Abstract
The World Health Organization has issued an early release revision to its antiretroviral guidelines in which PrEP (Pre-exposure prophylaxis in the form of daily oral, fixed dose combination tenofovir disoproxil fumarate/emtricitabine (TDF/FTC)) is recommended as a prevention option to all people at substantial risk of acquiring HIV. However, lack of effectiveness in two major women-only PrEP trials, VOICE and FEM PrEP, continue to be a cause for concern about achieving effectiveness for women in Southern Africa. We conducted a series of meta-analyses of oral TDF/FTC effectiveness in women including all five randomized placebo-controlled trials that included women. An adherence-based meta-analysis model showed that with high levels of adherence (75%), oral PrEP is estimated to be effective (RR=0.39, 95% CI 0.25 to 0.60). Provided that these results apply to women in Southern Africa, future prevention trial designs in that region should account for potentially reduced HIV incidence when PrEP is available.
Keywords: HIV prevention, pre-exposure prophylaxis, PrEP, tenofovir, Truvada
The World Health Organization has issued an early release revision to its antiretroviral guidelines in which pre-exposure prophylaxis (PrEP) is recommended as a prevention option to all people at substantial risk of acquiring HIV.1 While clinical trials in men who have sex with men have uniformly reported effectiveness, PrEP trials in women have had mixed results, raising concern as to whether oral PrEP should be recommended for all women at risk. Moreover, pharmacokinetic studies reporting lower concentrations of TDF and FTC in vaginal as compared to rectal mucosa suggest the possibility that biologic differences between men and women may, in part, explain the variable results in women.2 Here we provide a brief, quantitative synthesis of existing evidence regarding oral-PrEP effectiveness in women, and evaluate the importance of drug adherence. (Effectiveness is defined here as the impact on the risk of HIV-1 infection in practical settings, including trials, with incomplete and/or imperfect use.)
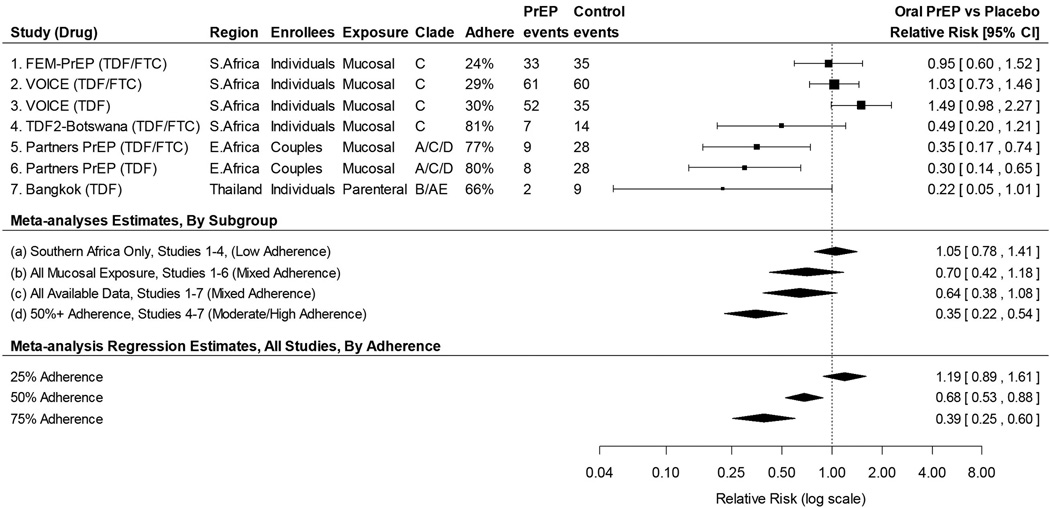
Five major randomized trials of oral PrEP have included women.3–7 Of these five, three reported evidence of effectiveness in the women’s subgroups (Figure 1). Partners PrEP3 showed a significant reduction in risk of HIV acquisition among women (both TDF and TDF/FTC), while both TDF2-Botswana4 and Bangkok-TDF5 showed non-significant trends toward reduced risk. The other two major trials, VOICE6 and FEM-PrEP7 were conducted only in women, and showed no reduction in risk. Various factors may explain the conflicting results observed in women, including drug adherence, differences in the study populations such as participant type (discordant couples versus uninfected individuals), demographic and behavioral characteristics, prevalent HIV subtypes, transmission mode (mucosal versus parenteral), and underlying host factors such as genital-tract inflammation and/or the presence of co-pathogens.
Figure 1.
Forest plot showing point estimates and 95% confidence intervals of PrEP effectiveness in preventing HIV-1 acquisition, meta-analysis estimates, and meta-analysis regression results for major PrEP trials in women.
We aggregate results in women from the five major trials using random-effects meta-analysis. Focusing first on Southern Africa trials and then expanding to other regions and exposure types, we consider three nested populations: (a) individually-enrolled Southern African women mucosally exposed in a clade C epidemic (i.e. the population most likely to be recruited for future trials); (b) women in (a) plus Eastern African women in discordant partnerships who were mucosally exposed in a clade A/C/D epidemic; and (c) women in (b) plus individually enrolled intravenous drug users in an AE/B clade epidemic (i.e. all available oral PrEP trials). The meta-analysis result in population (a) (VOICE, FEM-PrEP, TDF2-Botswana – overall low adherence) shows no effect, with an estimated PrEP vs Placebo relative risk of 1.05 (95% CI 0.78 to 1.71) (Figure 1). Including the Partners PrEP (high adherence) results into the analysis [population (b)] yields a lower relative risk (0.70, 95% CI 0.42 to 1.18), and adding Bangkok-TDF injection drug users [population (c), moderate adherence] strengthens the overall result to 0.64 (95% CI 0.38 to 1.08), although not to the level of statistical significance. The larger numbers of events in VOICE and FEM-PrEP cause the population (a) results to dominate the overall combined estimates; however, it is important to note that both of these studies had low rates of adherence.
To evaluate the importance of adherence we first limit the analysis to studies with relatively high adherence, as measured by the proportion of a sub-sample of participants with detectable drug in plasma. In our analyses adherence is defined as the proportion of participants having any detectable drug levels (>0.31 ng/mL), with the exception of FEM-PrEP which used a higher threshold of 10 ng/mL. Population (d) (Figure 1) is limited to studies with at least 50 percent adherence, and shows substantial and statistically significant effectiveness for oral PrEP (RR 0.35, 95% CI 0.22 to 0.54). Although self-reported adherence was also available in some studies, biased reporting of adherence is a common phenomenon and we therefore chose not to include self-reported adherence in the meta-analysis.
As a second approach we use a mixed-effects meta-analysis regression8 including all five trials, and model the association between plasma-assessed adherence and effectiveness. Predicted effectiveness for low (25%), moderate (50%), and high (75%) levels of adherence is presented at the bottom of Figure 1. For low adherence oral PrEP is estimated to have no effectiveness (RR 1.19, 95% CI 0.89 to 1.61). With moderate and high levels of adherence, oral PrEP is estimated to be effective, with a dose-response (Medium: RR=0.68, 95% CI 0.53 to 0.88; High: RR=0.39, 95% CI 0.25 to 0.60).
Although results vary widely across studies in women, our analyses suggest that oral PrEP can be highly effective when adherence is high. Lack of effectiveness in the VOICE and FEM PrEP studies in South Africa, where adherence was also lowest, continue to be a cause for concern about achieving effectiveness in this setting. Our analyses do not rule out biological factors as mediators of PrEP effectiveness, but our adherence-based meta-analysis shows a strong association between trial-level adherence and risk reduction, suggesting that greater adherence in Southern African women could be expected to translate into effectiveness. Multiple HIV prevention trials are currently planned or underway in Southern Africa, including studies of long-acting injectable PrEP, vaccines, and monoclonal antibody infusions. When planning these trials in populations where PrEP is available, evidence suggests that high adherence to oral PrEP would reduce HIV-1 incidence in women, and therefore trial design and sample size calculations should be adjusted accordingly.
Acknowledgments
Source of Funding: DJD reports grants from NIH, during the conduct of the study; grants from Bill and Melinda Gates Foundation and NIAID outside the submitted work.
Footnotes
Conflicts of Interest: BH, HEJ, PDG, YH, ERB, YQC, SMH, PBG have nothing to disclose.
References
- 1.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015 Sep; (ISBN: 978 92 4 150956 5). [PubMed]
- 2.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 5.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 6.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med. 2015;372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Damme L, Corneli A, Ahmed K, et al. Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for Meta-analysis in Medical Research. Wiley Series in Probability and Statistics, John Wiley & Sons; 2000. [Google Scholar]