Abstract
The gram-negative bacterium Legionella pneumophila causes a severe form of pneumonia called Legionnaires' disease, characterized by bacterial replication within alveolar macrophages. Prior to intracellular replication, the vacuole harboring the bacterium must first escape trafficking to the host lysosome, a process that is dependent on the Dot/Icm type IV secretion system. To identify genes required for intracellular growth, bacterial mutants were isolated that were delayed in escape from the macrophage but which retain a minimally functional Dot/Icm machinery. The mutations were found in eight distinct genes, including three genes known to be required for optimal intracellular growth. Two of these genes, icmF and dotU, are located at one end of a cluster of genes that encode the type IV secretion system, yet both icmF and dotU lack orthologs in other type IV translocons. DotU protein is degraded in the early postexponential phase in wild-type L. pneumophila and at all growth phases in an icmF mutant. IcmF contains an extracytoplasmic domain(s) based on accessibility to a membrane-impermeant amine-reactive reagent. In the absence of either gene, L. pneumophila targets inappropriately to LAMP-1-positive compartments during macrophage infection, is defective in the formation of replicative vacuoles, and is impaired in the translocation of the effector protein SidC. Therefore, although IcmF and DotU do not appear to be part of the core type IV secretion system, these proteins are necessary for an efficiently functioning secretion apparatus.
The gram-negative bacterium Legionella pneumophila is the causative agent of a severe form of pneumonia called Legionnaires' disease as well as the milder illness termed Pontiac Fever (32, 37). L. pneumophila normally inhabits aquatic environments such as lakes and ponds where it multiplies within fresh water amoebae, but it can also be found in many manmade aquatic environments (21). Humans encounter this pathogen upon inhalation of bacterium-containing aerosols that frequently originate from sources such as air conditioners and hot water heaters (40).
After inhalation, L. pneumophila is engulfed by alveolar macrophages and avoids destruction by preventing trafficking of the bacterium-containing vacuole into the endocytic pathway (8, 29). Instead, shortly after uptake, the vacuole associates with smooth vesicles and mitochondria and, within 10 min of entry, is surrounded by markers of the endoplasmic reticulum (ER) and early secretory pathway (16, 28). The interaction of the L. pneumophila vacuole with the ER may be initiated by the fusion of early secretory vesicles (31), and the vacuole eventually becomes surrounded by membranes derived from the ER (59). Bacterial cell division within the compartment begins approximately 4 h after uptake and continues for approximately 20 h further until the host cell lyses and numerous bacteria are released to infect additional macrophages (30).
Integral to the intracellular lifestyle of L. pneumophila is a group of genes termed dot/icm that are clustered in two regions of the genome that contain 7 and 18 genes, respectively (reviewed in references 50 and 60). Many of these genes (19 of the 25) show high sequence similarity to conjugative transfer systems and together define a type IV secretion system (T4SS) (51). T4SSs have also been found in several other pathogenic bacteria, including Agrobacterium tumefaciens, Brucella spp., Helicobacter pylori, Bordetella pertussis, and Rickettsia prowazekii (reviewed in reference 6). In most of these species, the T4SS is required for the transport of protein or DNA (or both) into the eukaryotic cell as a central feature of pathogenicity. In L. pneumophila, strains bearing mutations in the dot/icm genes are not competent for intracellular growth, and vacuoles containing these mutants colocalize with markers of the late endosomal system soon after entry into the mammalian cell (1, 2, 27, 48, 55, 57, 61). Translocated substrates of the T4SS, therefore, appear critical for the proper routing of the bacterium into a replicative vacuole. Consistent with a role in the early stages of intracellular growth, expression of the Dot/Icm complex was found to be required very early after contact with macrophages and dispensable following the successful evasion of fusion with endocytic compartments (10, 46).
Many Dot/Icm proteins have been characterized, including those that are homologous to T4SSs of other organisms and those that appear to be unique to L. pneumophila. DotA, a presumptive structural component of the T4SS, contains eight membrane-spanning domains and is located in the bacterial inner membrane (47). A fragment of DotA was also found to be exported into the extracellular milieu during growth in culture (42). DotG has orthologs in other T4SSs, and although DotG itself has not been extensively characterized, its H. pylori ortholog has recently been shown to localize both in discrete loci on the bacterial surface as well as on the type IV extracellular needle structure (45). The DotG ortholog in A. tumefaciens, VirB10, has been detected in the bacterial inner membrane, the periplasm, and the outer membrane (12, 22, 58). Several Dot/Icm proteins without T4SS homology have been closely characterized in an effort to understand L. pneumophila-specific pathogenicity. IcmW and IcmS form a protein complex thought to function within the bacterial cytoplasm, perhaps as chaperones for translocated effector proteins (9). IcmR is another protein with chaperone activity whose substrate, IcmQ, can multimerize and form discrete pores in artificial membranes in vitro (9, 17, 18). Finally, the L. pneumophila-specific protein IcmX is primarily found in the bacterial periplasm, and a small amount of a truncated product of IcmX is found in the supernatant of broth-grown bacteria (36).
Several substrates of the Dot/Icm T4SS have recently been identified. RalF, a protein with homology to mammalian Sec7, is translocated into macrophages and can function in vitro as an exchange factor for Arf, a small GTP-binding protein involved in several membrane trafficking events (41). RalF is essential for the localization of Arf to the L. pneumophila vacuole, yet RalF is not required for the growth of L. pneumophila in either macrophages or amoebae. Two other proteins, LidA and SidC, are each translocated across the vacuolar membrane and are associated with the cytoplasmic face of this compartment (11, 35). L. pneumophila strains lacking LidA are partially defective for growth within macrophages, but its function is presently unknown. Finally, two proteins required for L. pneumophila exit from amoeba, LepA and LepB, have been identified as translocated substrates (5).
Although substantial work has identified L. pneumophila genes involved in optimal virulence, many factors essential for the growth of the bacterium in human cells remain uncharacterized. Here we describe the isolation of L. pneumophila mutants that fail to multiply efficiently within macrophages but which do not exhibit the absolute growth defects caused by lesions in many of the dot/icm genes. Growth-defective mutants were enriched from a pool of mutagenized bacteria due to their block or delay in lysing out of macrophages at the termination of an infection cycle. The majority of the mutants obtained were in the previously identified gene icmF (44) and the gene directly upstream of icmF, dotU (19, 51).
MATERIALS AND METHODS
Media, plasmids, and strains.
L. pneumophila strains were grown in AYE broth [10 mg of N-(2-acetamido)-2-aminoethanesulfonic acid (ACES)-KOH (pH 6.9)/ml, 10 mg of yeast extract/ml, 0.4 mg of cysteine/ml, 0.135 mg of ferric nitrate/ml] and maintained on CYE plates (AYE including 2 mg of charcoal/ml and 18 mg of agar/ml) (20, 23). Media supplemented with 100 μg of thymidine/ml were designated AYET and CYET, respectively. CYET-Kan plates contained 20 μg of kanamycin/ml, CYET-Cm plates contained 5 μg of chloramphenicol/ml, and CYET-Suc plates contained 50 mg of sucrose/ml.
Plasmids and strains are listed in Table 1, and primers are listed in Table 2. Plasmid pJK211, a kind gift of J. Kirby (Beth Israel Deaconess Medical Center, Boston, Mass.), is a suicide plasmid containing a mini-Tn10 (Kanr) transposable element and transposase. To assist in cloning DNA surrounding the genomic integration of the element, the transposon contained an R6K origin of replication. Plasmid pQE-30 was obtained from Qiagen (Valencia, Calif.), and pGEX4T-1 was obtained from Amersham (Carlsbad, Calif.). Plasmid pJB908 was a kind gift of J. Vogel, University of Washington School of Medicine, St. Louis, Mo. Plasmid pAM239 expressed green fluorescent protein (GFP) under the control of the tac promoter (53).
TABLE 1.
Plasmids and strains used in this work
| Strain or plasmid | Genotype, relevant markers | Reference or source |
|---|---|---|
| L. pneumophila | ||
| Lp01 | Philadelphia-1 rpsL hsdR | 2 |
| Lp02 | Philadelphia-1 rpsL hsdR thyA mutant | 2 |
| Lp03 | Lp02 dotA3 | 2 |
| icmF1::miniTn10 | Lp02 icmF1::miniTn10 | This work |
| ΔdotU | Lp02 ΔdotU | This work |
| ΔicmF | Lp02 ΔicmF | This work |
| E. coli DH5α λpir | Stratagene | |
| D. discoideum AX3K | D. Knecht | |
| Plasmids | ||
| pAM239 | ori RSF1010, GFP | 52 |
| pGEX-4T-1 | E. coli GST fusion vector | Amersham |
| pGD5 | ΔdotU construct in pSR47s | This study |
| pGD33 | GST-DotU in pGEX-4T-1 | This study |
| pGD34 | His6-IcmF in pQE-30 | This study |
| pJB908 | ori RSF1010 (ΔoriT), Ampr, tdΔi | J. Vogel |
| pJK211 | mini-Tn10 | J. Kirby |
| pQE-30 | E. coli His6 fusion vector | Qiagen |
| pSR47s | ori R6K, ori TRP4, Kanr, SacB | 39 |
| pSV39 | ΔicmF construct in pSR47s | This study |
| pSV38 | dotU in pJB908 | This study |
| pSV36 | icmF in pJB908 | This study |
| pSV42 | His6-DotG C terminus in pQE-30 | This study |
TABLE 2.
Oligonucleotide primers used in this work
| Primer | Sequence (5′-3′)a |
|---|---|
| BIF1-1 | AAGGATCCATTTGGACATACCCAACTTGCTATG |
| BIF1-2 | AGAGCTCGTGCATTAAGATAATTCCTCGCATC |
| BIF2-1 | AGTGTCGACCCATTGAAAACATTGACATTGGTG |
| BIF2-2 | AAGGATCCGACGATTGACAAGTGAGGACGG |
| BIF3 | CGCTCTAGAGAAACCCATGGAGTAATATAATG |
| BIF4 | CGCTCTAGAAATGAATTGTCCATTAGTTTTCC |
| BIF5 | GGGAATTCATGACAACTGAGCAATACCC |
| BIF6 | CCGCTCGAGTTAGTTTTCCAGCATAGCAAG |
| DOTG1 | CGCGGGATCCGAATCAGCCGTTGCAGG |
| DOTG2 | CGCGGGATCCTTAAATTGTTGTAACATCCTGGG |
| ICMF1-1 | CGCGGATCCTAATGAATTGTCCATTAGTTTTCCAGC |
| ICMF1-2 | GCAGCGTCGACGTGGCAAGCAGAGCAAATGGG |
| ICMF2-1 | CGCGGATCCTTAAACGACCATATTGCATAATTATG |
| ICMF2-2 | GCGGAGCTCTTATTCAATCGATAGAAATTATGTCG |
| ICMF3 | CGCTCTAGACAACTTGCTATGCTGGAAAAC |
| ICMF4 | CGCTCTAGATTATGCAATATGGTCGTTTAAAG |
| ICMF5 | ACGCCCCGGGAATGGACAATTCATTACGCGC |
| ICMF6 | ACGCGTCGACTTATGCAATATGGTCGTTTAAAG |
| JK211MCS | GGATCTGGTACCGGATCC |
| JK211R6K | CGCCGGACGCATCGTGG |
Restriction enzyme sites are underlined.
The plasmid used to construct the dotU deletion strain, pGD5, was constructed by digesting pSR47s, a suicide plasmid that contains SacB (for sucrose sensitivity) and Kanr (39), with SacI and SalI followed by ligation to two PCR products, BIF1 and BIF2. BIF1 was generated from Lp02 genomic DNA with primers BIF1-1 and BIF1-2 and was digested with BamHI and SacI, and BIF2 was generated with primers BIF2-1 and BIF2-2 followed by digestion with BamHI and SalI. The plasmid used to construct the icmF deletion strain, pSV39, was produced by ligating SacI- and SalI-digested pSR47s with PCR products ICMF1 and ICMF2, which were digested with BamHI and SalI or BamHI and SacI, respectively. ICMF1 was generated from Lp02 genomic DNA with primers ICMF1-1 and ICMF1-2, and ICMF2 was generated with primers ICMF2-1 and ICMF2-2. Plasmid pSV38, which expresses dotU under the control of the tac promoter, was created by digesting pJB908 with XbaI followed by ligation to an XbaI-digested PCR product generated with primers BIF3 and BIF4. Plasmid pSV36, which expresses IcmF under the control of the tac promoter, was generated by ligating XbaI-digested pJB908 with an XbaI-digested PCR product generated with primers ICMF3 and ICMF4. The C-terminal 317 amino acids of DotG (termed DotG-CT) were expressed with an amino-terminal His6 tag from plasmid pSV42, which was engineered by digesting pQE-30 with BamHI followed by ligation to a similarly digested PCR product generated with primers DOTG1 and DOTG2. The correct directionality of the insert for pSV36, pSV38, and pSV42 was determined by restriction digest. Amino-terminally tagged glutathione S-transferase (GST)-DotU was expressed from pGD33 and was constructed by digesting pGEX-4T with EcoRI and XhoI followed by ligation to a similarly digested PCR product that had been generated with primers BIF5 and BIF6. IcmF was expressed with an N-terminal His6 tag from plasmid pGD34, developed by digesting pQE30 with XmaI and SalI followed by ligation to a similarly digested PCR product generated from primers ICMF5 and ICMF6. All PCRs utilized Vent polymerase (New England Biolabs, Beverly, Mass.). Plasmids obtained through isolation of genomic DNA surrounding each transposon insertion were isolated as follows. Genomic DNA was isolated from each strain, digested with EcoRI (a restriction enzyme that does not digest within the transposon sequence), ligated, and used to transform the Escherichia coli strain DH5α λpir (34). DNA was isolated from the resulting colonies, and sequences adjacent to the transposon were determined with primers JK211R6K and JK211MCS.
L. pneumophila strains Lp01 and Lp02, which contain defective restriction modification systems, are derivatives of strain Philadelphia-1. Lp02 is a thymine auxotroph, whereas Lp01 is a prototroph (2). Lp03 is an Lp02 strain bearing the dotA3 mutation (3). The icmF1::miniTn10 strain was engineered by introduction of the genomic plasmid bearing an icmF transposon integration after codon 50 (insertion site TGCCCGTA) into Lp02 by natural transformation (11, 54); the presence of the transposon within icmF was confirmed by PCR. Strains bearing a deletion of icmF or dotU were made by using pSV39 and pGD5, respectively, by standard procedures (39). In brief, plasmids were introduced via mating into L. pneumophila strain Lp02, selecting for plasmid integration by resistance to kanamycin on CYET-Kan plates. Purified colonies were then gown on media containing sucrose (CYET-Suc) to counterselect for the plasmid. Individual purified colonies were then screened for the presence of the deletion and the absence of the targeted gene locus by PCR. Dictyostelium discoideum strain AX3K, a kind gift of David Knecht (University of Connecticut, Storrs), was used for the depletion of dot/icm mutants.
L. pneumophila infections in murine macrophages.
Bone marrow-derived macrophages were isolated from the femurs of 6- to 8-week-old female A/J mice and prepared by standard procedures (56). Macrophages were maintained in RPMI medium supplemented with 10% heat-inactivated fetal bovine serum and 1 mM glutamine (Gibco, Carlsbad, Calif.). For L. pneumophila thymine auxotrophs, 100 μg of thymidine (Sigma-Aldrich, St. Louis, Mo.)/ml was added 24 h before the initiation of the infection and maintained throughout. L. pneumophila strains used for all infections were grown to early postexponential phase in AYET, as monitored by A600 and the appearance of motility (4). Strains bearing pJB908-derived plasmids were grown in AYE. Strains bearing pAM239(GFP) were patched onto CYET-Cm plates prior to growth in AYET supplemented with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). For monitoring intracellular growth, host cells were plated at 4 ×105 cells per well in 24-well tissue culture plates and were infected at a multiplicity of infection (MOI) of 0.05. After 2 h, the monolayer was washed twice with media. For each time point, the monolayer was lysed with 0.02% saponin and dilutions were plated onto CYET plates to determine the titer of CFU. For immunofluorescence analyses, macrophages were plated at a density of 1 ×105 to 2 ×105 cells per well and infected with L. pneumophila as described in the figure legends.
Enrichment for intracellular growth mutants.
Lp01 was transformed with a suicide vector containing a mini-Tn10 transposable element, pJK211, and plated on CYET-Kan. Between 500 and 1,000 individual colonies were pooled and introduced into U937 cells in the presence of either wortmannin or D. discoideum. For infections of U937 cells (26), the monolayers (2 × 106 cells per well in a 24-well plate) were pretreated with 5 μM wortmannin for 30 min and then inoculated with L. pneumophila at an MOI of 0.5 in the presence of fresh 5 μM wortmannin. After 1 h of incubation, gentamicin was added at 50 μg/ml for 30 min. The monolayer was washed three times with medium and lysed with 0.02% saponin, and dilutions of the lysate were plated onto CYET plates. D. discoideum was infected as previously described (53). Briefly, amoebae were plated at 106 cells/well and infected with L. pneumophila at an MOI of 5 for 30 min. Gentamicin was then added at 50 μg/ml, and after an additional 3.5 h, the monolayer was lysed with 0.02% saponin and dilutions were plated onto CYET plates. For either enrichment strategy, colonies that arose from an individual well were then pooled (>1,500 per pool) and grown in AYE to the early postexponential phase. Triplicate wells of bone marrow-derived macrophages were then incubated with each pool at an MOI of 0.5 for 60 min, after which 50 μg of gentamicin/ml was added for an additional 30 min. Washed cells were incubated for 5.5 h, and then 25 μg of gentamicin/ml was added to each well to kill any bacteria that were eventually liberated from macrophages after successful replication. Between 26 and 30 h after the initiation of the infection, the monolayer was washed several times to remove gentamicin and lysed with 0.02% saponin to isolate bacteria that had not lysed out from the macrophage. Lysates were plated onto CYET to recover L. pneumophila. Three to four colonies from each pool (when colonies were present) were tested for growth defects in bone marrow-derived macrophages from A/J mice. Briefly, murine macrophages were infected with L. pneumophila at an MOI of 1.0 for 90 min, and washed cells were lysed immediately, to determine total internalized bacteria, or lysed after further incubation of 48 h, to determine total yield of bacteria from each well. Dilutions were plated onto CYET plates, and the number of CFU was assessed for each time point.
Antibodies, Western blots, and immunofluorescence.
GST-DotU, His6-IcmF, and His6-DotG-CT were purified according to the manufacturers' instructions (Amersham; QIAGEN); His6-DotG-CT was purified under denaturing conditions with 6 M guanidine hydrochloride. Proteins were used to inoculate rabbits according to standard protocols (Pocono Rabbit Farm and Laboratory, Canadensis, Pa.). The antibodies against DotU and IcmF were affinity purified with the protein against which the antibody was made, whereas the serum from the DotG-CT immunization was used as the antibody. For analysis of L. pneumophila lysates, approximately 0.2 to 0.4 optical density (OD) units of cells grown in AYE were isolated by centrifugation, resuspended in sample buffer, and lysed by boiling. Equivalent amounts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride membranes, and processed for Western blot analysis by standard procedures (25). Serum specific for Bacillus subtilis isocitrate dehydrogenase (ICDH) was kindly provided by A. L. Sonenshein, Tufts University Medical School, Boston, Mass. For immunofluorescent analyses, cells were fixed (38) and stained (63) by standard procedures with 4% goat serum as a blocking agent. Affinity-purified antibody against SidC was a kind gift of Zhao-Qing Luo and was used at a dilution of 1:800. Rat anti-LAMP-1 antibody 1D4B (64) and rabbit anti-L. pneumophila antibody were used at dilutions of 1:100 and 1:10,000, respectively. To differentiate bacteria that had been internalized by macrophages from those that had not, a staining procedure was employed in which samples were stained with anti-L. pneumophila antibody both prior to and after permeabilization, with secondary antibodies conjugated to fluors of distinct color at each step (11).
Hydrophobicity plots and surface labeling.
Hydrophobicity plots were generated with the Kyte-Doolittle scale of the TopPred program by using a core window size of 19, a wedge window size of 5, and a critical loop length of 60 (7, 62). The cutoffs for certain and putative transmembrane segments are 1.0 and 0.6, respectively. To detect surface labeling, proteins exported across the L. pneumophila inner membrane were labeled with biotin as described previously (36) with several modifications. Briefly, L. pneumophila was grown to mid-logarithmic phase (OD of 1.3) in AYET, and 1.5 × 109 bacteria were washed twice in phosphate-buffered saline (PBS), resuspended in PBS containing 500 μg of EZ-link sulfo-NHS-LC-biotin (Pierce, Rockford, Ill.)/ml, and incubated for 30 min on ice. The reaction was then quenched by a 15-min incubation on ice with 50 mM ammonium chloride, and the cells were washed three times with PBS. Cells were lysed by boiling in 100 μl of buffer containing 1% SDS, 10 mM Tris-Cl (pH 8.0), and 1 mM EDTA. After the addition of 900 μl of buffer containing 2% Triton X-100, 150 mM NaCl, 50 mM Tris-Cl (pH 8.0), and 1 mM EDTA, lysates were cleared by centrifugation at 15,000 × g for 10 min. Streptavidin agarose (50 μl of a 50% slurry; Pierce) was added to 400 μl of cleared lysate, and the reaction mixture was incubated overnight at 4°C with rocking. The beads were washed four times with PBS, and bound proteins were released by boiling in the presence of sample buffer. Proteins in the bead supernatant prior to washing and cleared lysate prior to incubation with the beads (400 μl each) were precipitated with trichloroacetic acid and resuspended in sample buffer. Equivalent volumes were separated by SDS-PAGE and analyzed by Western blotting.
RESULTS
Isolation of L. pneumophila mutants defective for growth within macrophages.
We sought to identify L. pneumophila mutants that are defective for intracellular growth without isolating lesions in essential components of the Dot/Icm T4SS. L. pneumophila were mutagenized with a mini-Tn10 transposable element (11, 43), and then two independent strategies were employed to avoid reisolation of mutants in these dot/icm genes. The first method involves specifically interfering with uptake of strong dot/icm mutants by using the phosphatidylinositol 3-kinase inhibitor wortmannin, which has negligible effect on strains having an intact Dot/Icm system (33). To this end, mutagenized L. pneumophila were introduced into U937 cells in the presence of wortmannin, and internalized (dot/icm+) bacteria were isolated. The second method took advantage of the observation that mutants having lesions in essential components of the Dot/Icm T4SS do not survive internalization by D. discoideum (53). For this procedure, D. discoideum organisms were infected with mutagenized bacteria, and external bacteria were eliminated by the addition of gentamicin after a 30-min infection. Four hours after the initiation of infection, internalized bacteria were recovered. In both cases, preliminary experiments demonstrated that dot/icm+ bacteria were 100-fold enriched over the dotA3 strain (data not shown). Thirty-seven pools, each containing greater than 500 distinct transposon insertion mutants, were introduced into U937 cells in the presence of wortmannin (13 pools) or into D. discoideum (24 pools).
To isolate L. pneumophila mutants that were defective for intracellular growth, the pools of mutagenized bacteria depleted of mutations in essential Dot/Icm components were used to infect murine bone marrow-derived macrophages. After a 1-h pulse of infection, the membrane-impermeable antibiotic gentamicin was added to the media. By 24 h after initiation of the infection, L. pneumophila with wild-type growth rates had lysed the host cell and were killed upon contact with gentamicin. After the majority of the wild-type bacteria had lysed out, macrophages that harbored slow-growing bacteria were washed and mechanically lysed and the bacteria present in the lysate were recovered by plating on CYET. Initial experiments comparing a dot/icm+ strain with a strain defective for growth due to a mutation in dotA determined that this procedure enriched for intracellular growth mutants over 100-fold (data not shown). Bacteria from the 37 pools that survived the gentamicin enrichments were then tested individually for growth defects. Approximately 12% of the mutants thus tested exhibited a mild to severe growth defect, yielding 25 distinct intracellular growth mutants.
To identify the gene disrupted in each of the 25 mutants, the DNA surrounding the transposon insertion was obtained (see Materials and Methods) and end sequenced. The mutants were found to be contained in eight distinct genes, including five uncharacterized genes and three previously identified genes: milA (24), icmF (44, 49), and the gene immediately upstream of icmF, termed dotU (19, 51). Presuming that identical insertion sites from the same pool represented siblings and not independent insertion events, the number of distinct transposon insertions in each gene could also be determined: 15 of the 25 mutants were unique transposon insertions, and the remaining 10 mutants were siblings. The majority of the mutants isolated, 9 of the 15 unique transposon insertions, mapped to icmF or dotU. Since the majority of the mutations affected these poorly characterized genes, we focused our studies on the properties of these mutations and the behavior of their products.
icmF or dotU mutants are defective for growth in bone marrow-derived macrophages.
To characterize the intracellular growth of strains with mutations in icmF or dotU, bone marrow-derived macrophages were infected with wild-type L. pneumophila or strains bearing a mutation in dotA, icmF, or dotU. The dot/icm+ strain Lp02 increased in number over 1,000-fold within 3 days, whereas the dotA3 strain Lp03 decreased in number over the course of the infections (Fig. 1). Strains bearing a deletion of either icmF or dotU grew only 10-fold over the 3-day infection period. The growth rates of icmF and dotU deletion strains were nearly indistinguishable and were similar to the phenotype of icmF and dotU transposon mutants obtained in the gentamicin enrichment screen (data not shown). The growth defect of either mutant strain could be complemented by the presence of the corresponding gene expressed from a plasmid (Fig. 1). The intermediate growth defect in bone marrow-derived macrophages parallels that seen in earlier studies of icmF and dotU with distinct host cells. An icmF mutant strain was demonstrated to be partially defective for growth in and killing of the HL-60 macrophage-like cell line (44, 48, 65), and a dotU mutant was observed to grow weakly in guinea pig alveolar macrophages and HL-60 cells (19, 65). In both cases, the phenotype of icmF or dotU was intermediate between the wild-type strain and the majority of dot/icm mutants examined.
FIG. 1.
L. pneumophila strains bearing a deletion of either dotU or icmF are partially defective for growth in bone marrow-derived macrophages. Bone marrow-derived macrophages were infected at an MOI of 0.05 with each of the indicated strains for 2 h, after which the monolayer was washed to remove unbound bacteria. At each time point, the monolayer was lysed with 0.02% saponin, dilutions were plated onto CYET plates, and numbers of CFU were determined. The dot/icm+ strain Lp02 is the parent for both deletions as well as the dotA strain Lp03. All strains contained either a vector control (v; pJB908) or a complementing plasmid (picmF/pSV36 or pdotU/pSV38), as indicated. The experiment at each time point was completed in triplicate, and means and standard deviations are shown.
Since the icmF and dotU mutant strains grew steadily but poorly over the 3-day growth curve, the kinetics of growth during the first infection cycle were examined in detail. Bone marrow-derived macrophages were infected with a wild-type L. pneumophila strain or strains bearing a mutation in dotA, icmF, or dotU, and the number of bacteria per vacuole was examined either 1 or 14.5 h after the initiation of infection. After the initial 1-h pulse of infection, the majority of the macrophages contained a single bacterium (Fig. 2A). After 14.5 h, proliferation of the dot/icm+ strain was evident, with only 27% of macrophages still having 1 to 2 bacteria and approximately 36% of macrophages having greater than 12 bacteria (Fig. 2B). As seen previously, the dotA3 mutant displays a profound growth defect, with 100% of bacteria present in nonproliferative vacuoles. The icmF and dotU mutants showed clearly more efficient growth than the dotA3 strain, since 35 and 23% of vacuoles contain greater than 13 bacteria, respectively. The icmF and dotU mutant strains, however, produced a significant number vacuoles bearing 1 to 2 bacteria (41% for icmF and 52% dotU). L. pneumophila lacking icmF or dotU thus form replicative vacuoles at a decreased frequency relative to a wild-type strain. L. pneumophila strains lacking icmF or dotU therefore either are defective for initiation of replication or are unable to sustain an optimal intracellular niche. The mild defects observed in Fig. 1 appear to be due to the fact that although a fraction of bacteria are unable to replicate intracellularly, those organisms that do initiate replication appear to grow at rates close to those of wild-type strains (Fig. 2).
FIG. 2.
icmF and dotU mutants are defective for formation of replicative vacuoles. Bone marrow-derived macrophages were infected at an MOI of 1.0 for 1 h, after which they were washed and either fixed or incubated for an additional 13.5 h and then fixed. L. pneumophila were stained with antibody as described in the text (see Materials and Methods). The number of bacteria within each macrophage was assessed microscopically at each time point and assigned to one of the following four categories: 1 to 2 bacteria per macrophage, 3 to 6 bacteria per macrophage, 7 to 12 bacteria per macrophage, or more than 12 bacteria per macrophage. Approximately 100 infected macrophages were counted per coverslip (except for the dotA strain Lp03, for which 50 macrophages per coverslip were examined), and three coverslips were analyzed for each strain at each time point. Means and standard deviations are shown.
icmF and dotU mutants fail to avoid fusion with endosomal compartments.
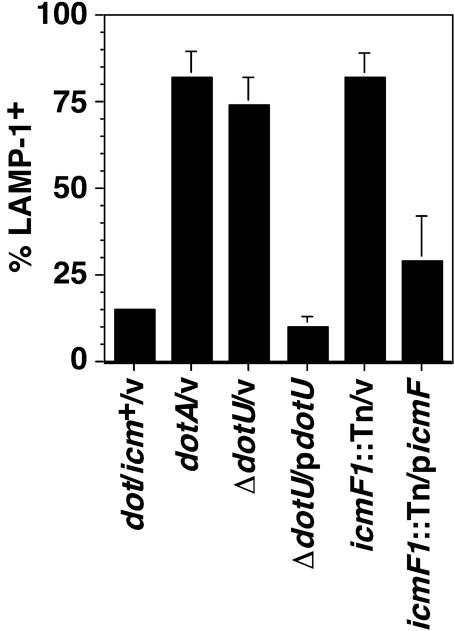
The inability of a subset of internalized icmF and dotU mutants to initiate multiplication may be due to their failure to escape the endocytic pathway. To assess targeting to late endosomal compartments, vacuoles were examined for the presence of LAMP-1, a membrane protein present on lysosomal compartments. Bone marrow-derived macrophages infected for 30 min with wild-type L. pneumophila or L. pneumophila strains bearing mutations in dotA, icmF, or dotU were thus stained with an antibody against LAMP-1. The dot/icm+ L. pneumophila strain was targeted efficiently, and only 15% of internalized bacteria were found in a LAMP-1-positive compartment. The dotA3 mutant Lp03, however, was unable to evade the endocytic pathway, and greater that 80% of bacteria colocalized with LAMP-1 (Fig. 3). The icmF and dotU mutant strains also displayed severe targeting defects, with 82 and 74% of internalized bacteria colocalizing with LAMP-1, respectively. The defect in either strain could be complemented to near wild-type levels by inclusion of a plasmid expressing icmF or dotU in the strain (Fig. 3).
FIG. 3.
L. pneumophila strains lacking dotU or icmF are internalized into vacuoles that associate with the endocytic pathway. Bone marrow-derived macrophages were infected for 30 min at an MOI of 10 with each of the indicated strains, and then the cells were washed, fixed, and probed with antibodies against L. pneumophila and LAMP-1. The icmF1::miniTn10 (icmF1::Tn) strain bears a transposon mutation near the amino terminus of icmF. Each strain was analyzed in triplicate, and means and standard deviations are shown. v, vector.
The strong defect in targeting (∼75 to 80% of icmF or dotU mutants colocalize with LAMP-1) (Fig. 3) contrasts with a less severe defect in the formation of replication vacuoles (∼50% of icmF or dotU mutants are capable of initiating replication) (Fig. 2), so we explored the possibility that icmF and dotU mutants could multiply within LAMP-1-positive compartments. Bone marrow-derived macrophages were thus infected with wild-type L. pneumophila or strains bearing a mutation in dotA, icmF, or dotU, and the vacuoles were examined for LAMP-1 colocalization 8 h after the initiation of infection. At this time point, dot/icm+ L. pneumophila strains had undergone considerable replication and vacuoles bearing multiple bacteria did not colocalize with LAMP-1 (Fig. 4). The dotA3 strain Lp03, however, failed to multiply, and approximately 50% of the bacteria colocalized with LAMP-1 (Fig. 4). This value is lower than that seen in the previous experiment (Fig. 3) due to the small loss of LAMP-1-positive vacuoles observed for each strain between 1 and 8 h that is consistently observed (data not shown). A similar percentage of vacuoles containing a single dotU or icmF (Fig. 4) mutant bacterium also stained positively for LAMP-1, yet replication vacuoles (those containing multiple bacteria) were LAMP-1 negative. From these data, growth of the icmF or dotU mutants (Fig. 1 and 2) appears to be due either to the 20 to 25% of bacteria that target into a vacuole that avoids localization in LAMP-1-positive compartments or to the ability of these mutants to escape the late endosomal compartment and subsequently replicate.
FIG. 4.
Neither the icmF nor the dotU strain replicates in LAMP-1-positive compartments. Bone marrow-derived macrophages were infected for 75 min at an MOI of 1.0 with either the dot/icm+ strain Lp02 (filled bars), the dotA3 strain Lp03 (open bars), the ΔdotU strain (narrow-hatched bars), or the icmF1::miniTn10 (icmF1::Tn) strain (wide-hatched bars). Washed monolayers were then incubated for an additional 7 h prior to fixation and probing with anti-L. pneumophila and anti-LAMP-1 antibodies. Each strain contained the vector pJB908, and identical results were obtained with strains lacking this plasmid (data not shown). Three coverslips were analyzed per strain, and ∼50 vacuoles were scored per coverslip. For each strain, the percentage of phagosomes containing 1 to 2, 3 to 6, or 7 or more bacteria per phagosome (A) and the percentage of phagosomes staining positive for LAMP-1 in each category (B) were scored. Only singly infected macrophages were analyzed. Means and standard deviations are shown.
The presence of DotU in L. pneumophila lysates requires IcmF and is growth phase dependent.
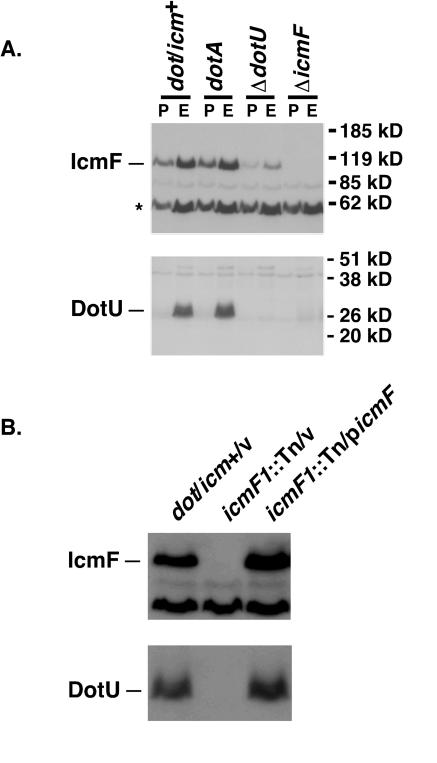
To analyze IcmF and DotU proteins in broth-grown L. pneumophila, antibodies against each protein were produced in rabbits and affinity purified. Wild-type (dot/icm+) L. pneumophila strains as well as strains bearing mutations in icmF or dotU were then grown to either mid-logarithmic phase or postexponential phase, lysed, and analyzed by Western blotting. The antibody against His6-IcmF recognized a protein in wild-type bacteria that migrated at approximately 110 kDa, consistent with its predicted molecular mass, in both exponential- and postexponential-phase bacteria (Fig. 5A, upper panel). This band was absent in a strain bearing a deletion of icmF and was decreased in a ΔdotU strain. Because the reduction of IcmF in the ΔdotU strain was not complemented by the expression of DotU from a plasmid, there is most likely a polar effect of the ΔdotU mutation on the downstream gene, icmF (data not shown). The anti-DotU antibody recognized a protein of approximately 30 kDa (predicted molecular mass, 30 kDa) in exponentially growing, wild-type bacteria, and this band was absent in strains with a deletion of dotU (Fig. 5A, lower panel). DotU was not present, however, in lysates of wild-type bacteria that were grown to postexponential phase. DotU was not found in stationary-phase culture supernatants, indicating that the protein was not secreted from the bacterium (data not shown). However, the regulation appears to be posttranscriptional because DotU expressed from a plasmid under control of the tac promoter was also absent in stationary-phase bacteria (data not shown). The disappearance of DotU in L. pneumophila lysates occurs rapidly and correlates with the acquisition of motility, which occurs around an OD of 3.2 (Fig. 5; data not shown).
FIG. 5.
The presence of DotU in L. pneumophila lysates is dependent on IcmF and growth phase. (A) L. pneumophila strains were grown in AYET to either exponential phase (OD of 1.5 to 2.5; E) or postexponential phase (OD > 4.0; P), and equivalent amounts were resuspended in sample buffer and lysed by boiling. Proteins were resolved by SDS-PAGE, transferred to a membrane support, and probed with antibodies against DotU or IcmF. (B) L. pneumophila strains bearing the indicated plasmids (v, pJB908; picmF, pSV36) were grown to mid-exponential phase in AYE and processed as described for panel A. The migration of DotU and IcmF is shown on the left, and the migration of molecular mass markers is shown on the right for panel A. The IcmF antibody recognizes a second protein (marked with an asterisk) that is unrelated to IcmF.
In addition to being dependent on growth phase, the stability of DotU was also dependent on the presence of IcmF: DotU was absent in bacteria that contained either a transposon mutation in or a complete deletion of icmF (Fig. 5, lower panels). The disappearance of DotU was not due to the loss of icmF having cis effects on the transcript encoding dotU because DotU was detectable in exponential-phase bacteria when icmF was expressed in trans from a plasmid (Fig. 5B). Therefore, the accumulation of DotU appears to depend on the presence of IcmF in the cell.
IcmF is accessible to a membrane-impermeant reagent in broth-grown L. pneumophila.
Analysis of IcmF and DotU with the TopPred program (7, 62) revealed that each protein contains at least one predicted transmembrane domain (Fig. 6A and B). IcmF has a single region, centered at codon 325, that is predicted to be a transmembrane domain, and DotU has two probable transmembrane domains, centered at codons 41 and 229. To determine whether either protein is inserted into the inner membrane or has any domain localized in the periplasm or outer membrane, surface-exposed proteins of exponential-phase wild-type L. pneumophila were labeled with an amine-reactive, membrane-impermeant biotinylation reagent (NHS-LC-biotin) and lysed, and biotinylated proteins were isolated with streptavidin-conjugated beads. Proteins from the total, supernatant, and bead-associated samples were separated by SDS-PAGE and probed with antibodies against specific proteins. Only very small amounts of the cytoplasmic protein, ICDH, were present in the bead fraction (Fig. 6C). In contrast, DotG, which has a single transmembrane domain and is predicted to span the inner membrane as part of the T4SS, was highly enriched on the beads. IcmF behaved like DotG, showing depletion from the supernatant fraction and strong enrichment in the bead fraction, indicating that IcmF and DotG are surface exposed. DotU, however, displayed a fractionation pattern similar to that of ICDH, with no depletion of the protein seen in the supernatant fraction and only small amounts in the bead fraction, most likely due to contamination (Fig. 6C). IcmF, therefore, is localized to the bacterial inner membrane or transported into or past the periplasmic space, whereas DotU was largely inaccessible to the probe, presumably because its large hydrophilic region is cytoplasmically exposed or is blocked by interactions with other proteins.
FIG. 6.
IcmF and DotU each contain a putative transmembrane domain(s), and IcmF is accessible to a membrane-impermeant reagent in broth-grown L. pneumophila. Hydrophobicity plots of IcmF (A) and DotU (B) were generated with the TopPred program by using the Kyte-Doolittle scale and a window size of 29. The solid line indicates the hydrophobicity value, and the short-dashed and long-dashed lines represent the cutoff values for certain and putative transmembrane segments, respectively. (C) Wild-type L. pneumophila was grown to mid-logarithmic phase in AYE, and surface-exposed proteins were labeled with membrane-impermeant sulfo-NHS-biotin for 30 min. Labeled cells were then lysed by boiling, and biotinylated proteins were isolated on streptavidin-conjugated agarose beads. Equivalent amounts of the total extract (T) as well as supernatant (S) and bead (B) samples were resolved by SDS-PAGE and probed for the presence of IcmF, DotU, DotG, and ICDH with antibodies specific for each of these proteins. The migration of each protein is indicated on the left.
Strains with deletions of either icmF or dotU display decreased translocation of the effector protein, SidC.
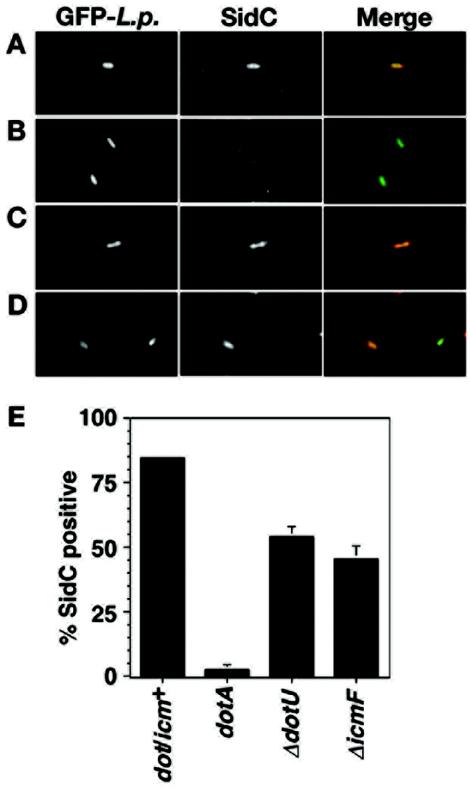
Our observations suggest that IcmF and DotU may be important for the functionality of the T4SS. To test this hypothesis in an assay that examines the function of the T4SS during host infection, translocation of the Dot/Icm substrate protein SidC into the host cell was monitored in L. pneumophila strains lacking icmF or dotU. Bone marrow-derived macrophages were infected for 1 h with wild-type L. pneumophila or strains bearing a mutation in dotA, icmF, or dotU and analyzed for SidC translocation by probing fixed cells with antibody against SidC. In the dot/icm+ strain, approximately 85% of L. pneumophila-containing vacuoles stained positively for SidC (Fig. 7A and E). In contrast, less than 5% of dotA bacteria colocalized with SidC (Fig. 7B and E). L. pneumophila lacking dotU (Fig. 7C and E) or icmF (Fig. 7D and E) displayed an intermediate phenotype, with 54 and 46% colocalization, respectively. In three separate experiments, the percentage of icmF or dotU vacuoles that colocalized with SidC varied, ranging from 19 to 46% for the icmF strain and from 11 to 54% for the dotU strain. This probably reflects the fact that the mutations cause an incomplete block in Dot/Icm-promoted processes. In contrast, the percentage of vacuoles that stained positively for SidC were consistent for dot/icm+ and dotA strains (75 to 85% of SidC-positive vacuoles for the wild type; 2 to 5% of SidC-positive vacuoles for dotA). It is clear that IcmF and DotU are required for optimal translocation of SidC into the host cell, indicating that these proteins have a role in assisting the assembly or maintaining the stability of the Dot/Icm translocon.
FIG. 7.
Translocation of effector protein SidC is reduced in strains with deletions of dotU or icmF. Bone marrow-derived macrophages were infected with the indicated strains for 1 h at an MOI of 2.0. Each strain expressed GFP from plasmid pAM239. After washing, monolayers were fixed and stained for external bacteria with an anti-L. pneumophila antibody prior to permeabilization and then stained with an anti-SidC antibody after permeabilization. (A to D) SidC staining of GFP-expressing L. pneumophila strains Lp02 (dot/icm+) (A), Lp03 (dotA3) (B), ΔdotU (C), and ΔicmF (D). GFP stains are shown in the left panels (GFP-L.p.), SidC stains are shown in the center panels (SidC), and merged images are shown in the right panels (merge). (E) Quantitation of SidC colocalization. For the experiment shown, three coverslips were examined per strain and approximately 100 vacuoles were characterized per coverslip. Means and standard deviations are indicated.
DISCUSSION
A double enrichment strategy that selected against strong defects in the Dot/Icm complex led to the identification of eight genes that are required for efficient intracellular growth of L. pneumophila in bone marrow-derived macrophages. Interestingly, all of the mutants displayed growth rates that were intermediate between wild-type L. pneumophila and a dotA3 mutant, and the majority of the mutants exhibited a relatively mild defect (data not shown). Mutants with strong growth defects were likely lost either in the first enrichment, because they contained lesions in the Dot/Icm cluster, or in the second enrichment, because faster-growing mutants make up a higher proportion of the bacteria recovered from the macrophage lysate. Thus, although mutants defective at any stage of intracellular growth are candidates for isolation, the strategy presented here makes those that are capable of considerable replication more likely to be obtained.
One of the mutants obtained corresponds to the previously characterized gene, milA (24). milA was previously isolated as a mutant defective for growth within and killing of U937 cells and was an anticipated target of our screen. Although milA mutants successfully evade compartments that label with the lysosomal proteins LAMP-1 or cathepsin D and acquire wild-type levels of ER markers at 4 h postinfection, the mutants colocalize with a distinct lysosomal marker LAMP-2 to a small extent and display decreased colocalization with ER markers relative to wild-type strains after 6 h of infection (24). MilA, therefore, appears to be required for the maintenance of a replicative vacuole. Homology to permeases from many bacterial species indicates that MilA may function as a transport protein.
Strains with mutations in icmF and dotU were isolated because they have less severe defects in intracellular growth than the majority of dot/icm mutants. Reconstruction experiments, in which the survival of selected mutants was compared to that of wild-type strains by using the two enrichment strategies described in this work (treatment with wortmannin or exposure to D. discoideum), revealed that although dotA mutants were strongly depleted, icmF or dotU mutants survived, although to a lesser extent than wild-type L. pneumophila (data not shown). The genes encoding IcmF and DotU are adjacent to one another in the L. pneumophila genome at one end of the major cluster of dot/icm genes but are separated from the cluster by a single gene, citA, that has no role in L. pneumophila intracellular growth (44). Previous results indicate that icmF, dotU, and citA are cotranscribed (65). The icmF gene was isolated in the original icm (defective in intracellular multiplication) screen in which mutagenized L. pneumophila strains were examined for strains that were defective in killing a macrophage-like cell line, HL-60 (44). The dotU mutant was isolated in an independent study by using signature-tagged mutagenesis with the guinea pig infection model system (19) and was shown to have a mild growth defect in isolated guinea pig macrophages compared to that of a wild-type strain. An intermediate growth defect in HL-60 cells has been described for both icmF and dotU mutants (65), although the growth defects observed in the model amoebic organism Acanthameba castellani were significantly more severe than what we have observed with the amoeba D. discoideum incubated with either mutant (data not shown).
Orthologs of icmF and dotU exist in at least 17 distinct gram-negative bacterial strains (data not shown) (15). Interestingly, many icmF and dotU orthologs are in bacterial strains that closely associate with eukaryotic cells, and several are found in other pathogenic bacteria, including Pseudomonas aeruginosa, Salmonella enterica serovar Typhimurium, Vibrio cholerae, E. coli O157:H7, Coxiella burnetii, and Yersinia pestis (15). With the exception of C. burnetii, icmF and dotU are not found in other species that contain T4SS. Some of the species with icmF and dotU orthologs contain distinct specialized secretion systems, such as type III secretion systems. The observation that each strain with icmF and dotU orthologs associates with eukaryotic cells hints that these proteins may stabilize protein complexes essential for interkingdom interactions. The most extensively characterized icmF/dotU ortholog is the icmF ortholog of V. cholerae, which was found to be induced in vivo in a mouse model system (14). Further study demonstrated that the V. cholerae mutant is less motile than the wild-type strain but displays increased adherence to cultured epithelial cells and enhanced frequency of mating when used as a recipient (13). These phenotypes indicate a possible role in the structural integrity of surface-exposed complexes. Interestingly, in 10 of the strains that contain icmF and dotU orthologs, these genes are part of a cluster of 15 linked genes with orthologs in each species (15). Although L. pneumophila lacks this cluster, this finding indicates that IcmF and DotU orthologs may function in the context of a larger group of proteins in other organisms.
In our study, the icmF and dotU mutant strains displayed only mild growth defects in bone marrow-derived macrophages but were found to be strongly defective in avoiding LAMP-1-positive compartments, displaying colocalization rates with LAMP-1-containing compartments that were nearly identical to a dotA mutant strain. Since the majority of L. pneumophila mutants that colocalize with LAMP-1 are completely defective for intracellular growth, the icmF and dotU mutants exhibit a rare and interesting phenotype. We tested whether icmF and dotU mutants are capable of growth within a LAMP-1-positive compartment, but at 4 and 8 h postinfection, replication vacuoles did not stain positively for LAMP-1. It appeared, then, that a fraction of icmF and dotU mutants that were initially within a LAMP-1-positive compartment were capable of escaping this compartment and subsequently replicating. The alternate possibility is that many of the bacteria found in the LAMP-1 compartment at the 1 h time point get degraded, enriching for bacteria lacking this marker at 4 and 8 h postinfection. The discovery that early in the infection only 20% of the vacuoles harboring icmF or dotU mutants avoid a LAMP-1-positive compartment yet as many as 50% can be SidC-positive indicates that there may be vacuoles that are both LAMP-1 positive and capable of translocating SidC. In fact, when vacuoles were double stained for LAMP-1 and SidC, the icmF and dotU strains displayed a low percentage of vacuoles that were strongly positive for both markers (data not shown), consistent with at least some of the LAMP-1 compartments being different in nature from compartments harboring previously characterized dot/icm mutants.
The icmF and dotU mutants may interact more weakly with the endosomal network than does a dotA mutant, resulting in a lower LAMP-1 density on vacuoles containing icmF or dotU mutants than found with a dotA mutant. We were unable, however, to detect a significant difference in the intensity of LAMP-1 stain between vacuoles containing dotA and icmF or dotU through immunofluorescence analysis (data not shown), although the density of this marker may not be a true reflection of the extent of maturation within the endosomal network. On the other hand, partially purified vacuoles from bone marrow-derived macrophages infected with icmF or dotU mutants were found to be considerably more resistant to physical stress than vacuoles containing dotA mutants, indicating that the membrane composition of icmF/dotU-containing vacuoles differs from those of a dotA vacuole (data not shown). This observation supports the idea that, although icmF and dotU mutants are found in LAMP-1-positive compartments, these compartments do not share all properties of a dotA compartment. It appears, therefore, that vacuoles bearing icmF and dotU mutants are LAMP-1 positive, but at least a fraction of these compartments are distinct from vacuoles containing dotA mutant bacteria based on the partial colocalization with SidC and the decreased fragility of these compartments compared to a dotA mutant.
In each assay that monitors different aspects of the L. pneumophila infection, strains bearing mutations in icmF or dotU behave nearly identically. This observation, combined with the discovery that high steady-state levels of DotU are dependent on the presence of IcmF, indicate that these two proteins define a functional unit and may physically interact. In addition, with the exception of the LAMP-1 targeting assay, icmF and dotU mutants display phenotypes intermediate between wild-type bacteria and a classical dot/icm mutant including growth in bone marrow-derived macrophages (Fig. 1), formation of replicative vacuoles (Fig. 2), translocation of SidC (Fig. 7), plasmid conjugation (data not shown), and pore formation (data not shown). IcmF and DotU, therefore, may play a role in stabilizing or assisting in the assembly of the Dot/Icm complex. The extracytoplasmic localization of IcmF places the protein in an appropriate context to perform such a function.
The discovery that DotU is degraded as L. pneumophila enters the stationary phase is intriguing because it occurs concurrently with the growth phase associated with peak virulence (4). Many of the traits associated with increased virulence, including salt sensitivity, accurate targeting, and enhanced survival after uptake, are eliminated in dot/icm mutant strains, indicating that these traits are dependent on an intact Dot/Icm T4SS. It is possible that the Dot/Icm T4SS undergoes a conformational change in the early stationary phase that increases the virulence of L. pneumophila in macrophages. IcmF and DotU may function together to stabilize the T4SS in logarithmically growing bacteria, and once DotU is degraded, the translocon may be released into a more active conformation. Alternatively, IcmF may assist in the assembly of a translocon conformation that is necessary for full virulence but be prevented from functioning in exponentially growing bacteria through association with DotU. Since a strain lacking DotU confers an intracellular growth defect, this protein must have a positive role in addition to its inhibitory function on IcmF. The presence of DotU in the exponential phase may be required for the appropriate association of IcmF with the Dot/Icm complex in the early postexponential phase. Preliminary data indicate that in icmF or dotU mutant strains at least one component of the T4SS is inappropriately degraded in stationary phase, supporting a role for IcmF and DotU in stabilizing the postexponential phase or virulent form of the apparatus. Since the Dot/Icm T4SS is able to function to a large extent in the absence of IcmF or DotU, these proteins are certainly not essential components of the translocon. Instead, as may be the case for their orthologs in other bacterial species, these proteins are necessary for optimal function of this apparatus.
Acknowledgments
We are grateful to A. L. Sonenshein (Tufts University Medical School), J. Kirby (Beth Israel Deaconess Medical Center), and J. Vogel (University of Washington) for generously supplying reagents. We thank May Tang for assistance in protein purification and members of the Isberg laboratory, past and present, for advice and stimulating discussions. We are grateful to Molly Bergman, Isabelle Derré, Marion Dorer, Zhao-Qing Luo, and Matthias Machner for critical reading of the manuscript.
This work was supported by the Jane Coffin Childs Memorial Fund for Cancer Research (S.M.V.), the Irvington Institute for Immunological Research (G.D.), and the Howard Hughes Medical Institute (R.R.I.).
Editor: J. T. Barbieri
REFERENCES
- 1.Andrews, H. L., J. P. Vogel, and R. R. Isberg. 1998. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect. Immun. 66:950-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger, K. H., and R. R. Isberg. 1993. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7:7-19. [DOI] [PubMed] [Google Scholar]
- 3.Berger, K. H., J. J. Merriam, and R. R. Isberg. 1994. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol. Microbiol. 14:809-822. [DOI] [PubMed] [Google Scholar]
- 4.Byrne, B., and M. S. Swanson. 1998. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, J., K. S. de Felipe, M. Clarke, H. Lu, O. R. Anderson, G. Segal, and H. A. Shuman. 2004. Legionella effectors that promote nonlytic release from protozoa. Science 303:1358-1361. [DOI] [PubMed] [Google Scholar]
- 6.Christie, P. J., and J. P. Vogel. 2000. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 8:354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claros, M. G., and G. von Heijne. 1994. TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci. 10:685-686. [DOI] [PubMed] [Google Scholar]
- 8.Clemens, D. L., and M. A. Horwitz. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coers, J., J. C. Kagan, M. Matthews, H. Nagai, D. M. Zuckman, and C. R. Roy. 2000. Identification of Icm protein complexes that play distinct roles in the biogenesis of an organelle permissive for Legionella pneumophila intracellular growth. Mol. Microbiol. 38:719-736. [DOI] [PubMed] [Google Scholar]
- 10.Coers, J., C. Monahan, and C. R. Roy. 1999. Modulation of phagosome biogenesis by Legionella pneumophila creates an organelle permissive for intracellular growth. Nat. Cell Biol. 1:451-453. [DOI] [PubMed] [Google Scholar]
- 11.Conover, G. M., I. Derre, J. P. Vogel, and R. R. Isberg. 2003. The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol. Microbiol. 48:305-321. [DOI] [PubMed] [Google Scholar]
- 12.Das, A., and Y. H. Xie. 2000. The Agrobacterium T-DNA transport pore proteins VirB8, VirB9, and VirB10 interact with one another. J. Bacteriol. 182:758-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das, S., A. Chakrabortty, R. Banerjee, and K. Chaudhuri. 2002. Involvement of in vivo induced icmF gene of Vibrio cholerae in motility, adherence to epithelial cells, and conjugation frequency. Biochem. Biophys. Res. Commun. 295:922-928. [DOI] [PubMed] [Google Scholar]
- 14.Das, S., A. Chakrabortty, R. Banerjee, S. Roychoudhury, and K. Chaudhuri. 2000. Comparison of global transcription responses allows identification of Vibrio cholerae genes differentially expressed following infection. FEMS Microbiol. Lett. 190:87-91. [DOI] [PubMed] [Google Scholar]
- 15.Das, S., and K. Chaudhuri. 2003. Identification of a unique IAHP (IcmF associated homologous proteins) cluster in Vibrio cholerae and other proteobacteria through in silico analysis. In Silico Biol. 3:0025. [PubMed] [Google Scholar]
- 16.Derré, I., and R. R. Isberg. 2004. Legionella pneumophila replication vacuole formation involved rapid recruitment of proteins of the early secretory system. Infect. Immun. 72:3048-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumenil, G., and R. R. Isberg. 2001. The Legionella pneumophila IcmR protein exhibits chaperone activity for IcmQ by preventing its participation in high-molecular-weight complexes. Mol. Microbiol. 40:1113-1127. [DOI] [PubMed] [Google Scholar]
- 18.Dumenil, G., T. P. Montminy, M. Tang, and R. R. Isberg. 2004. IcmR-regulated membrane insertion and efflux by Legionella pneumophila IcmQ protein. J. Biol. Chem. 279:4686-4695. [DOI] [PubMed] [Google Scholar]
- 19.Edelstein, P. H., M. A. Edelstein, F. Higa, and S. Falkow. 1999. Discovery of virulence genes of Legionella pneumophila by using signature tagged mutagenesis in a guinea pig pneumonia model. Proc. Natl. Acad. Sci. USA 96:8190-8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feeley, J. C., R. J. Gibson, G. W. Gorman, N. C. Langford, J. K. Rasheed, D. C. Mackel, and W. B. Baine. 1979. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J. Clin. Microbiol. 10:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fields, B. S. 1996. The molecular ecology of legionellae. Trends Microbiol. 4:286-290. [DOI] [PubMed] [Google Scholar]
- 22.Finberg, K. E., T. R. Muth, S. P. Young, J. B. Maken, S. M. Heitritter, A. N. Binns, and L. M. Banta. 1995. Interactions of VirB9, -10, and -11 with the membrane fraction of Agrobacterium tumefaciens: solubility studies provide evidence for tight associations. J. Bacteriol. 177:4881-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabay, J. E., M. Blake, W. D. Niles, and M. A. Horwitz. 1985. Purification of Legionella pneumophila major outer membrane protein and demonstration that it is a porin. J. Bacteriol. 162:85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harb, O. S., and Y. Abu Kwaik. 2000. Characterization of a macrophage-specific infectivity locus (milA) of Legionella pneumophila. Infect. Immun. 68:368-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harlow, E., and D. Lane. 1999. Using antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Harris, P., and P. Ralph. 1985. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J. Leukoc. Biol. 37:407-422. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz, M. A. 1987. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J. Exp. Med. 166:1310-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horwitz, M. A. 1983. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158:1319-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horwitz, M. A. 1983. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158:2108-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwitz, M. A., and S. C. Silverstein. 1980. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J. Clin. Investig. 66:441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagan, J. C., and C. R. Roy. 2002. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 4:945-954. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann, A. F., J. E. McDade, C. M. Patton, J. V. Bennett, P. Skaliy, J. C. Feeley, D. C. Anderson, M. E. Potter, V. F. Newhouse, M. B. Gregg, and P. S. Brachman. 1981. Pontiac fever: isolation of the etiologic agent (Legionella pneumophilia) and demonstration of its mode of transmission. Am. J. Epidemiol. 114:337-347. [DOI] [PubMed] [Google Scholar]
- 33.Khelef, N., H. A. Shuman, and F. R. Maxfield. 2001. Phagocytosis of wild-type Legionella pneumophila occurs through a wortmannin-insensitive pathway. Infect. Immun. 69:5157-5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolter, R., M. Inuzuka, and D. R. Helinski. 1978. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell 15:1199-1208. [DOI] [PubMed] [Google Scholar]
- 35.Luo, Z.-Q., and R. R. Isberg. 2003. Multiple novel substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. USA 101:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthews, M., and C. R. Roy. 2000. Identification and subcellular localization of the Legionella pneumophila IcmX protein: a factor essential for establishment of a replicative organelle in eukaryotic host cells. Infect. Immun. 68:3971-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDade, J. E., C. C. Shepard, D. W. Fraser, T. R. Tsai, M. A. Redus, and W. R. Dowdle. 1977. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N. Engl. J. Med. 297:1197-1203. [DOI] [PubMed] [Google Scholar]
- 38.McLean, I. W., and P. K. Nakane. 1974. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J. Histochem. Cytochem. 22:1077-1083. [DOI] [PubMed] [Google Scholar]
- 39.Merriam, J. J., R. Mathur, R. Maxfield-Boumil, and R. R. Isberg. 1997. Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect. Immun. 65:2497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muder, R. R., V. L. Yu, and A. H. Woo. 1986. Mode of transmission of Legionella pneumophila. A critical review. Arch. Intern. Med. 146:1607-1612. [PubMed] [Google Scholar]
- 41.Nagai, H., J. C. Kagan, X. Zhu, R. A. Kahn, and C. R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295:679-682. [DOI] [PubMed] [Google Scholar]
- 42.Nagai, H., and C. R. Roy. 2001. The DotA protein from Legionella pneumophila is secreted by a novel process that requires the Dot/Icm transporter. EMBO J. 20:5962-5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pope, C. D., L. Dhand, and N. P. Cianciotto. 1994. Random mutagenesis of Legionella pneumophila with mini-Tn10. FEMS Microbiol. Lett. 124:107-111. [DOI] [PubMed] [Google Scholar]
- 44.Purcell, M., and H. A. Shuman. 1998. The Legionella pneumophila icmGCDJBF genes are required for killing of human macrophages. Infect. Immun. 66:2245-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rohde, M., J. Puls, R. Buhrdorf, W. Fischer, and R. Haas. 2003. A novel sheathed surface organelle of the Helicobacter pylori cag type IV secretion system. Mol. Microbiol. 49:219-234. [DOI] [PubMed] [Google Scholar]
- 46.Roy, C. R., K. H. Berger, and R. R. Isberg. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663-674. [DOI] [PubMed] [Google Scholar]
- 47.Roy, C. R., and R. R. Isberg. 1997. Topology of Legionella pneumophila DotA: an inner membrane protein required for replication in macrophages. Infect. Immun. 65:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadosky, A. B., L. A. Wiater, and H. A. Shuman. 1993. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect. Immun. 61:5361-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segal, G., M. Purcell, and H. A. Shuman. 1998. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. USA 95:1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segal, G., and H. A. Shuman. 1998. How is the intracellular fate of the Legionella pneumophila phagosome determined? Trends Microbiol. 6:253-255. [DOI] [PubMed] [Google Scholar]
- 51.Sexton, J. A., and J. P. Vogel. 2002. Type IVB secretion by intracellular pathogens. Traffic 3:178-185. [DOI] [PubMed] [Google Scholar]
- 52.Solomon, J. M., and R. R. Isberg. 2000. Growth of Legionella pneumophila in Dictyostelium discoideum: a novel system for genetic analysis of host-pathogen interactions. Trends Microbiol. 8:478-480. [DOI] [PubMed] [Google Scholar]
- 53.Solomon, J. M., A. Rupper, J. A. Cardelli, and R. R. Isberg. 2000. Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions. Infect. Immun. 68:2939-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stone, B. J., and Y. Abu Kwaik. 1999. Natural competence for DNA transformation by Legionella pneumophila and its association with expression of type IV pili. J. Bacteriol. 181:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swanson, M. S., and R. R. Isberg. 1996. Analysis of the intracellular fate of Legionella pneumophila mutants. Ann. N. Y. Acad. Sci. 797:8-18. [DOI] [PubMed] [Google Scholar]
- 56.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swanson, M. S., and R. R. Isberg. 1996. Identification of Legionella pneumophila mutants that have aberrant intracellular fates. Infect. Immun. 64:2585-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thorstenson, Y. R., G. A. Kuldau, and P. C. Zambryski. 1993. Subcellular localization of seven VirB proteins of Agrobacterium tumefaciens: implications for the formation of a T-DNA transport structure. J. Bacteriol. 175:5233-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tilney, L. G., O. S. Harb, P. S. Connelly, C. G. Robinson, and C. R. Roy. 2001. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J. Cell Sci. 114:4637-4650. [DOI] [PubMed] [Google Scholar]
- 60.Vogel, J. P., and R. R. Isberg. 1999. Cell biology of Legionella pneumophila. Curr. Opin. Microbiol. 2:30-34. [DOI] [PubMed] [Google Scholar]
- 61.Vogel, J. P., C. Roy, and R. R. Isberg. 1996. Use of salt to isolate Legionella pneumophila mutants unable to replicate in macrophages. Ann. N. Y. Acad. Sci. 797:271-272. [DOI] [PubMed] [Google Scholar]
- 62.von Heijne, G. 1992. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225:487-494. [DOI] [PubMed] [Google Scholar]
- 63.Watarai, M., H. L. Andrews, and R. R. Isberg. 2001. Formation of a fibrous structure on the surface of Legionella pneumophila associated with exposure of DotH and DotO proteins after intracellular growth. Mol. Microbiol. 39:313-329. [DOI] [PubMed] [Google Scholar]
- 64.Watarai, M., I. Derre, J. Kirby, J. D. Growney, W. F. Dietrich, and R. R. Isberg. 2001. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J. Exp. Med. 194:1081-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zusman, T., M. Feldman, E. Halperin, and G. Segal. 2004. Characterizatin of the icmH and icmF genes required for Legionella pneumophila intracellular growth, genes that are present in many bacteria associated with eukaryotic cells. Infect. Immun. 72:3398-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]