Abstract
Background: Increased numbers of bone marrow–derived progenitor cells, known as fibrocytes, populate the peripheral circulation, orbit, and thyroid of patients with Graves' disease (GD). These cells have been implicated in the development of thyroid-associated ophthalmopathy. They can differentiate into myofibroblasts or adipocytes, produce inflammatory cytokines, and remodel tissue. This study sought to determine whether thyrotropin (TSH) and CD40 ligand (CD40L), implicated in the pathogenesis of GD, induce interleukin-12 (IL-12) in human fibrocytes.
Materials and Methods: IL-12 protein concentrations and mRNA levels were measured by Luminex and real-time polymerase chain reaction, respectively. Flow cytometry assessed intracellular IL-12 concentrations. Vector containing IL-12p40 promoter was transfected into cultured fibrocytes, and promoter activity was monitored using luciferase assay.
Results: TSH and CD40L stimulated intracellular IL-12 protein accumulation in peripheral blood fibrocytes. Inhibiting Akt and nuclear factor-κB (NF-κB) activity diminished IL-12 expression in fibrocytes, while TSH did not induce promoter activity. TSH-mediated IL-12 production required de novo synthesized proteins and augmented IL-12 mRNA stability. IL-12 production mediated by CD40L required tumor necrosis factor receptor–associated factor 6.
Conclusion: TSH and CD40L induce IL-12 expression in fibrocytes, and Akt and NF-κB mediate this activity. Given the importance of IL-12 in immune function, its production by fibrocytes may promote an inflammatory immune response and tissue remodeling in thyroid-associated ophthalmopathy.
Keywords: : Graves' disease, thyroid-associated ophthalmopathy, fibrocyte, autoimmunity, interleukin-12
Introduction
Graves' disease (GD) is a systemic autoimmune disease involving the thyroid gland, skin, and orbit (1). Thyrotropin (TSH) binds the TSH receptor (TSHR) expressed on thyroid epithelial cells, stimulating the production of thyroid hormone (2). Activating autoantibodies targeting the TSHR and infiltrating lymphocytes cause thyroid gland dysfunction by driving overproduction of thyroid hormone and potentiating inflammation. Despite progress in understanding the endocrine manifestations of GD, the pathogenesis of thyroid-associated ophthalmopathy (TAO) and the role of the TSHR remain largely unknown (3). Orbital tissues from patients with TAO express several putative autoantigens, including the TSHR (4), which may mediate immune activation.
Fibrocytes, bone marrow–derived progenitor cells, migrate to inflamed tissue and have a characteristic phenotype expressing markers of myeloid cells (CD45), hematopoietic cells (CD34), and fibroblasts (type 1 collagen) (5–7). Fibrocytes display remarkable functional plasticity, antigen presentation, and cytokine and chemokine secretion, and can differentiate into thyroid epithelium, myofibroblasts, or fat cells (2,6,7). Frequencies of circulating TSHR+ and CD40+ fibrocytes are greater in patients with GD than they are in healthy controls (8). It is hypothesized that these fibrocytes selectively infiltrate TAO tissue, since fibroblasts with the analogous phenotype are identified in orbital tissues in TAO but not in healthy tissue (8). Recent studies have characterized the phenotype of early progenitors of fibrocytes in the peripheral blood, which may facilitate insight into their contribution to TAO (9,10).
TSHR and CD40 activation of fibroblasts and fibrocytes have been implicated in the pathogenesis of TAO. The TSHR is expressed at a markedly higher level on fibrocytes than on fibroblasts (8). Furthermore, stimulating fibrocytes with TSH or an activating autoantibody to the TSHR, such as M22, results in the production of several proinflammatory cytokines (11).
CD40, a transmembrane receptor of the tumor necrosis factor (TNF) receptor superfamily, is expressed on various cell types (12–16). Aberrant signaling through the CD40 pathways has been implicated in the pathogenesis of several autoimmune diseases, including GD (17–19). Fibrocytes from patients with TAO have higher levels of CD40 than do those from healthy donors (20).
Interleukin-12 (IL-12) is a heterodimeric protein composed of 35 and 40 kDa subunits forming IL-12 p70 (p35 and p40) (21). IL-12 expression, which is regulated by CD40–CD40L binding during interactions between T lymphocytes and antigen-presenting cells (22,23), has been directly and indirectly implicated in the pathogenesis of GD (24–27). Thyroid tissue from patients with GD express abundant IL-12p40 mRNA, and their serum IL-12 concentrations are significantly higher than those are in healthy controls (28,29). The relative overabundance of IL-12 appears to be related to GD severity. However, the site of the increased IL-12 production is unknown (29,30). This study sought to determine whether fibrocytes activated by TSH and CD40L express IL-12 and to describe the mechanisms of activation.
Methods
Patient samples
Patients with GD (n = 7) were recruited from the Kellogg Eye Center at the University of Michigan. Informed consent was obtained in compliance with policies of the Institutional Review Board of the University of Michigan Health System. Research methods followed the tenets of the Declaration of Helsinki. Leukocyte reduction filters provided by the American Red Cross were the source of fibrocytes of healthy controls.
Fibrocyte preparation and treatments
Fibrocytes were assayed directly from peripheral blood mononuclear cells (PBMCs) or after in vitro differentiation (8,31). PBMCs were isolated using Ficoll-Paque Plus (GE Healthcare Bio-Sciences, Pittsburgh, PA; catalog no. 17-1440-03). They were then incubated in Gibco™ Dulbecco's modified Eagle's medium with 10% fetal bovine serum (FBS) and Gibco™ 1% penicillin-streptomycin mixture (Pen Strep) until differentiation. Culture purity was >90% fibrocytes, as determined by flow cytometry.
Peripheral blood fibrocytes were identified within 24 h of acquisition among PBMCs according to their expression of CD45, CD34, and type I collagen (Col1) (11,20). CD45-PerCP, CD34-APC, isotype control-FITC, isotype controlPerCP, and isotype controlAPC were used to assay phenotypes (catalog nos. 347464, 560940, 555748, 340762, and 555751, respectively; BD Biosciences, San Jose, CA), and collagen type I FITC (catalog no. FCMAB412F; EMD Millipore, Temecula, CA).
Fibrocyte treatments
Cultured and peripheral blood fibrocytes were treated with bovine TSH (5 mIU/mL) from Calbiochem–EMD Biosciences (La Jolla, CA), CD40L (100 ng/mL soluble, recombinant human, MegaCD40L) from Enzo Life Sciences (Farmingdale, NY), and TSHR stimulating autoantibody (M22, 10 ng/mL) from Kronus, Inc. (Star, ID). In some experiments, cells were pretreated with 500 nM of Akt inhibitor IV (AKTi, Calbiochem–EMD Biosciences) or 5 μg/mL of carbobenzoxy-Leu-Leuleucinal (MG132) from Cayman Chemical (Ann Arbor, MI). Inhibitors were added 1 h before TSH or CD40L stimulation.
A TNF receptor–associated factor 2 (TRAF2) inhibitor peptide (DRQIKIWFQNRRMKWKKNTAAPVQETLHGCQPVTQ) (32) was synthesized by New England Peptide (Gardner, MA). A TRAF6 inhibitor and a control peptide were purchased from Imgenex (San Diego, CA). For studies assessing response to these peptides, cells were treated with peptides (50 μM) and added 24 h and 1 h before stimulation.
5,6-dichlorobenzimidazole (DRB) from Cayman Chemical or cycloheximide (Sigma, St. Louis, MO) was added at a final concentration of 50 μM or 10 μg/mL, respectively, to cultured fibrocytes.
IL-12 protein production
Extracellular IL-12 concentration was measured using Luminex analysis (Invitrogen IL-12 Human Singleplex Bead Kit; Life Technologies, Grand Island, NY; catalog no. LHC0121). Intracellular IL-12 was tested with two antibodies: one specific to p40 monomers and p70 heterodimers (IL-12p40/p70-PE; BD Biosciences; catalog no. 559329), and the other specific for p70 heterodimers (IL-12p70-PE; BD Biosciences; catalog no. 559325. PBMCs were stimulated with nothing (control), TSH, or CD40L, and treated with GolgiStop (BD Biosciences; catalog no. 554724) after 6 h of stimulation. After 18 h in culture, cells were collected and treated first with the surface markers of fibrocytes. For the intracellular IL-12 detection, fibrocytes were permeabilized and fixed in Cytofix/Cytoperm solution (BD Biosciences; catalog no. 554722) At least 106 events were collected. Mean fluorescent intensity (MFI) was calculated as a ratio of geometric mean fluorescence of the sample to the geometric mean fluorescence of the isotype.
IL-12 mRNA production
IL-12p40 subunit mRNA was quantified by real-time polymerase chain reaction (PCR). Total RNA was isolated by using Aurum Total RNA Mini Kit (Bio-Rad Laboratories, Hercules, CA) and reverse transcribed with QuantiTect Reverse Transcription Kit (Qiagen, Germantown, MD). Quantitative PCR was performed by using a SYBR Green kit from Bio-Rad, with 5′-ACA AAG GAG GCG AGG TTC TAA-3′ and 5′-CCC TTG GGG GTC AGA AGA G-3′ as the forward and reverse primers, respectively, for IL-12. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene, with forward, 5′-TTG CCA TCA ATG ACC CCT T-3′, and reverse, 5′-CGC CCC ACT TGA TTT TGG A-3′, primers.
Study of Il-12 promoter activity
A promoter region fragment (1544 bp spanning from −138 to +161) of IL-12p40 cloned into pGL3 basic vector was purchased from GeneCopoeia (Rockville, MD). The promoterless pGL3 vector was used as a control. Vectors were transfected into cultured fibrocytes using Human CD34+ Nucleofection Kit from Lonza (Walkersville, MD) according to the procedure described by Raychaudhuri et al. (33) The luciferase assay reagents were from Promega (Madison, WI; catalog no. E1980).
Statistical analysis
Each experiment was performed in triplicate. Unless otherwise stated, data are reported as means and standard deviations, and were analyzed with analysis of variance. All experiments were performed at least three times.
Results
TSH and CD40L increase IL-12 production
IL-12 production in peripheral blood fibrocytes was studied by using two antibodies: one specific for IL-12p40/p70, and the other specific for IL-12 p70. TSH induced significant intracellular accumulation of IL-12p40/p70 after 18 h (1.54-fold increase in MFI; Fig. 1A). CD40L provoked a 1.25-fold increase in IL-12p40/p70 production (Fig. 1A). Peripheral blood fibrocytes also expressed significantly increased IL-12p70 in response to TSH and CD40L (2.43-fold and 2.33-fold increases, respectively; Fig. 1A).
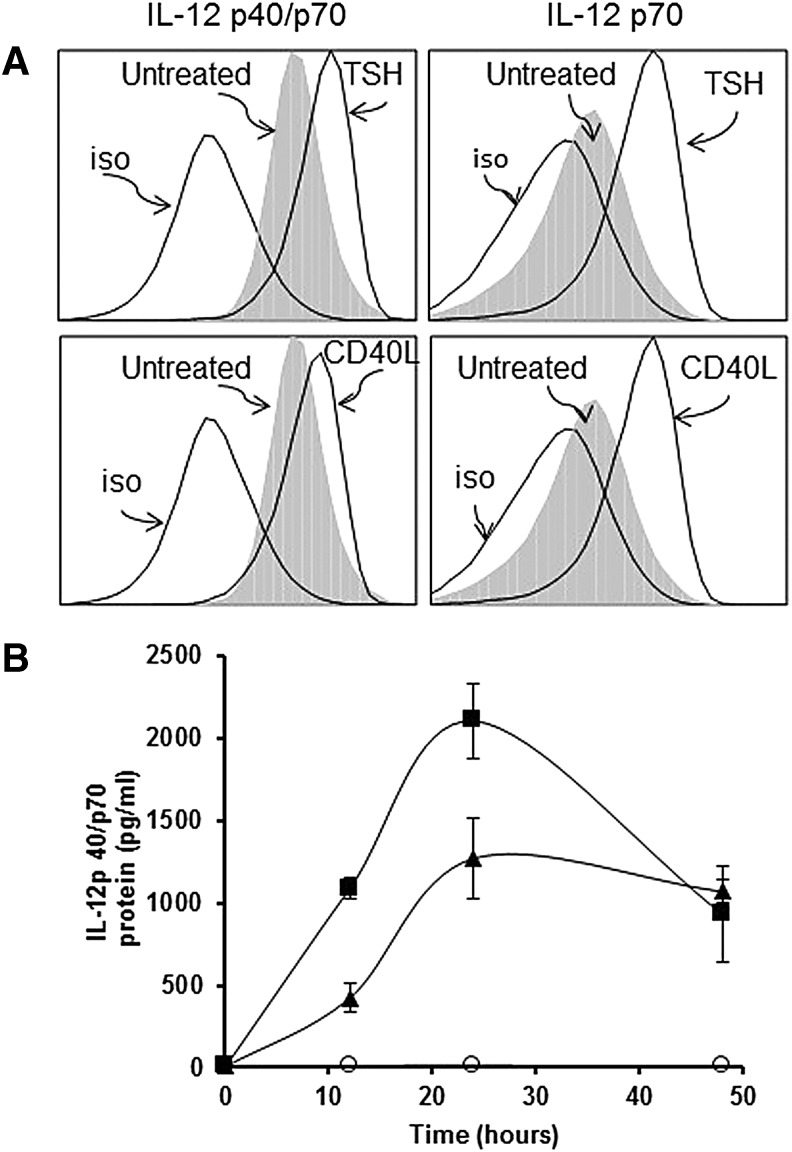
FIG. 1.
Thyrotropin (TSH) and CD40L induced interleukin-12 (IL-12) production in circulating fibrocytes (A) and in cultured fibrocytes (B). (A) Freshly isolated peripheral blood mononuclear cells (PBMCs) were stimulated for 18 h. Flow cytometry showed that TSH and CD40L induced IL-12p40/p70 (the combined p40 monomer and p70 heterodimer) in circulating fibrocytes, resulting in MFI values that were 1.54- and 1.25-fold higher, respectively, than in untreated cells. The MFIs of IL-12 p70 (heterodimer) were 2.43- and 2.33-fold higher after TSH or CD40L was added (right panels). (B) The fibrocytes in cultures were treated with TSH and CD40L for 48 h. The time course of extracellular IL-12p40/p70 concentrations shows that TSH (▲) and CD40L (■) stimulated IL-12 production, whereas unstimulated cells (◯) produced negligible amounts. Peak production was reached at 24 h (p < 0.0001 for TSH and CD40L).
Cultured fibrocytes produced negligible amounts of IL-12p40/p70 (10 pg/mL) without stimulation, but concentrations were increased by TSH and CD40L after 24 h (1271 ± 248 pg/mL, p < 0.001, and 2106 ± 227 pg/mL, p < 0.0001, respectively; Fig. 1B).
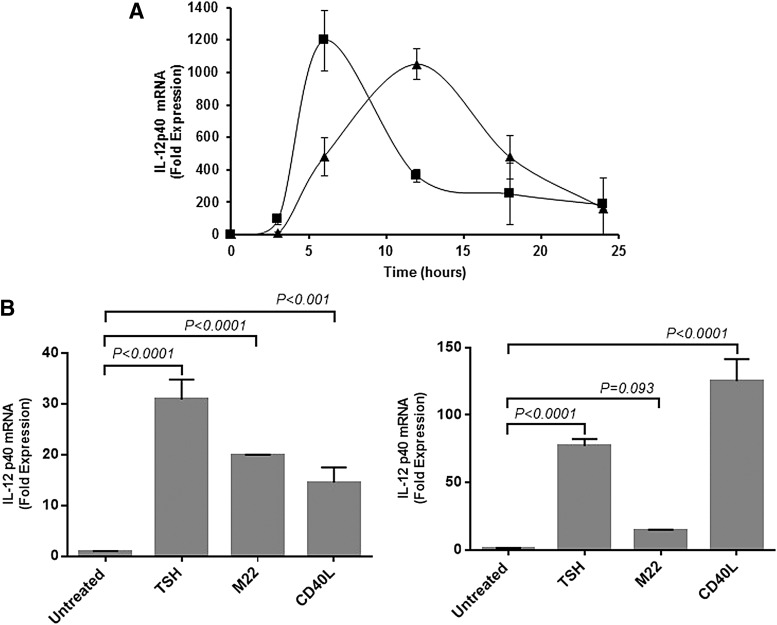
IL-12p40 mRNA was induced by CD40L (1197-fold increase; p < 0.0001 vs. control) and TSH (1051-fold increase; p < 0.0001 vs. control; Fig. 2A). Thus, TSH and CD40 appear to act at the pre-translational level. IL-12p40 mRNA was similarly stimulated with TSH, M22, and CD40L in fibrocytes from healthy controls or from patients with GD, as previously observed in the case of other cytokines (Fig. 2B).
FIG. 2.
TSH and CD40L induced IL-12 at the pre-translational level. Steady-state IL-12p40 mRNA expression stimulated with TSH (▲) or CD40L (■) was followed by real-time polymerase chain reaction (PCR) in cultured fibrocytes for 24 h (A). IL-12p40 mRNA production increased responding to TSH, M22, and CD40L stimulation in fibrocytes from healthy controls (B, left panel) and from patients with GD (B, right panel).
TSH and CD40L induce IL-12 production through Akt and NF-κB
This study sought to determine if Akt and NF-κB are involved in TSH- and CD40-mediated IL-12 signaling in fibrocytes. AKTi and MG132 were added to TSH- and CD40L-stimulated fibrocytes to inhibit these respective pathways.
Fibrocytes, which are undifferentiated, were identified in whole blood using multiparameter flow cytometry. The addition of AKTi and MG132 to peripheral blood fibrocytes markedly reduced IL-12p40/p70 and IL-12p70 after CD40L and TSH stimulation, as shown in Figure 3A and Table 1.
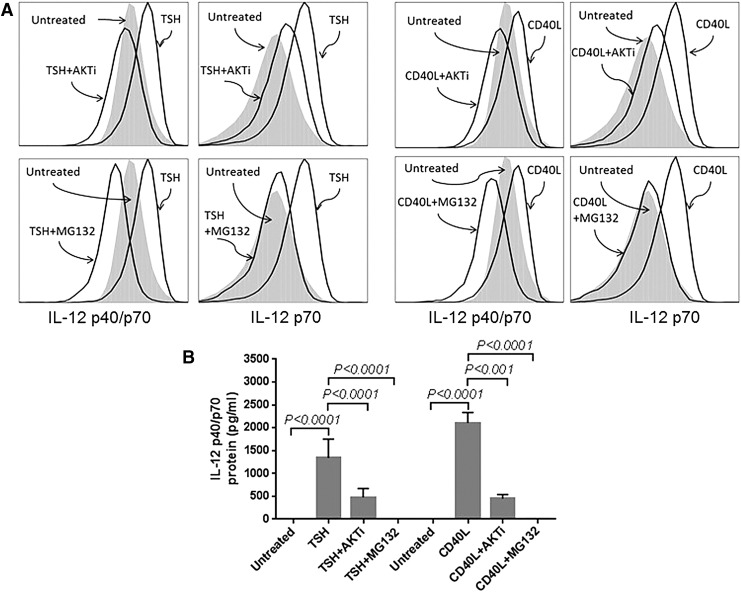
FIG. 3.
IL-12 protein production was stimulated by TSH or CD40L induced through the Akt–NF-κB pathway in circulating (A) and cultured (B) fibrocytes. Intracellular IL-12p40/p70 (the combined monomer and heterodimer) and IL-12p70 (the heterodimer) were measured by flow cytometry in circulating fibrocytes 18 h after TSH or CD40L was added. Extracellular IL-12p40/p70 concentration was measured by Luminex technology in cultured fibrocytes after 24 h of stimulation. Akt inhibitor (AKTi) and NF-κB inhibitor (MG132) were added to cells 1 h before stimulation. The stimulatory effect of TSH or CD40L was markedly diminished by adding AKTi or MG132.
Table 1.
Akt and NF-κB Are Involved in TSHR and CD40 Signaling for IL-12 Production in Circulating Fibrocytes
| IL-12 p40/p70 (MFI) | IL-12 p70 (MFI) | |
|---|---|---|
| Control | 3.56 | 1.59 |
| TSH | 5.09 | 3.87 |
| TSH + AKTi | 2.81 | 2.37 |
| TSH + MG132 | 1.99 | 1.74 |
| CD40L | 4.11 | 3.71 |
| CD40L + AKTi | 2.69 | 2.16 |
| CD40L + MG132 | 2.06 | 1.69 |
Intracellular IL-12 p40/p70 (combined monomer and heterodimer) and IL-12 p70 (heterodimer) were measured by flow cytometry 18 h after TSH or CD40L was added. Akt inhibitor (AKTi) and NF-κB inhibitor (MG132) were added to cells 1 h before stimulation. TSH or CD40L stimulation was markedly reduced by adding AKTi or MG132.
TSHR, thyrotropin receptor; IL-12, interleukin-12; MFI, mean fluorescent intensity; TSH, thyrotropin.
Fibrocytes were also isolated, and these cells were differentiated in culture, as described in the Methods. Analogously, AKTi reduced CD40L and TSH mediated expression of IL-12p40/p70 (Fig. 3B; from 2100 ± 220 pg/mL to 453 ± 89 pg/mL for CD40+AKTi, p < 0.0001; and from 1340 ± 411 g/mL to 479 ± 192 pg/mL for TSH + AKTi, p < 0.001). MG132 also reduced IL-12 production by CD40L (p < 0.0001) and TSH (p < 0.0001) to the concentration of unstimulated cells (Fig. 3B).
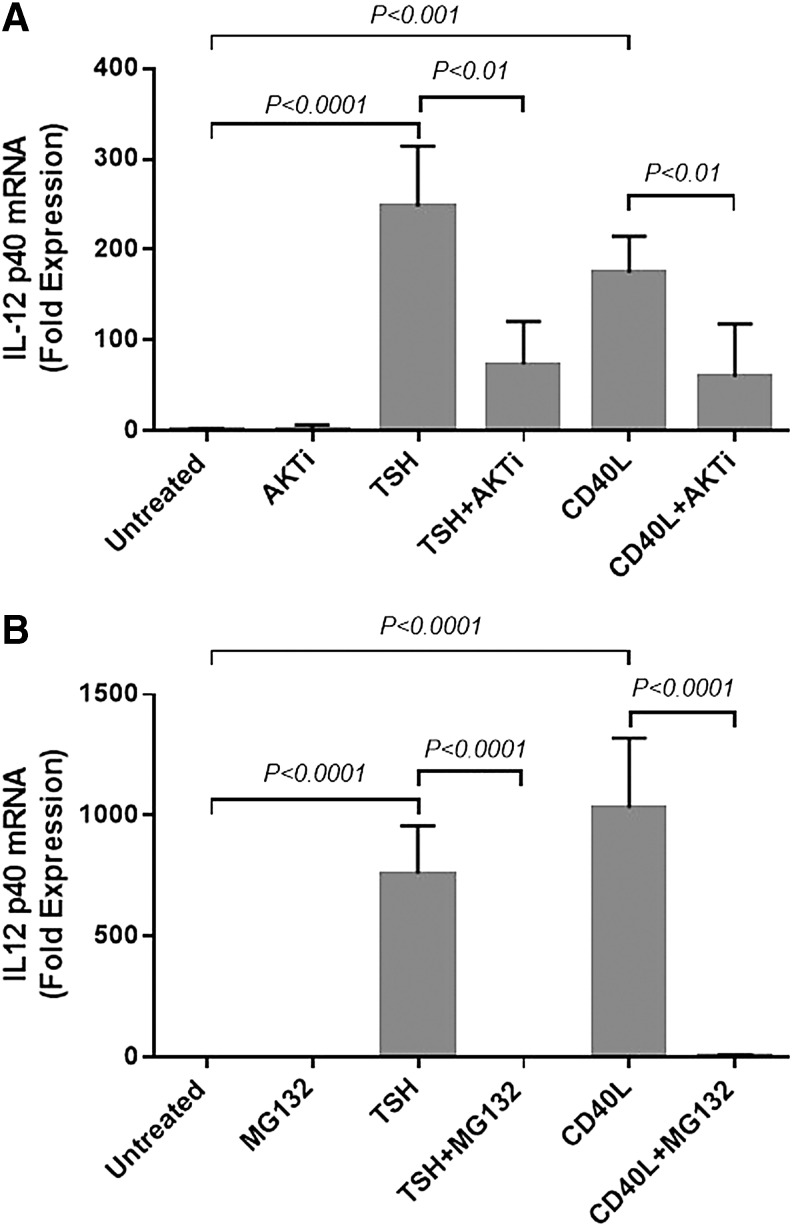
Next, this study sought to determine the effect of Akt and NF-κB in CD40- and TSH-mediated IL-12 mRNA induction (Fig. 4). AKTi significantly reduced CD40L- and TSH-stimulated IL-12p40 mRNA by 65% (p < 0.05) and 70% (p < 0.01), respectively (Fig. 4A). MG132 also inhibited this induction by CD40L or TSH to basal levels (p < 0.001; Fig. 4B). These data show that fibrocyte production of IL-12 provoked by CD40L and TSH is dependent upon Akt and NF-κB.
FIG. 4.
TSH and CD40L induced IL-12p40 mRNA expression through Akt (A) and NF-κB (B) signaling in cultured fibrocytes. Akt inhibitor (AKTi) and NF-κB inhibitor (MG132) were added 1 h before TSH or CD40L stimulation. RNA was isolated after 6 h of induction. AKTi and MG132 blocked IL-12p40 mRNA expression induced by TSH (p < 0.01 and p < 0.001, respectively) or CD40L (p < 0.01 and p < 0.0001, respectively).
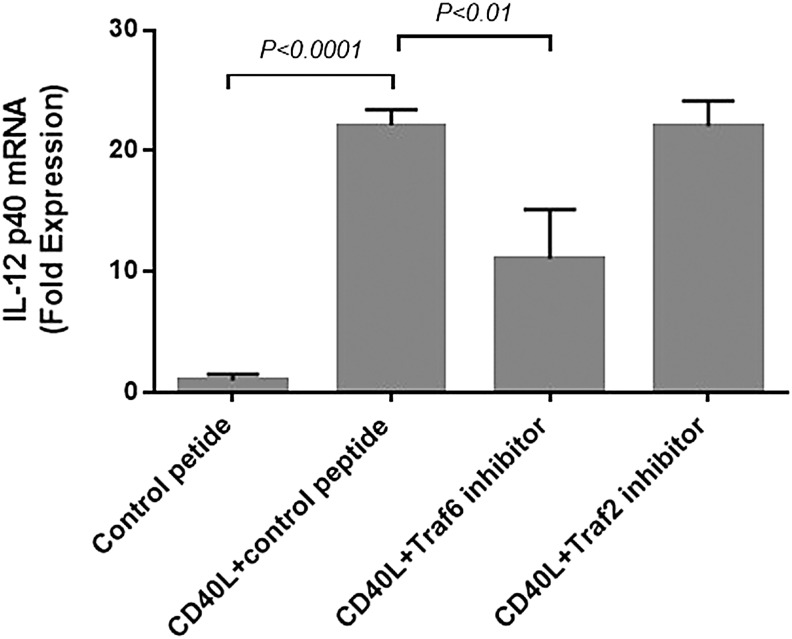
CD40-mediated IL-12 production is dependent on TRAF6
This study determined whether canonical signaling pathways, including TRAF6 or TRAF2, are involved in CD40-mediated IL-12 induction (Fig. 5). Cultured fibrocytes were pre-incubated with control peptide, TRAF6, or TRAF2 inhibitor peptide for 24 h before CD40L stimulation. In the presence of control peptide, adding CD40L increased the IL-12p40 mRNA level by 22-fold (p < 0.0001). Adding TRAF6 inhibitory peptide attenuated the effect of CD40L stimulation (50% inhibition; p < 0.001). The inhibitory peptide to TRAF2 had no effect.
FIG. 5.
CD40 signaling of IL-12 production proceeded through TRAF6 in fibrocytes. Two doses of 50 μM control peptide or TRAF6 or TRAF2 inhibitor peptide were added to cells 24 h and 1 h before CD40L stimulation. Six hours after CD40L stimulation, RNA was isolated, and IL-12p40 mRNA levels were measured by real-time PCR. TRAF6 inhibitor peptide significantly inhibited the action of CD40L, whereas the TRAF2 peptide had no effect.
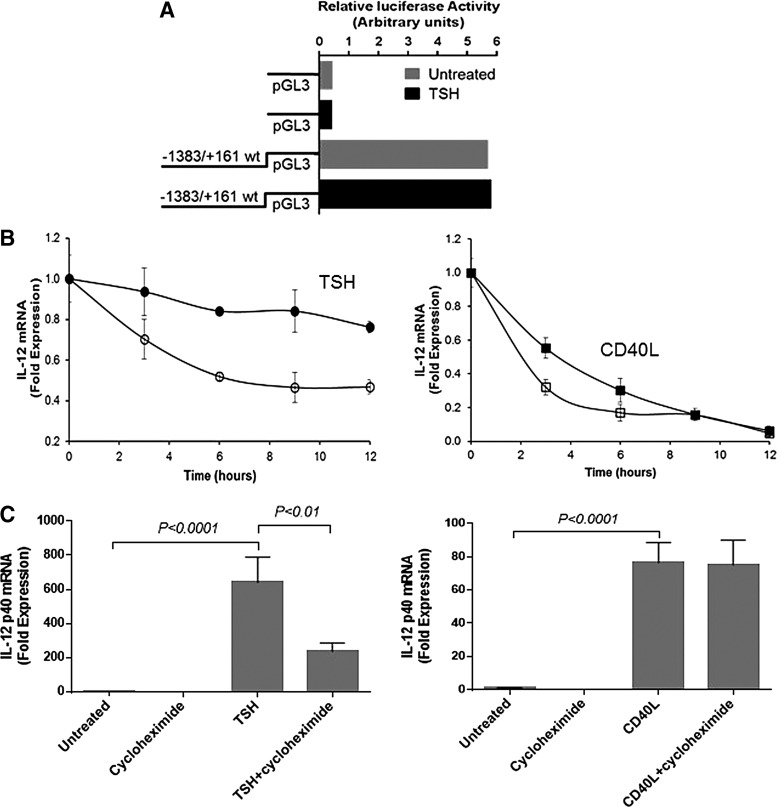
TSH stabilizes IL-12p40 mRNA but requires de novo protein synthesis
To determine the mechanism of mRNA stimulation by TSH, cultured fibrocytes were transfected with IL-12p40 promoter containing pGL3 vector. Transfected cells were stimulated with either nothing or TSH for 2 h. Promoter activity was marked, even without adding TSH (Fig. 6A). TSH did not enhance promoter activity. The experiment was repeated, and cells were incubated with TSH for 4 and 8 h. Promoter activity did not differ between untreated and TSH-treated cells (data not shown).
FIG. 6.
TSH did not induce IL-12 promoter activity (A). The vector containing the promoter was transfected into fibrocytes and cells were incubated with TSH for 2 h. TSH stabilized IL-12p40 mRNA (B, left panel), whereas CD40L did not affect mRNA stability (B, right panel). IL-12 mRNA production was stimulated for 12 h with TSH. Cells were then washed and treated with 50 μM of DRB alone, DRB and TSH, or DRB and CD40L (time zero). Degradation of existing mRNA was slower in the presence of TSH and DRB (●) than in the presence of DRB alone (◯), whereas mRNA degradation proceeded at similar rate in the absence (□) and presence (■) of CD40L. TSH stimulation of IL-12p40 mRNA depended on de novo protein production (C, left panel). Adding cycloheximide (10 μg/mL) 1 h before TSH stimulation significantly lowered the TSH-induced IL-12p40 mRNA expression. Adding cycloheximide did not affect CD40L stimulation of IL-12p40 mRNA (C, right panel), indicating that CD40L does not depend on de novo protein synthesis.
Since TSH can stabilize IL-6 mRNA in fibrocytes (33), this study determined whether TSH also affects IL-12p40 mRNA stability using the RNA polymerase II inhibitor, 5,6-dichlorobenzimidazole (DRB). Steady-state IL-12 mRNA levels were assayed in the presence of DRB for 12 h, with or without TSH (Fig. 6B). Without TSH, IL-12p40 mRNA level fell 50% after 12 h, whereas in its presence, mRNA levels decayed by 25%. CD40L did not affect IL-12p40 mRNA stability (Fig. 6B).
Next, this study evaluated whether de novo protein synthesis was necessary for IL-12 mRNA induction by TSH and CD40L. TSH-mediated IL-12 expression decreased after adding cycloheximide (from 640-fold to 236-fold; p < 0.01; Fig. 6C). In contrast, cycloheximide had no effect on CD40L-mediated IL-12 expression (Fig. 6C). Thus, maximal induction of IL-12p40 mRNA by TSH requires de novo protein synthesis.
Discussion
GD is a systemic autoimmune disorder targeting the thyroid gland, skin, and orbital connective tissues. Although it is widely accepted that thyroid-stimulating immunoglobulins (TSIs) are responsible for the hyperthyroidism associated with GD, several other potential autoantigens and co-stimulatory molecules, such as the insulin-like growth factor 1 receptor (IGF-1R), CD40, and thyroglobulin, may also be involved in the pathogenesis of TAO (2,11,34,35).
The immune mechanisms, which lead to orbital involvement, are unknown. Skewed cytokine production promoting immune cell activation has been proposed as a factor in GD and TAO. The understanding of cytokines networks (Th1, Th2, Th17, etc.) and their interplay and temporal relation to disease have become more detailed in the last several years. Several authors have described how these cytokine networks can function antagonistically. Thus, the balance between these subpopulations may determine the outcome of autoimmune diseases (36). Th1 cytokines, such as IFN-γ and TNF-α, are preferentially induced in primary cultures of thymocytes from patients with GD (37,38). The Th1 cytokine response predominated by IFN-γ has been found to dominate in the active state of GD, whereas the Th2 cytokine response (IL-4) is thought to dominate in the non-active state of GD (38,39). Serum levels of IL17 indicative of a Th17 response were elevated in GD patients but not in TAO patients compared with control donors (38). These studies are likely incomplete in regards to the relation of cytokine network, but they do highlight the fact that shifts in cytokine production can alter disease state.
IL-12 induces the Th1 cytokine IFN-γ in T and NK cells, enhancing the generation and cytotoxic activity of T lymphocytes, promoting Th1 cell differentiation with specific T-cell–mediated immune response (40,41). Furthermore, Th1 responses are involved in host defense against infectious agents as a consequence of IL-12 actions on T-cell priming (42). IL-12 is involved in initiating autoimmunity in several murine models (43), and it is believed to provide critical co-stimulation of the Th1 paradigm (44,45). IL-12–/– mice have a markedly deficient Th1 response and are thus resistant to certain forms of autoimmunity (46). This response suggests that IL-12 is critical in the pathogenesis of Th1-mediated autoimmune diseases.
IL-12 also has been implicated in human autoimmune disease (47–50), and a significant increase in serum IL-12 level was observed in patients with GD (51,52). CD40–CD40L signaling of dendritic cells is considered a major source of IL-12 (21). Now it is demonstrated that TSHR signaling promotes IL-12 production in fibrocytes. It can be speculated that circulating fibrocytes may markedly contribute to serum IL-12 levels due to increased fibrocyte frequency in GD (8).
Fibrocytes express high levels of autoantigens (TSHR and IGF-1R), together with human leucocyte antigen D–related (HLA-DR), suggesting that these cells may present autoantigens and possibly initiate an antigen-specific immune response (53). The frequency of circulating TSHR+ fibrocytes is increased in patients with GD, and TSH induces the production of IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1), and TNF-α (11). Orbital fibroblasts from patients with GD express high levels of CD40, and in response to CD40L, these cells produce hyaluronan and cytokines, such as IL-6, IL-8, and prostaglandin E2 (PGE2) (54). Moreover, an increased frequency of CD40+ fibrocytes has been observed in the blood and orbital tissues of patients with GD compared with controls, and the CD40–CD40L signaling pathway in these cells can induce production of IL-6, IL-8, MCP-1, and TNF-α (20). This study reports that TSH and CD40L can induce the production of IL-12 in cultured and circulating fibrocytes and that their effects may occur at a pre-translational level. TSHR- and CD40-mediated fibrocyte responses may therefore be important in the development of GD.
The function of Akt and NF-κB in the CD40-mediated signaling pathway of IL-6 production in cultured fibrocytes has been previously described (20). The present study found that Akt and NF-κB are involved in TSH- and CD40L-mediated signaling of IL-12 production in cultured and circulating fibrocytes. It was determined that TRAF6, but not TRAF2, is necessary for IL-12 production as in CD40L-induced dendritic cells (55). Stimulating IL-6 and IL-8 required neither TRAF6 nor TRAF2 by CD40L in cultured fibrocytes (Mester and Douglas, unpublished data).
The IL-12 p40 promoter contains several regulatory elements, including one for NF-κB (56–58). Although TSH induces NF-κB activity (33) and NF-κB inhibitor reduces IL-12 protein IL-12 p40 mRNA production, TSH did not stimulate IL-12 p40 promoter activity. TSH may act directly or indirectly through a de novo synthesized protein on the IL-12 p40 enhancer region, thus priming IL-12 promoter induction. The function of an enhancer region in stimulation by IFN-γ has been described (59). The mRNA stabilizing effect of TSH may also increase IL-12 p40 mRNA production, a finding also observed with other transcripts (33,60).
The IL-12 pathway remains an attractive potential target for interventions inhibiting downstream immune responses. Acetyl salicylic acid, HIV-1 Vpr, and parthenolide can decrease IL-12 production and Th1 development by inhibiting NF-κB activation (61–63). Treating autoimmune diseases with IL-12 antagonists may diminish the Th1 response, thereby decreasing the inflammatory response (64).
Treatments for multiple sclerosis and rheumatoid arthritis by blocking IL-12 production have recently received widespread attention (65). In one study, the binding of recombinant IL-12p40 homodimer to the IL-12 receptor served as an antagonist for IL-12-mediated signaling (66), a finding that may be useful in treating some autoimmune and systemic inflammatory diseases. Thus, blocking IL-12 or interrupting the signaling pathways of TSHR- and CD40-mediated IL-12 production could provide new therapies for patients with GD.
In the treatment of TAO, there are several potential targets for novel treatments, including the putative antigen and signaling cascades (IGFR and TSHR), cellular constituents such as B cells (rituximab treatment), and cytokine networks. The heterogeneity seen in GD may be due to alterations in cytokine predominance or the contribution of all of these components to varying degrees. Unlike in other autoimmune diseases, a singular target may not be appropriate for all patients. A protocol for TAO is envisaged where treatment is personalized based upon predictive testing. Among potential cytokines, IL-12 is often responsible for downstream activation of other cytokines. Therefore, it may be a useful target.
Acknowledgments
This work was supported in part by National Institutes of Health grants EY008976, EY016339, and EY021197, and an EY007003 Eye Core Grant; an unrestricted grant from Research to Prevent Blindness; a Research to Prevent Blindness Career Development Award; a Research to Prevent Blindness Lew Wasserman Merit Award; and the Bell Charitable Foundation.
Author Disclosure Statement
The authors have no proprietary or commercial interest in any material discussed in this article.
References
- 1.Bartalena L, Fatourechi V. 2014. Extrathyroidal manifestations of Graves' disease: a 2014 update. J Endocrinol Invest 37:691–700 [DOI] [PubMed] [Google Scholar]
- 2.Fernando R, Atkins S, Raychaudhuri N, Lu Y, Li B, Douglas RS, Smith TJ. 2012. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci U S A 109:7427–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Smith TJ. 2014. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci 55:1735–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith TJ, Padovani-Claudio DA, Lu Y, Raychaudhuri N, Fernando R, Atkins S, Gillespie EF, Gianoukakis AG, Miller BS, Gauger PG, Doherty GM, Douglas RS. 2011. Fibroblasts expressing the thyrotropin receptor overarch thyroid and orbit in Graves' disease. J Clin Endocrinol Metab 96:3827–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellini A, Mattoli S. 2007. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest 87:858–870 [DOI] [PubMed] [Google Scholar]
- 6.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. 1998. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol 160:419–425 [PubMed] [Google Scholar]
- 7.Herzog EL, Bucala R. 2010. Fibrocytes in health and disease. Exp Hematol 38:548–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas RS, Afifiyan NF, Hwang CJ, Chong K, Haider U, Richards P, Gianoukakis AG, Smith TJ. 2010. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 95:430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathai SK, Gulati M, Peng XY, Russell TR, Shaw AC, Rubinowitz AN, Murray LA, Siner JM, Antin-Ozerkis DE, Montgomery RR, Reilkoff RAS, Bucala RJ, Herzog EL. 2010. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest 90:812–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilling D, Buckley CD, Salmon M, Gomer RH. 2003. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol 171:5537–5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillespie EF, Papageorgiou KI, Fernando R, Raychaudhuri N, Cockerham KP, Charara LK, Goncalves AC, Zhao SX, Ginter A, Lu Y, Smith TJ, Douglas RS. 2012. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Endocrinol Metab 97:E740–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schonbeck U, Libby P. 2001. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci 58:4–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. 1998. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391:591–594 [DOI] [PubMed] [Google Scholar]
- 14.Mach F, Schonbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P. 1997. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40–CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci U S A 94:1931–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Chen J, Xiong Y, Da Q, Xu Y, Jiang X, Tang H. 2006. Internalization of CD40 regulates its signal transduction in vascular endothelial cells. Biochem Biophys Res Commun 345:106–117 [DOI] [PubMed] [Google Scholar]
- 16.Yellin MJ, Winikoff S, Fortune SM, Baum D, Crow MK, Lederman S, Chess L. 1995. Ligation of CD40 on fibroblasts induces CD54 (ICAM-1) and CD106 (VCAM-1) up-regulation and IL-6 production and proliferation. J Leukoc Biol 58:209–216 [DOI] [PubMed] [Google Scholar]
- 17.Munroe ME, Bishop GA. 2007. A costimulatory function for T cell CD40. J Immunol 178:671–682 [DOI] [PubMed] [Google Scholar]
- 18.Laman JD, De Boer M, Hart BA. 1998. CD40 in clinical inflammation: from multiple sclerosis to atherosclerosis. Dev Immunol 6:215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toubi E, Shoenfeld Y. 2004. The role of CD40-CD154 interactions in autoimmunity and the benefit of disrupting this pathway. Autoimmunity 37:457–464 [DOI] [PubMed] [Google Scholar]
- 20.Gillespie EF, Raychaudhuri N, Papageorgiou KI, Atkins SJ, Lu Y, Charara LK, Mester T, Smith TJ, Douglas RS. 2012. Interleukin-6 production in CD40-engaged fibrocytes in thyroid-associated ophthalmopathy: involvement of Akt and NF-kappaB. Invest Ophthalmol Vis Sci 53:7746–7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Wang Q. 2008. Factors determining the formation and release of bioactive IL-12: regulatory mechanisms for IL-12p70 synthesis and inhibition. Biochem Biophys Res Commun 372:509–512 [DOI] [PubMed] [Google Scholar]
- 22.Ma X, Trinchieri G. 2001. Regulation of interleukin-12 production in antigen-presenting cells. Adv Immunol 79:55–92 [DOI] [PubMed] [Google Scholar]
- 23.Shu U, Kiniwa M, Wu CY, Maliszewski C, Vezzio N, Hakimi J, Gately M, Delespesse G. 1995. Activated T cells induce interleukin-12 production by monocytes via CD40–CD40 ligand interaction. Eur J Immunol 25:1125–1128 [DOI] [PubMed] [Google Scholar]
- 24.Kallmann BA, Huther M, Tubes M, Feldkamp J, Bertrams J, Gries FA, Lampeter EF, Kolb H. 1997. Systemic bias of cytokine production toward cell-mediated immune regulation in IDDM and toward humoral immunity in Graves' disease. Diabetes 46:237–243 [DOI] [PubMed] [Google Scholar]
- 25.Heuer M, Aust G, Ode-Hakim S, Scherbaum WA. 1996. Different cytokine mRNA profiles in Graves' disease, Hashimoto's thyroiditis, and nonautoimmune thyroid disorders determined by quantitative reverse transcriptase polymerase chain reaction (RT-PCR). Thyroid 6:97–106 [DOI] [PubMed] [Google Scholar]
- 26.Roura-Mir C, Catalfamo M, Sospedra M, Alcalde L, Pujol-Borrell R, Jaraquemada D. 1997. Single-cell analysis of intrathyroidal lymphocytes shows differential cytokine expression in Hashimoto's and Graves' disease. Eur J Immunol 27:3290–3302 [DOI] [PubMed] [Google Scholar]
- 27.Itoh M, Uchimura K, Yamamoto K, Makino M, Imamura S, Kobayashi T, Fujiwara K, Kato T, Hayakawa N, Sawai Y, Nagasaka A, Iwase K, Nomura T, Hagino Y. 2002. Distinctive response of thyroid-infiltrating mononuclear cells to B cell activation through CD40 and interleukin-4 in Graves' patients. Cytokine 19:107–114 [DOI] [PubMed] [Google Scholar]
- 28.Ajjan RA, Watson PF, Weetman AP. 1997. Detection of IL-12, IL-13, and IL-15 messenger ribonucleic acid in the thyroid of patients with autoimmune thyroid disease. J Clin Endocrinol Metab 82:666–669 [DOI] [PubMed] [Google Scholar]
- 29.Murakami S, Okubo K, Tsuji Y, Sakata H, Kikuchi M, Takahashi T, Kato T, Hirayama R. 2005. Serum levels of interleukin-12 in Graves' disease and their dynamic changes after surgery. Surg Today 35:1016–1020 [DOI] [PubMed] [Google Scholar]
- 30.Tamaru M, Matsuura B, Onji M. 1999. Increased levels of serum interleukin-12 in Graves' disease. Eur J Endocrinol 141:111–116 [DOI] [PubMed] [Google Scholar]
- 31.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. 1994. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1:71–81 [PMC free article] [PubMed] [Google Scholar]
- 32.Ye H, Park YC, Kreishman M, Kieff E, Wu H. 1999. The structural basis for the recognition of diverse receptor sequences by TRAF2. Molecular Cell 4:321–330 [DOI] [PubMed] [Google Scholar]
- 33.Raychaudhuri N, Fernando R, Smith TJ. 2013. Thyrotropin regulates IL-6 expression in CD34(+) fibrocytes: clear delineation of its cAMP-independent actions. PLOS ONE 8:e75100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witebsky E, Rose NR, Shulman S. 1958. The autoantibody nature of the thyroiditis and the role of thyroglobulin in the reaction. Lancet 1:808–810 [DOI] [PubMed] [Google Scholar]
- 35.Smith TJ. 2013. Is IGF-I receptor a target for autoantibody generation in Graves' disease? J Clin Endocrinol Metab 98:515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholson LB, Kuchroo VK. 1996. Manipulation of the Th1/Th2 balance in autoimmune disease. Curr Opin Immunol 8:837–842 [DOI] [PubMed] [Google Scholar]
- 37.Berger JP, Akiyama TE, Meinke PT. 2005. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci 26:244–251 [DOI] [PubMed] [Google Scholar]
- 38.Shen J, Li Z, Li W, Ge Y, Xie M, Lv M, Fan Y, Chen Z, Zhao D, Han Y. 2015. Th1, Th2, and Th17 cytokine involvement in thyroid associated ophthalmopathy. Dis Markers 2015:609593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakelkamp IM, Bakker O, Baldeschi L, Wiersinga WM, Prummel MF. 2003. TSH-R expression and cytokine profile in orbital tissue of active vs. inactive Graves' ophthalmopathy patients. Clin Endocrinol (Oxf) 58:280–287 [DOI] [PubMed] [Google Scholar]
- 40.Trinchieri G. 1998. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol 70:83–243 [DOI] [PubMed] [Google Scholar]
- 41.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. 1998. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol 16:495–521 [DOI] [PubMed] [Google Scholar]
- 42.Romagnani S. 1992. Induction of TH1 and TH2 responses: a key role for the “natural” immune response? Immunol Today 13:379–381 [DOI] [PubMed] [Google Scholar]
- 43.Trembleau S, Germann T, Gately MK, Adorini L. 1995. The role of IL-12 in the induction of organ-specific autoimmune diseases. Immunol Today 16:383–386 [DOI] [PubMed] [Google Scholar]
- 44.Kubin M, Kamoun M, Trinchieri G. 1994. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med 180:211–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy EE, Terres G, Macatonia SE, Hsieh CS, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O'Garra A. 1994. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med 180:223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK. 1996. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 4:471–481 [DOI] [PubMed] [Google Scholar]
- 47.Leonard JP, Waldburger KE, Goldman SJ. 1995. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med 181:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. 1995. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med 182:1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nitta Y, Kawamoto S, Tashiro F, Aihara H, Yoshimoto T, Nariuchi H, Tabayashi K, Miyazaki J. 2001. IL-12 plays a pathologic role at the inflammatory loci in the development of diabetes in NOD mice. J Autoimmun 16:97–104 [DOI] [PubMed] [Google Scholar]
- 50.Adorini L, Aloisi F, Galbiati F, Gately MK, Gregori S, Penna G, Ria F, Smiroldo S, Trembleau S. 1997. Targeting IL-12, the key cytokine driving Th1-mediated autoimmune diseases. Chem Immunol 68:175–197 [DOI] [PubMed] [Google Scholar]
- 51.Poplawska-Kita A, Siewko K, Telejko B, Modzelewska A, Mysliwiec J, Milewski R, Gorska M, Szelachowska M, gorzata 2013. The changes in the endothelial function and haemostatic and inflammatory parameters in subclinical and overt hyperthyroidism. Int J Endocrinol 2013:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murakami S, Okubo K, Tsuji Y, Sakata H, Kikuchi M, Takahashi T, Kato T, Hirayama R. 2005. Serum levels of interleukin-12 in Graves' disease and their dynamic changes after surgery. Surg Today 35:1016–1020 [DOI] [PubMed] [Google Scholar]
- 53.Smith TJ. 2010. Potential role for bone marrow-derived fibrocytes in the orbital fibroblast heterogeneity associated with thyroid-associated ophthalmopathy. Clin Exp Immunol 162:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang CJ, Afifiyan N, Sand D, Naik V, Said J, Pollock SJ, Chen B, Phipps RP, Goldberg RA, Smith TJ, Douglas RS. 2009. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci 50:2262–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mackey MF, Wang Z, Eichelberg K, Germain RN. 2003. Distinct contributions of different CD40 TRAF binding sites to CD154-induced dendritic cell maturation and IL-12 secretion. Eur J Immunol 33:779–789 [DOI] [PubMed] [Google Scholar]
- 56.Becker C, Wirtz S, Ma XJ, Blessing M, Galle PR, Neurath MF. 2001. Regulation of IL-12 p40 promoter activity in primary human monocytes: Roles of NF-kappa B, CCAAT/enhancer-binding protein beta, and PU.1 and identification of a novel repressor element (GA-12) that responds to IL-4 and prostaglandin E-2. J Immunol 167:2608–2618 [DOI] [PubMed] [Google Scholar]
- 57.Ma W, Gee K, Lim W, Chambers K, Angel JB, Kumar A. 2004. Dexamethasone inhibits IL-12p40 production in lipopolysaccharide-stimulated human monocytic cells by down-regulating the activity of c-jun N-terminal kinase, the activation protein-1, and NF-kappa B transcription factors. J Immunol 172:318–330 [DOI] [PubMed] [Google Scholar]
- 58.Ma XJ, Chow JM, Gri G, Carra G, Gerosa F, Wolf SE, Dzialo R, Trinchieri G. 1996. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med 183:147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koshiba R, Yanai H, Matsuda A, Goto A, Nakajima A, Negishi H, Nishio J, Smale ST, Taniguchi T. 2013. Regulation of cooperative function of the Il12b enhancer and promoter by the interferon regulatory factors 3 and 5. Biochem Biophys Res Commun 430:95–100 [DOI] [PubMed] [Google Scholar]
- 60.Li B, Smith TJ. 2014. Regulation of IL-1 Receptor antagonist by TSH in fibrocytes and orbital fibroblasts. J Clin Endocrinol Metab 99:E625–E633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazzeo D, Panina-Bordignon P, Recalde H, Sinigaglia F, D'Ambrosio D. 1998. Decreased IL-12 production and Th1 cell development by acetyl salicylic acid-mediated inhibition of NF-kappaB. Eur J Immunol 28:3205–3213 [DOI] [PubMed] [Google Scholar]
- 62.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams WV, Green DR, Weiner DB. 1997. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat Med 3:1117–1123 [DOI] [PubMed] [Google Scholar]
- 63.Kang BY, Chung SW, Kim TS. 2001. Inhibition of interleukin-12 production in lipopolysaccharide-activated mouse macrophages by parthenolide, a predominant sesquiterpene lactone in Tanacetum parthenium: involvement of nuclear factor-kappaB. Immunol Lett 77:159–163 [DOI] [PubMed] [Google Scholar]
- 64.Schmidt C, Marth T, Wittig BM, Hombach A, Abken H, Stallmach A. 2002. Interleukin-12 antagonists as new therapeutic agents in inflammatory bowel disease. Pathobiology 70:177–183 [DOI] [PubMed] [Google Scholar]
- 65.Vandenbroeck K, Alloza I, Gadina M, Matthys P. 2004. Inhibiting cytokines of the interleukin-12 family: recent advances and novel challenges. J Pharm Pharmacol 56:145–160 [DOI] [PubMed] [Google Scholar]
- 66.'t Hart BA, Brok HP, Remarque E, Benson J, Treacy G, Amor S, Hintzen RQ, Laman JD, Bauer J, Blezer EL. 2005. Suppression of ongoing disease in a nonhuman primate model of multiple sclerosis by a human-anti-human IL-12p40 antibody. J Immunol 175:4761–4768 [DOI] [PubMed] [Google Scholar]