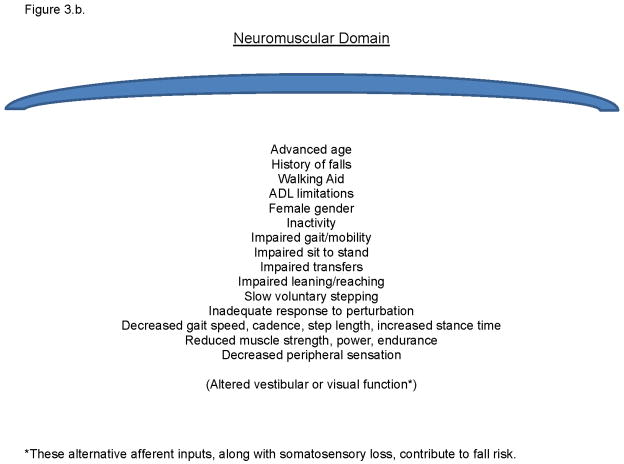
Figure 3.b. A list of identified fall risk factors which are associated with impairments in lower limb neuromuscular function.
There is growing recognition that neurocognitive attributes powerfully influence fall risk.13 Executive function, and more specifically inhibitory executive function, has been identified as a key neurocognitive factor for minimizing fall risk. Executive function likely resides anatomically within the dorsolateral prefrontal cortex (DLPFC), and is responsible for the rapid allocation of cognitive resources. Giordani and Prasad describe inhibitory executive function as the ability to “…prevent distracting information from entering working memory… and prevent pre-potent (automatic) responses that may not be appropriate to the current situation.”14 The advantages of being able to ignore distracting information and quickly arrest a pre-planned step while walking so as to institute an alternative strategy are evident with regard to fall and injury prevention.
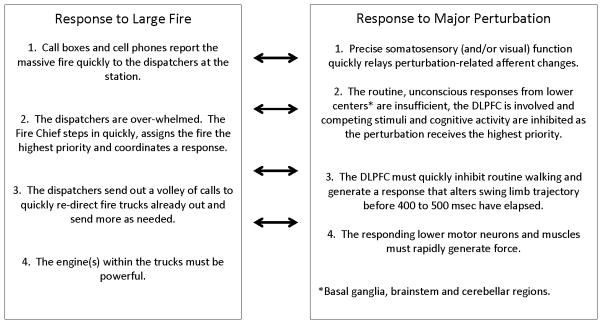
Combining our work with that of others suggests that rapidly available neuromuscular and neurocognitive attributes are critical to balance. This invites the inclusion, and linking, of both sets of attributes into a single clinically available conceptual model. A functionally pragmatic definition of human balance can be: “The ability to respond quickly to a perturbation such that postural equilibrium is maintained or restored.” What then, are the specific attributes necessary to do this? Perhaps it is easier to work with analogy and ask: What specific attributes are required to quickly respond to a large fire, and then relate this to response to a major perturbation. (Figures 1 and 2)
Figure 1.
Essential elements for rapid response to a fire (left), and the analogous essential attributes for rapid response to a postural perturbation.
Figure 2.
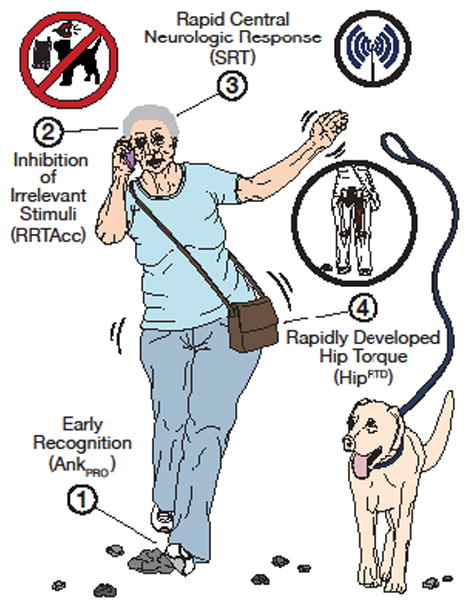
A model of the key attributes required for the ability to respond quickly to a perturbation.
Note: The afferent process shown is proprioceptive in nature, but vision and vestibular input must be accounted for as well. The central processes are related to inhibitory executive function and attention/simple reaction time. Although Complex RTclin Accuracy (as RRTAcc) is depicted in the Figure, other measures of this critical neurocognitive attribute such as Stroop or Trails B testing is acceptable.
In the same manner that small fires are handled by dispatchers without fire chief involvement, small perturbations are likely handled by the basal ganglia, brainstem gait centers, and cerebellum in those with intact neuromuscular function. This absence of cortical involvement allows the healthy to walk in most environments with minimal conscious effort. However, the fire chief and the cerebral cortex must become involved when: a) There is a major fire or the perturbation is sufficiently large; or b) The dispatchers/lower gait centers are insufficient to the recovery task. With regard to falls this could be due to absence of precise afferent inflow (e.g., neuropathy or vision loss), weakness (e.g., myopathic process) or primary disorders of the lower centers themselves (e.g., Parkinson’s disease). Under such circumstances the cerebral cortex/fire chief must become involved by quickly inhibiting irrelevant afferent input and distracting cognitive processes, weighing the multiple inputs, and rapidly generating a coordinated response via the lower centers/dispatchers. Evidence suggests that the dorsolateral prefrontal cortex (DLPFC) is involved in executive functions,15 and imaging studies show this area to be highly active during recovery from large perturbations16 and in the compensation for ataxic gait following cerebellar injury.17 Finally, the fire chief’s rapid planning is for naught if the trucks are slow and, similarly, there must be sufficient strength in key muscles to generate torque quickly enough for the perturbation to be successfully rejected.
The model’s validity is supported if the myriad accepted fall risk factors are accounted for within it, and if accepted interventions are present as well. Figures 3.a. and b. list previously identified risk factors which share an association with the neurocognitive or neuromuscular attributes within the model. Although two known risk factors, incontinence and pain, are not primarily within the model they appear to interact with executive impairments to increase fall risk as such patients impulsively move into precarious circumstances to avoid pain or bladder accident.18 With respect to known successful interventions, optimizing vision and augmenting somatosensory function can decrease fall risk and improve response to perturbation,19,20 and cognitive training and attention-enhancing medications may improve gait parameters associated with fall risk.3 Finally, increasing lower limb strength appears to decrease fall risk.21
Model validity is also supported if poor performance in an attribute delays appropriate response to perturbation, or relative strength in another compensates for limitations. Hereditary neuropathy patients demonstrate delayed neuromuscular response to perturbation that is proportionate to the sensory deficit.22 We found that hip strength can compensate for poor ankle proprioception7 and the companion article, along with others, note that neurocognitive factors are critically important when neuromuscular factors are sub-optimal.23 The clinical relevance of these relationships is that improvement in modifiable attributes may help compensate for those irreversibly impaired.
This clinically applicable model is a first approximation that is not intended to perfectly reflect known basic science. For example, there is likely central nervous system-mediated variability in perturbation recovery efficiency, analogous to the fire truck’s ability to find the most direct route to the blaze,24 and the critical muscles to be evaluated doe recovery have not been clearly identified and accounted for.25 In addition, feed forward mechanisms of anticipatory postural control are likely mediated by communication from the temporoparietal cortex to lower centers,26 and no means of discretely and clinically evaluating this capaciy is offered. Finally, the concepts described have been derived from subjects with diabetic neuropathy and so generalization to other clinical groups is less certain.
The critical point is that the model links a few essential neuromuscular and neurocognitive traits required for speed of response to perturbation (Figure 2), and so has the potential to streamline and objectify clinical decision-making. In a succinct application, as is invariably required in clinic visits, practitioners confronted with patients reporting a history of a perturbation-induced fall or loss of balance may observe unipedal stance or a timed up and go or some other functional task that allows observation of gait and balance performance. Then clinicians can profitably focus on reliable measures of somatosensory function and vision, and proximal muscle strength.27 Next they can evaluate the lower centers (brainstem, basal ganglia, and cerebellum) via examination of cranial nerves, evaluation for rigidity, tremor and multiple steps when turning, along with altered heel-to-shin and finger-nose-finger testing and dysdiakokinesia. If one or more of these sub-cortical or peripheral neuromuscular functions are sub-optimal and cannot be treated or reliably accommodated for then the patient will likely need optimal neurocognitive executive function in order to avoid falls. These can be evaluated through simple reaction time testing28 and classic Stroop or Trails B evaluations. It can then be predicted that patients with sub-optimal performance on these evaluations, along with poor neuromuscular or sub-cortical functions, will be least likely to respond successfully to perturbations, and so they should be targets of the most intense interventional therapy, medication review,29 and environmental modification.
Clinicians deserve a more logical and simplified strategy for evaluating fall risk, rather than being perplexed by self-evident, overlapping, and ever-multiplying risk factors, and patients/families deserve a better understanding of fall risk than “old age”.7 The challenge is to further test the model, and bring increasingly reliable tools to the bedside for evaluating the specific physiologic capacities that predict the ability to respond to a perturbation within the 300 to 400 msec window available prior to inappropriate swing limb placement and potential injury. With this more fundamental perspective in mind, rational therapy and specific strategies with which to guide patients and rehabilitation colleagues can be recommended with confidence, and generic referrals for “balance training” diminished, with corresponding reductions in costs and futility. Our patients and society deserve no less after the extensive resources that have been invested into fall prevention research.
References
- 1.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35:ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 2.Rejeski WJ, Ip EH, Bertoni Ag, Bray GA, Evans G, Gregg EW, Zhang Q. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012 Mar 29;366(13):1209–17. doi: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51–61. doi: 10.1016/j.maturitas.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Tinetti ME, Kumar C. The patient who falls. JAMA. 2010;303(3):258–66. doi: 10.1001/jama.2009.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lord SR, Ward JA, Williams P, Anstey KJ. Physiological factors associated with falls in older community-dwelling women. J An Geriatr Soc. 1994;42:1110–7. doi: 10.1111/j.1532-5415.1994.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 6.Pasma JH, Engelhart D, Schouten AC, vander Kooij H, Maier AB, Meskers CGM. Impaired standing balance: the clinical need for closing the loop. Neuroscience. 2014;267:157–65. doi: 10.1016/j.neuroscience.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Allet LA, Kim H, Ashton-Miller JA, De Mott T, Richardson JK. Frontal plane hip and ankle sensorimotor function, not age, predicts unipedal stance time. Muscle Nerve. 2012 Apr;45(4):578–85. doi: 10.1002/mus.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson JK, DeMott T, Allet L, Kim H, Ashton-Miller JA. The hip strength: Ankle proprioceptive threshold ratio predicts falls and injury in diabetic neuropathy. Muscle Nerve. 2013 Nov 26; doi: 10.1002/mus.24134. (Epub ahead of print) NIHMS545761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacKinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. J Biomech. 1993 Jun;26(6):633–44. doi: 10.1016/0021-9290(93)90027-c. [DOI] [PubMed] [Google Scholar]
- 10.Nevitt MC, Cummings SR. Type of fall and risk of hip and wrist fractures: the study of osteoporotic fr. doi: 10.1111/j.1532-5415.1994.tb06576.x. [DOI] [PubMed] [Google Scholar]
- 11.Richardson JK, Eckner JT, Allet L, Kim H, Ashton-Miller JA. Complex and simple clinical reaction times are associated with gait, balance, and major fall injury in older subjects with diabetic peripheral neuropathy. Am J Phys Med Rehabil. doi: 10.1097/PHM.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo J, Ashton-Miller JA. Effect of pre-impact movement strategies on the impact forces resulting from a lateral fall. J Biomech. 2008;41:1969–77. doi: 10.1016/j.jbiomech.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearney FC, Harwood RH, Gladman JRF, Lindoln N, Masud T. The relationship between executive function and falls and gait abnormalities in older adults: a systematic review. Dement Geriatr Cogn Disord. 2013;36:2035. doi: 10.1159/000350031. [DOI] [PubMed] [Google Scholar]
- 14.Giordani B, Prasad CC. Neuropsychological influences on gait in the elderly. In: Hausdorff JM, Alexander NB, editors. Gait disorders: evaluation and management. Boca Raton, FL: Taylor & Francis Group; 2005. [Google Scholar]
- 15.Lague-Beauvais M, Brunet J, Gagnon L, Lesage F, Bherer L. A fNIRS investigation of switching and inhibition during the modified Strook task in younger and older adults. NeuroImage. 2013;64:485–95. doi: 10.1016/j.neuroimage.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 16.Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Role of the prefrontal cortex in human balance control. NeuroImage. 2008;43:329–36. doi: 10.1016/j.neuroimage.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke? NeuroImage. 2007;37:1338, 45. doi: 10.1016/j.neuroimage.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Leveille Suzanne G, PhD, RN, Jones Richard N, ScD, Kiely Dan K, MPH, Hausdorff Jeffrey M, PhD, Shmerling Robert H, MD, Guralnik Jack M, PhD, MD, Kiel Douglas P, MD, Lipsitz Lewis A, MD, Bean Jonathan F., MD Chronic Musculoskeletal Pain and the Occurrence of Falls in an Older Population. JAMA. 2009;302(20):2214–2221. doi: 10.1001/jama.2009.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lord SR, Smith ST, Menant JC. Vision and fall risk in older people: risk factors and intervention strategies. Clin Geriatr Med. 2010;26:569–81. doi: 10.1016/j.cger.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Maki BE, Sibley KM, Jaglas SB, Bayley M, Brooks D, Fernie GR, Flint AJ, Gage W, Liu BA, McIlroy WE, Mihailidis A, Perry SD, Popovic MR, Pratt J, Zettel JL. Reducing fall risk by improving balance control; development, evaluation and knowledge-transition of new approaches. Journal of Safety Research. 2011;442:473–485. doi: 10.1016/j.jsr.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Sherrington C, Whitney JC, Lord SR, Herbert RD, Cumming RG, Close JCT. Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc. 2008:572234–43. doi: 10.1111/j.1532-5415.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 22.Van der Linden MH, Kam Dd, van Engelen BGM, Hendricks HT, Duysens J. Fast responses to stepping on an unexpected surface height depend on intact large-diameter nerve fibers: a study on Charcot-Marie-Tooth Type IA disease. J Neurophysiol. 2009;102:1684–98. doi: 10.1152/jn.91142.2008. [DOI] [PubMed] [Google Scholar]
- 23.Martin KL, Blizzard L, Srikanth VK, Wood A, Thomson R, Sanders LM, Callisaya ML. Cognitive function modifies the effect of physiologic function on the risk of multiple falls—a population-based study. J Gerontol A Biol Sci Med Sci. 2013;68(9):1091–97. doi: 10.1093/gerona/glt010. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira ASC, Gizzi L, Kersting UG, Farina D. Modular organization of balance control following perturbations during walking. J Neurophysiol. 2012;108:1895–906. doi: 10.1152/jn.00217.2012. [DOI] [PubMed] [Google Scholar]
- 25.Thomas JC, Odonkor C, Griffith L, Holt N, Percac-Lima S, Leveille S, Ni P, Latham NK, Jette AM, Bean JF. Reconceptualizing balance; attributes associated with balance performance. Experimental Gerontology. 2014;57:218–23. doi: 10.1016/j.exger.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Movement Dis. 2013;28(11):1483–91. doi: 10.1002/mds.25669. [DOI] [PubMed] [Google Scholar]
- 27.Donaghy A, Demott T, Allet L, Kim H, Ashton-Miller J, Richardson JK. Accuracy of clinical techniques for evaluating lower limb sensorimotor functions associated with increased fall risk. PM&R Journal. 2016;8:331–39. doi: 10.1016/j.pmrj.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckner JT, Whitacre RD, Kirsch NL, Richardson JK. Evaluating a clinical measure of reaction time: an observational study. Percept Mot Skills. 2009 Jun;108(3):717–20. doi: 10.2466/PMS.108.3.717-720. [DOI] [PubMed] [Google Scholar]
- 29.Ziere G, DIeleman JP, Hofman A, Pols HAP, van der Cammen TJM, Stricker BH. Polypharmacy and falls in the middle age and elderly population. Br J Clin Pharmacol. 2005;61:2, 218–23. doi: 10.1111/j.1365-2125.2005.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]