Abstract
Neutrophils are implicated in the damage of lung tissue in many disease states, including infectious diseases and environmental insults. These effects may be due to oxidative or nonoxidative functions of the neutrophil or both. We examined the role of neutrophils in pulmonary damage during infection with the opportunistic fungal pathogen Pneumocystis sp. in four mouse models of neutrophil dysfunction. These were (i) a knockout of the gp91phox component of NADPH oxidase, in which reactive oxygen species (ROS) production is greatly reduced; (ii) a double knockout of gp91phox and inducible nitric oxide synthase, in which ROS and nitric oxide production is greatly decreased; (iii) a knockout of the chemokine receptor CXCR2, in which accumulation of intra-alveolar neutrophils is severely diminished; and (iv) antibody depletion of circulating neutrophils in wild-type mice with the monoclonal antibody RB6. Surprisingly, in each case, indicators of pulmonary damage (respiratory rates, arterial oxygen partial pressures, and intra-alveolar albumin concentrations) were the same in knockout mice and comparable wild-type mice. Therefore, whereas neutrophils are a valid correlative marker of lung damage during Pneumocystis infection, neither neutrophils nor ROS appear to be the causative agent of tissue damage. We also show that there is no difference in Pneumocystis burdens between wild-type and knockout mice, which supports the idea that neutrophils do not have a major role in the clearance of this organism.
Although neutrophils have crucial host defense functions in pulmonary infections, their presence in lung tissue can also have injurious effects. When bacterial pathogens such as Streptococcus pneumoniae (12, 19), Pseudomonas aeruginosa (63), and Haemophilus influenzae (31) proliferate in the lung, neutrophils are recruited to aid in the clearance of these organisms. This is also the case with fungal pathogens such as Candida albicans (51) and Aspergillus fumigatus (36). However, there is some evidence that the damage that can occur to pulmonary tissue in these conditions is due in part to neutrophil-mediated mechanisms, including both the production of reactive oxygen species (ROS) such as superoxide and hydrogen peroxide and nonoxidative mechanisms such as those involving proteolytic enzymes and antimicrobial proteins (58). This is especially evident in some cases of acute respiratory distress syndrome, in which increased permeability of endothelial and/or epithelial barriers, cellular influx, and other inflammatory changes often lead to death (30).
Another disease state in which neutrophils are suspected to be involved in host tissue damage is Pneumocystis pneumonia. This illness is caused by infection with the opportunistic fungal pathogen Pneumocystis sp. Although this organism is endemic, it does not cause overt illness in immunocompetent hosts; indeed, most people have antibodies against Pneumocystis by the age of three (69). However, in immunocompromised individuals such as those with AIDS and transplant patients receiving immunosuppressant treatments, Pneumocystis infection can lead to a serious and sometimes fatal disease (43, 54). This is also the case in experimental rodent models in which animals are rendered susceptible to the disease by depletion of CD4+ lymphocytes (10, 29), sustained glucocorticoid treatment (6, 70), or genetic deletion such as in the case of the severe combined immunodeficient (SCID) mouse (17, 53) or the CD40 lymphocyte receptor knockout mouse (23, 71). In these immunosuppressed animals, the Pneumocystis organisms slowly propagate in the alveoli until they fill the airspace and respiratory failure occurs (17). Pathophysiological changes such as increased alveolar permeability, decreased lung compliance, increased respiratory rate, and decreased arterial oxygen partial pressure typically occur only during the later stages of the disease, when the alveoli are largely full of Pneumocystis. Unlike that in diseases caused by faster-growing bacterial pathogens, neutrophil influx into the alveoli occurs primarily during the later stages of Pneumocystis infection, concurrent with increases in numbers of lymphocytes and macrophages (9, 17). Because this influx coincides with the appearance of lung pathology, it has been speculated that the neutrophils have a causative role in the lung damage that occurs in the later stages of Pneumocystis pneumonia (68). This speculation is supported by evidence from human Pneumocystis patients that the severity of the disease correlates with the relative numbers of neutrophils in lavage samples (4, 39, 42, 61).
In light of these observations, we wished to test the hypothesis that elimination or reduction of some neutrophil accumulation or functions would result in reduced pulmonary tissue damage over the course of Pneumocystis infection. We utilized four experimental mouse models to test this hypothesis: a knockout of the chemokine receptor CXCR2, in which accumulation of neutrophils at sites of infection is severely diminished (CXCR2-KO); a knockout of the gp91phox component of NADPH oxidase, in which production of ROS by phagocytic cells is greatly reduced (gp91phox-KO); a double knockout of gp91phox and inducible nitric oxide synthase (iNOS), in which production of both ROS and nitric oxide (NO) by phagocytes is greatly decreased (phox/iNOS-KO); and RB6 depletion, in which mice were repeatedly injected with an antineutrophil antibody to deplete circulating neutrophils (RB6 depletion). We report here that there was virtually no difference in indicators of lung damage during Pneumocystis infections in mice with these neutrophil functional impairments compared to those in wild-type mice. Additionally, although there were some interesting differences in the patterns of Pneumocystis growth in these models, the overall burden of Pneumocystis was not different from that in wild-type animals.
MATERIALS AND METHODS
Animals.
Breeder mice with the chemokine receptor CXCR2 knockout [C.129S2(B6)-Il8rbtm1Mwm] were obtained from the Jackson Laboratory (Bar Harbor, Maine) and were bred in our colony at Montana State University. Mice with a null allele corresponding to the gp91phox component of NADPH oxidase (B6.129S6-Cybbtm1Din) were also obtained from the Jackson Laboratory. Breeder phox/iNOS-KO mice were a generous gift of Robert North at Trudeau Institute, Saranac Lake, N.Y. Stock mice were bred in our colony at Montana State University, and animals were given prophylactic antibiotic treatment with itraconazole and sulfamethoxazole-trimethoprim (Bactrim) as previously described (57) until 4 days prior to the start of an experiment. Mice used as wild-type infected and uninfected control animals were either BALB/c mice (for the CXCR2-KO model experiments) obtained from the Jackson Laboratory or C57BL/6 mice (for gp91phox-KO and phox/iNOS-KO model experiments) obtained from either the Jackson Laboratory or the National Cancer Institute (Fredrick, Md.). All mice were given autoclaved food and acidified water, which during experiments was supplemented with tetracycline to reduce the possibility of bacterial superinfections.
Cellular depletions and Pneumocystis infection.
To induce susceptibility to Pneumocystis, some groups of mice were depleted of CD4+ lymphocytes with twice weekly intraperitoneal injections of 300 μg of the anti-CD antibody GK1.5 (American Type Culture Collection). These injections began 2 to 4 days before inoculation and continued through the entire experimental period. During the same period, groups of BALB/c mice that were to be depleted of circulating neutrophils were given intraperitoneal injections of 250 μg of the antibody RB6 twice a week. Based on differential staining of lavage fluids of depleted and nondepleted mice, we have found that RB6 treatment seems to give effective depletion of neutrophils for only 9 days (after this period, neutrophils began to appear in the lavage fluids of RB6-depleted mice), and so for mice sacrificed on day 21 (postinoculation), RB6 treatment was begun on day 12, for mice sacrificed on day 29, RB6 treatment was begun on day 20, and for mice sacrificed on day 35, RB6 treatment was begun on day 26. Inoculation of mice with Pneumocystis was performed using lung homogenates from previously infected C.B17 scid/scid mice. The lungs of these source mice were placed in 5 ml of Hanks balanced salt solution (HBSS) and homogenized by being pushed through a stainless steel mesh. Material passing through the mesh was centrifuged (1,500 × g for 25 min), and the pellet was resuspended in a minimal volume of sterile HBSS. Pneumocystis nuclei in the material were enumerated as described below and diluted to a concentration of 108 Pneumocystis nuclei per ml. Mice to be infected were anesthetized with isoflurane and then given intratracheal injections of 107 Pneumocystis organisms with a blunted 20-gauge needle (25).
Respiratory measurements.
Prior to tissue sampling, respiratory rates in conscious mice were measured using a whole-body plethysmograph (Buxco Electronics, Sharon, Conn.). Arterial blood gas levels were determined by warming the mice for 5 min at 39°C, carefully nicking the ventral tail arteries, and collecting 150 μl of blood into a heparinized capillary tube. After mixing of the blood with the use of a small steel bar and magnet, the samples were analyzed on a clinical blood gas analyzer (Omni AVL; Roche Diagnostics, Indianapolis, Ind.) within 1 h of sampling. In some experiments, dynamic lung compliance was measured in live anesthetized mice as previously described (75) and expressed as milliliters per centimeter of H2O per kilogram of body weight.
BAL.
Mice were sacrificed by deep pentobarbital anesthesia followed by exsanguination. The trachea of each mouse was nicked, and a tube was inserted to lavage the lungs with five 1-ml aliquots of HBSS with 3 mM EDTA (28). Samples from each 5-ml pooled lavage fluid were spun onto a slide with a cytospin centrifuge and stained with Diff-Quick dye (Dade Behring, Newark, Del.), and the relative numbers of each cell type were determined using a 100× lens objective. The remaining lavage cells were concentrated by centrifugation at 900 × g for 10 min. The supernatant was tested for albumin concentration with a diagnostic assay (catalog no. 631-2; Sigma Diagnostics, St. Louis, Mo.) and in some cases for lactate dehydrogenase (LDH) concentration with the CytoTox 96 assay (Promega, Madison, Wis.). The bronchoalveolar lavage (BAL) cells were resuspended in a minimal volume of phosphate-buffered saline with 2% calf serum and an anti-mouse Fc receptor antibody (Trudeau Institute, Saranac Lake, N.Y.) to block nonspecific binding. These cells were then stained with fluorophore-conjugated antibodies against mouse CD4 (to verify that no CD4+ lymphocytes were present in samples from CD4-depleted animals) and CD8 (to detect CD8+ lymphocytes; PharMingen, San Diego, Calif.) and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, Calif.).
Enumeration of Pneumocystis nuclei.
After lavage, the trachea was ligated and two-thirds of the lung was removed, placed into 5 ml of sterile HBSS, and disrupted by being pushed through a metal screen. An aliquot of this material was diluted 1:20 and applied to a glass slide by using a cytospin centrifuge. After drying, the slides were stained for an extended period of time (20 to 40 min) in Diff-Quick dye. Pneumocystis nuclei (both cysts and trophozoites) were then counted in a minimum of 5 and up to a maximum of 50 (if nuclei were not readily apparent) oil immersion fields. The average counts were then converted to log numbers of Pneumocystis nuclei per lung; with this technique in this lab, the limit of detection was (log) 4.40 or 4.24 (depending on the lens used) when 50 fields were counted. Although the previous lavage does remove some Pneumocystis organisms from the lung, they are a small fraction (<10%) of what is present in the lung, and this is consistent among different groups of mice.
Histology.
One-third of the lung tissue was fixed for 24 h in phosphate-buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin by using standard histological techniques.
Statistical analysis.
The software program Prism (GraphPad, San Diego, Calif.) was used for all statistical tests of significance (to P values of ≤0.05). Normally, a two-sided t test was used to compare two groups of data, with Welch's correction being used if the groups had unequal variances. In cases in which a deviation from a normal distribution was suspected, a nonparametric test (Mann-Whitney test) was also applied. In those cases, we found that both the t test and Mann-Whitney test indicated the same results (i.e., both indicated significance or insignificance); however, typically one test gave a more conservative (larger, but still <0.05) P value. The P values we report are always the conservative values.
RESULTS
Compliance versus alveolar neutrophil accumulation.
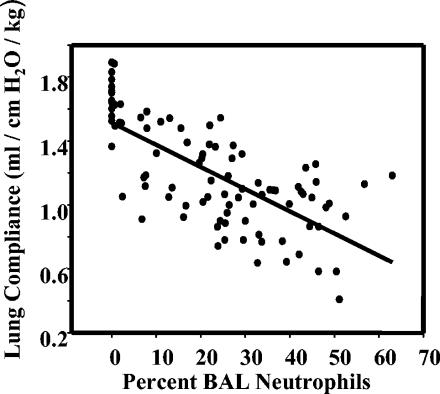
In humans with Pneumocystis pneumonia, there is a well-known correlation between the severity of pulmonary symptoms and the numbers of neutrophils found in BAL fluids. We show here a similar relationship in C57BL/6 mice in that lung compliance (which decreases with increasing pulmonary pathology) in Pneumocystis-infected animals was inversely and significantly correlated with the relative numbers of neutrophils in the alveolar compartments (Fig. 1) (F = 91.484; P < 0.001; r2 = 0.504). There was also significant correlation between the numbers of Pneumocystis organisms and the dynamic lung compliance in these same animals, although that model had a much poorer goodness of fit (data not shown) (F = 22.11; P < 0.001; r2 = 0.196).
FIG. 1.
Dynamic lung compliance in CD4+ lymphocyte-depleted and Pneumocystis-infected C57BL/6 mice versus percentages of neutrophils found in BAL fluids. Values from several experiments with mice at different stages of infection (3, 4, 5, and 6 weeks postinoculation) are pooled. The cluster of values at 0% neutrophils represents measurements in uninfected mice.
CXCR2-KO.
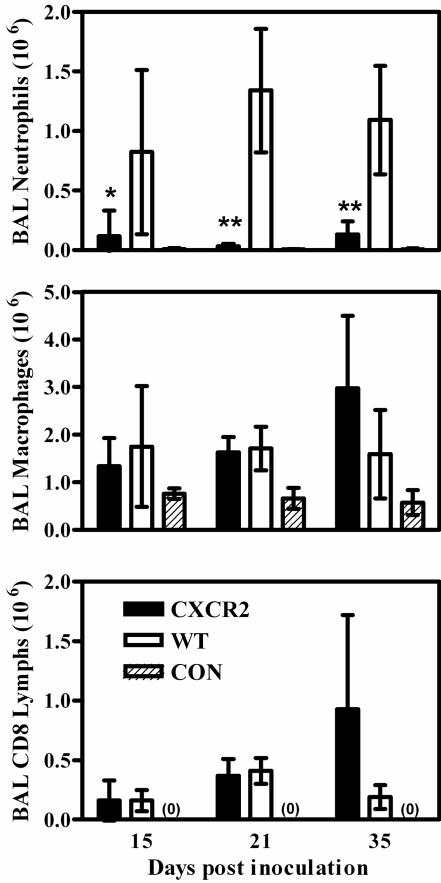
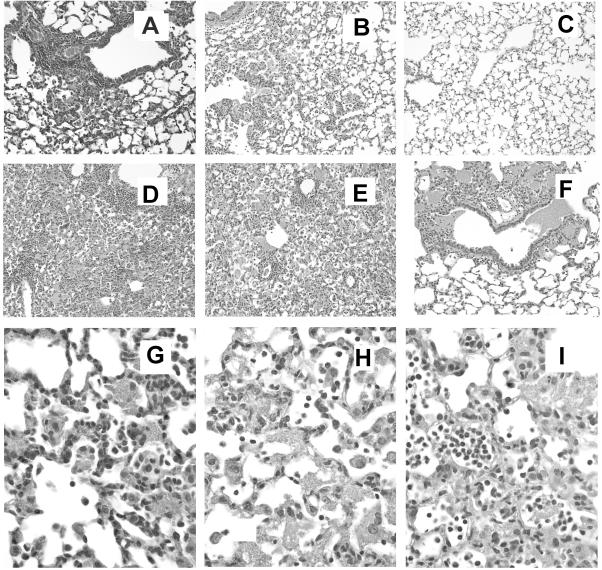
In mice with the genetic knockout of the chemokine receptor CXCR2, granulocytes are unresponsive to chemokines that are analogous to interleukin-8, such as macrophage inflammatory protein 2 and KC (15, 37). When wild-type mice were depleted of CD4+ lymphocytes to make them susceptible to infection and then inoculated with Pneumocystis, there was considerable accumulation of neutrophils in the alveoli during the course of infection; however, in similarly treated CXCR2-KO mice, only 5 to 10% of the number of neutrophils in wild-type mice were found in lavage fluids (Fig. 2). The levels of other alveolar inflammatory cells such as macrophages and CD8+ lymphocytes (no CD4+ lymphocytes were found in samples from CD4+-depleted mice) were similar in CXCR2-KO and wild-type animals; at the final stage of infection, there was a trend towards higher levels of macrophages and CD8+ lymphocytes in the alveoli of CXCR2-KO mice, but this was not statistically significant (Fig. 2). Histological examination of lung tissue from the CXCR2-KO mice confirmed that neutrophils were virtually absent from the alveolar spaces, although they could be seen marginated in pulmonary capillaries and venules and in the interstitial spaces. There were evident localized patches of lymphocytes and macrophages nearby growths of Pneumocystis in early stages of the disease and widespread infiltration by CD8+ lymphocytes and macrophages in later stages (Fig. 3A, B, and G).
FIG. 2.
Levels of inflammatory cells in BAL samples from CXCR2-KO and BALB/c wild-type (WT) mice depleted of CD4+ lymphocytes and inoculated with 107 Pneumocystis organisms, as well as those in samples from nondepleted, noninfected BALB/c control mice (CON). CD8 Lymphs, CD8+ lymphocytes. Mice were sampled at 15, 21, and 35 days postinoculation. Values are means ± standard deviations (SD). n = 5, except for CXCR2-KO mice at 21 days, where n = 3; *, P < 0.05; **, P < 0.01 (comparing CXCR2-KO to WT mice). Results shown are representative of those from two independent experiments.
FIG. 3.
Hematoxylin and eosin-stained cross sections of lung tissues from knockout and wild-type mice. (A) Tissue from CXCR2-KO mouse 21 days after inoculation with Pneumocystis (magnification, ×200). Note the perivascular cuffing of inflammatory cells and Pneumocystis. (B) Tissue from wild-type BALB/c mouse 21 days after inoculation with Pneumocystis, showing more-diffuse inflammation and Pneumocystis growth (magnification, ×200). (C) Tissue from uninfected BALB/c mouse (magnification, ×200). (D) Tissue from gp91phox-KO mouse 35 days after inoculation with Pneumocystis (magnification, ×200). (E) Tissue from wild-type C57BL/6 mouse 35 days after inoculation with Pneumocystis (magnification, ×200). (F) Tissue from phox/iNOS-KO mouse 35 days postinoculation, showing tight clusters of Pneumocystis pushing into airways (magnification, ×200). (G) Tissue from CXCR2-KO mouse 35 days after inoculation with Pneumocystis (magnification, ×600). Pneumocystis, macrophages, and lymphocytes are present in the alveolar space, while neutrophils are virtually absent from the alveoli but present in pulmonary blood vessels and the interstitial space. (H) Tissue from C57BL/6 wild-type mouse 35 days after inoculation with Pneumocystis (magnification, ×600), showing mixed alveolar infiltrate of neutrophils, macrophages, and lymphocytes. (I) Tissue from phox/iNOS-KO mouse 35 days after inoculation with Pneumocystis (magnification, ×600), showing large numbers of neutrophils, as well as macrophages and lymphocytes, in the alveolar space.
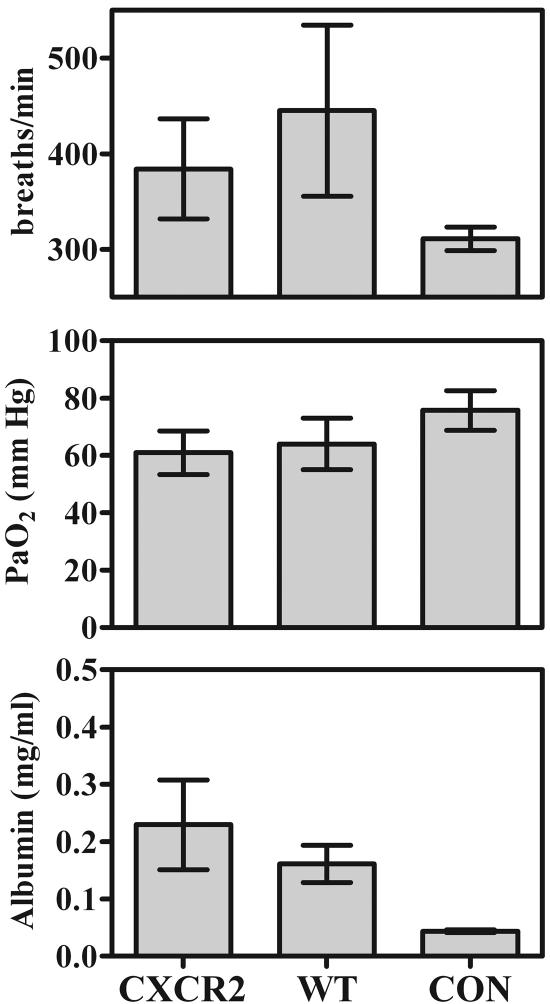
In spite of this lack of neutrophils in the alveolar spaces, measurements of lung damage were not lower in CXCR2-KO mice than in wild-type mice: no significant differences were seen in respiratory rates, arterial oxygen partial pressures, or BAL fluid albumin concentrations at any stage of infection (representative day-35 data are shown in Fig. 4). Finally, in spite of the difference in the pattern of Pneumocystis growth that was evident histologically in the CXCR2-KO mice (at least at early stages of infection), there were never any significant differences in total Pneumocystis burdens between CXCR2-KO and wild-type mice during the course of infection (Table 1).
FIG. 4.
Indicators of lung damage in CD4+-depleted CXCR2-KO and BALB/c wild-type (WT) mice 35 days after inoculation with 107 Pneumocystis organisms, as well as those in nondepleted, noninfected BALB/c control mice (CON). (Upper panel) Respiratory rates determined by whole-body plethysmography, expressed in breaths per minute. (Center panel) Oxygen partial pressures in arterial blood (PaO2), measured in millimeters of mercury (mm Hg). (Bottom panel) Albumin concentrations in BAL fluid in milligrams per milliliter. Values are means ± SD. n = 5. Results shown are representative of those from two independent experiments.
TABLE 1.
Pneumocystis burdens in lungs of mice
| Mouse model | Time point (days postinoculation) | Log10 no.a of Pneumocystis nuclei per lung (no. of mice) in:
|
||
|---|---|---|---|---|
| KO (+CD4)b mice | KO (−CD4)c mice | WT (−CD4)d mice | ||
| CXCR2-KO | 15 | NDe | 7.01 ± 0.22 (4) | 6.96 ± 0.91 (5) |
| 21 | ND | 7.06 ± 0.31 (6) | 7.66 ± 0.24 (5) | |
| 35 | ND | 7.38 ± 0.75 (3) | 7.76 ± 0.21 (5) | |
| 15 | ND | 6.31 ± 0.31 (5) | 6.26 ± 0.49 (5) | |
| gp91phox-KO | 28 | ND | 7.77 ± 0.70 (3) | 7.94 ± 0.08 (3) |
| 35 | 4.43 ± 0.00 (5) | 7.96 ± 0.31 (5) | 7.76 ± 0.60 (5) | |
| phox/iNOS-KO | 21 | 4.98 ± 0.72 (4) | 6.96 ± 0.08 (3) | 6.46 ± 0.81 (4) |
| 28 | 4.47 ± 0.45 (4) | 7.77 ± 0.60 (5) | 8.26 ± 0.08 (5) | |
| 35 | 4.91 ± 0.91 (5) | 8.32 ± 0.06 (5) | 8.14 ± 0.14 (5) | |
Values are means ± SD. No Pneumocystis nuclei were found in wild-type mice used as controls that were not depleted of CD4+ lymphocytes or infected with Pneumocystis (n = 5). This result translates to a log 4.40 or 4.24, which is the limit of detection in this method of counting depending on the microscope lens used.
KO (+CD4), knockout mice corresponding to the indicated model in which CD4+ lymphocytes were not depleted but that were inoculated with Pneumocystis.
KO (−CD4), knockout mice corresponding to the indicated model that were depleted of CD4+ lymphocytes during the experiment and inoculated with Pneumocystis.
WT (−CD4), wild-type mice appropriate for the indicated model that were depleted of CD4+ lymphocytes and infected with the same dose as the corresponding knockout mice.
Not done.
gp91phox-KO.
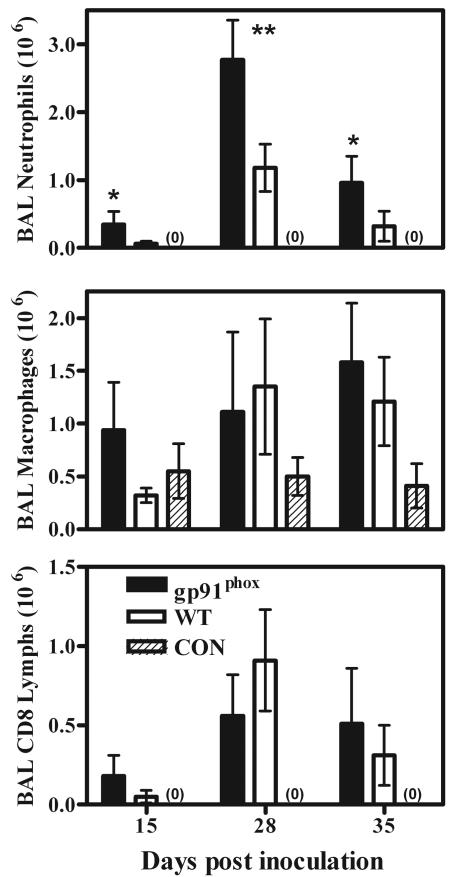
Neutrophils in mice that have the gp91phox component of the NADPH oxidase enzyme complex genetically deleted have normal responses to chemokines (unlike those in the CXCR2-KO mice); however, they are unable to produce significant ROS in response to inflammatory stimuli (47). When these mice were infected with Pneumocystis, neutrophil recruitment into the alveolar spaces was significantly enhanced over that seen in wild-type mice; at 15 days postinfection, there were five times as many neutrophils in the lungs of gp91phox-KO mice, and at 35 days the expected influx of neutrophils into the lungs of wild-type mice reduced this to only a fourfold difference (Fig. 5). There was also a tendency for other inflammatory cells to be at higher concentrations in the lungs of gp91phox-KO mice early in the course of Pneumocystis infection. Again, at 15 days postinfection there were significantly greater numbers of macrophages and CD8+ lymphocytes in the lavage fluids of gp91phox-KO mice than in those of wild-type mice (Fig. 5), but this discrepancy was not seen later in the infection.
FIG. 5.
Levels of inflammatory cells in BAL samples from gp91phox-KO and C57BL/6 wild-type (WT) mice depleted of CD4+ lymphocytes and inoculated with 107 Pneumocystis organisms, as well as those in samples from nondepleted, noninfected C57BL/6 control mice (CON). CD8 Lymphs, CD8+ lymphocytes. Mice were sampled at 15, 28, and 35 days postinoculation. Values are means ± SD. n = 5; *, P < 0.05; **, P < 0.01 (comparing gp91phox-KO to WT mice). Results shown are representative of those from two independent experiments.
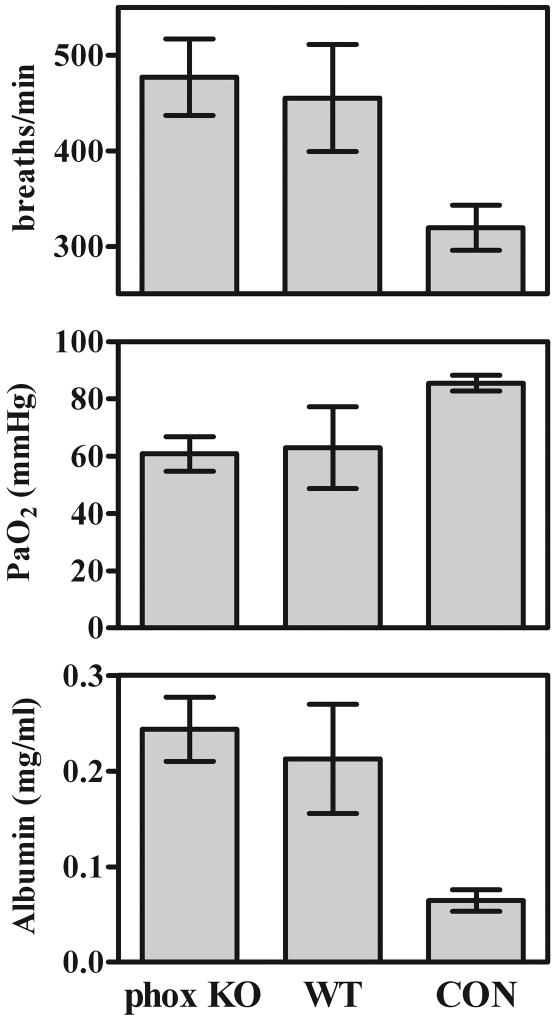
As with the CXCR2-KO mice, pulmonary damage in the gp91phox-KO mice during Pneumocystis infection was not significantly different from that seen in wild-type mice. Neither arterial oxygen partial pressures nor respiratory rates were significantly different between gp91phox-KO mice and wild-type mice at any point we sampled (Fig. 6 shows representative day-35 data). There were also no significant differences in pulmonary lavage fluid albumin concentrations between gp91phox-KO and wild-type mice at any time point (Fig. 6). Finally, there were no histologically obvious differences between the gp91phox-KO mice and wild-type mice in terms of levels of pulmonary damage or patterns of Pneumocystis growth (Fig. 3D and E).
FIG. 6.
Indicators of lung damage in CD4+-depleted gp91phox-KO (phox KO) and C57BL/6 wild-type (WT) mice 35 days after inoculation with 107 Pneumocystis organisms, as well as those in nondepleted, noninfected C57BL/6 control mice (CON). See the legend to Fig. 4 for explanation. Values are means ± SD. n = 5. Results shown are representative of those from two independent experiments.
The lack of a functional NADPH oxidase did not by itself make mice susceptible to Pneumocystis. gp91phox-KO mice that were not depleted of CD4+ lymphocytes and were inoculated with Pneumocystis completely cleared the organism and showed no signs of residual infection or pulmonary damage (Table 1). In mice that were depleted of CD4+ lymphocytes, levels of growth of Pneumocystis were identical in gp91phox-KO and wild-type mice (Table 1).
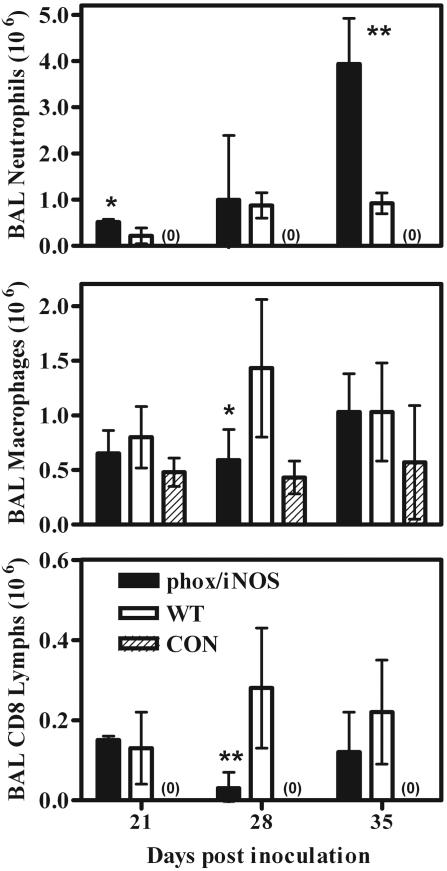
phox/iNOS-KO.
phox/iNOS-KO mice are genetically deficient in gp91phox and therefore lack a functional NADPH oxidase to produce ROS. Additionally, they are deficient in iNOS and so are also lacking in the production of reactive nitrogen species (RNS). As that in the gp91phox-KO mice, infection with Pneumocystis resulted in the recruitment of large numbers of neutrophils into the alveolar spaces; at later stages of the disease, there were almost six times as many neutrophils in the BAL fluids of phox/iNOS-KO mice as in those of wild-type mice (Fig. 7 and 3I). This discrepancy was not exclusively limited to neutrophils; at 28 days postinfection, levels of both macrophages and CD8+ lymphocytes were significantly lower in the BAL fluids of phox/iNOS-KO mice than in those of wild-type infected mice. However, there were no consistent differences in levels of cells other than neutrophils (Fig. 7).
FIG. 7.
Levels of inflammatory cells in BAL samples from phox/iNOS-KO and C57BL/6 wild-type (WT) mice depleted of CD4+ lymphocytes and inoculated with 107 Pneumocystis organisms, as well as those in samples from nondepleted, noninfected C57BL/6 control mice (CON). CD8 Lymphs, CD8+ lymphocytes. Mice were sampled at 21, 28, and 35 days postinoculation. Values are means ± SD. n = 5; *, P < 0.05; **, P < 0.01 (comparing phox/iNOS-KO to WT mice). Results shown are representative of those from three independent experiments.
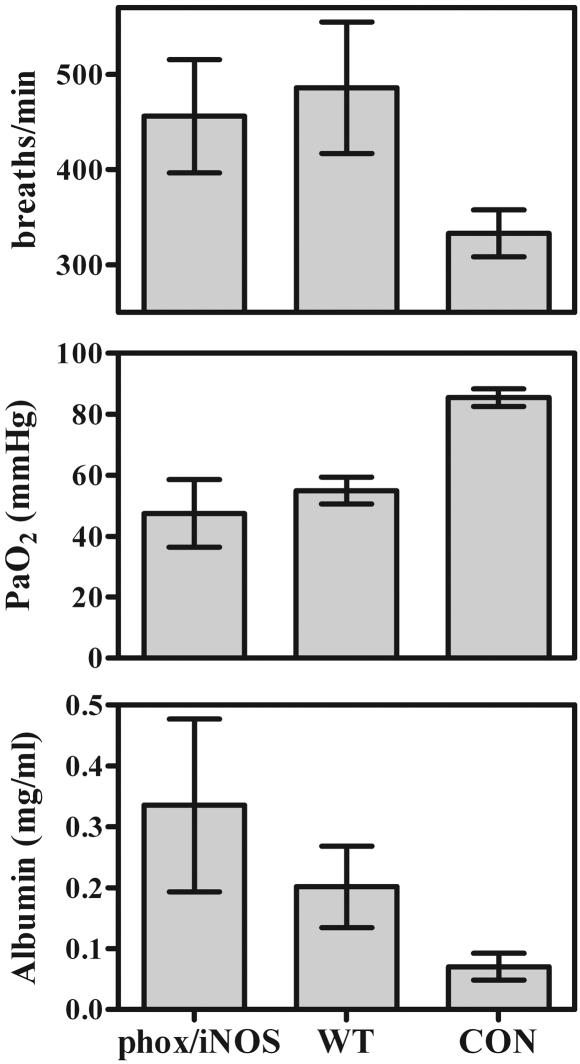
As in the previously described mouse models, there was very little difference in indicators of lung injury in the lungs of Pneumocystis-infected phox/iNOS-KO mice compared to those in wild-type mice. Although arterial oxygen partial pressures were low in infected mice and respiratory rates were elevated, there were no significant differences between phox/iNOS-KO and wild-type mice (Fig. 8). The same was true for BAL fluid albumin concentrations, which were slightly elevated but not significantly so at 35 days postinfection (Fig. 8). In this regard, BAL fluid LDH concentrations were significantly higher in samples from phox/iNOS-KO mice than in those from wild-type mice (0.025 ± 0.0023 U/ml [n = 4] versus 0.0146 ± 0.0017 U/ml [n = 5]), suggesting greater damage in lung cells in the phox/iNOS-KO mice. Although we did not conduct LDH assays in all experiments over the course of the study, this was the only case in which a significant difference was seen.
FIG. 8.
Indicators of lung damage in CD4+-depleted phox/iNOS-KO and C57BL/6 wild-type (WT) mice 35 days after inoculation with 107 Pneumocystis organisms, as well as those in nondepleted, noninfected C57BL/6 control mice (CON). See the legend to Fig. 4 for explanation. Values are means ± SD. n = 5. Results shown are representative of those from three independent experiments.
One unusual and consistent difference seen in phox/iNOS-KO mice was the pattern of Pneumocystis growth in the lung, as observed histologically. Pneumocystis in the lungs of these animals was less disperse than that seen in the lungs of wild-type mice; however, the clumps of Pneumocystis were much more dense in the alveoli and were often seen pushing out into distal airways (Fig. 3F).
RB6 depletion of neutrophils.
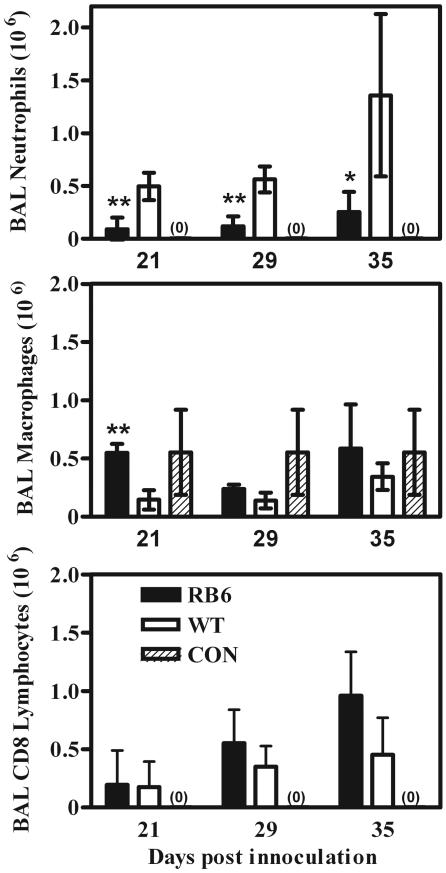
Depletion of circulating neutrophils resulted in a dramatic reduction of neutrophils in the alveolar compartment during Pneumocystis infection, to at least 25% of the level seen in wild-type animals (Fig. 9). At the same time, there was a slight increase in the numbers of CD8+ lymphocytes in the alveoli of neutrophil-depleted mice during the later stages of Pneumocystis infection relative to those in wild-type animals (Fig. 9).
FIG. 9.
Levels of inflammatory cells in BAL samples from BALB/c mice in which neutrophils were depleted with the antibody RB6 (RB6) and BALB/c wild-type mice (WT) both depleted of CD4+ lymphocytes and inoculated with 107 Pneumocystis organisms, as well as those in samples from nondepleted, noninfected BALB/c control mice (CON). Mice were sampled at 15, 28, and 35 days postinoculation. Values are means ± SD. n = 5; *, P < 0.05; **, P < 0.01 (comparing RB6 to WT mice). Results shown are representative of those from two independent experiments.
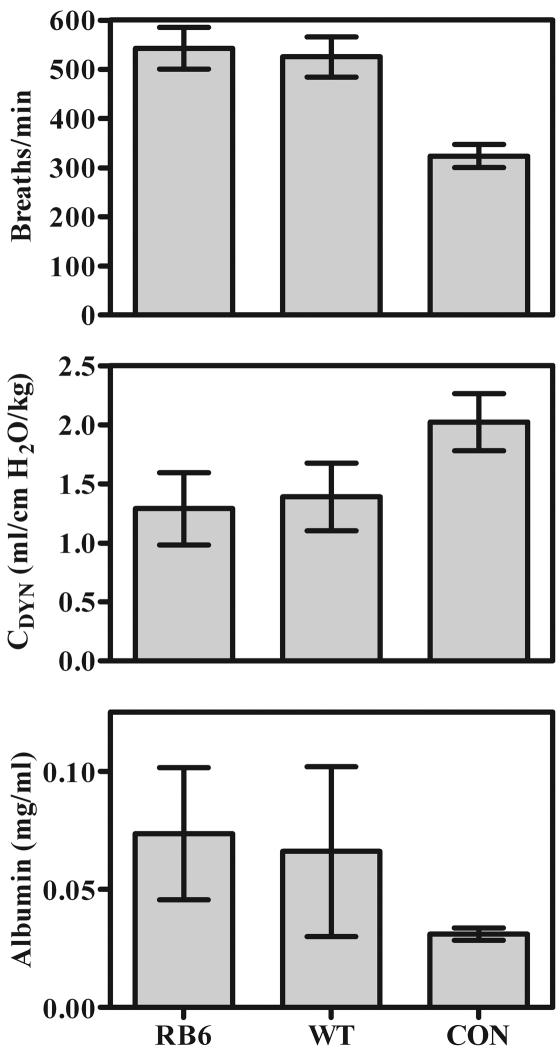
In the RB6 deletion experiments, the indicators of pulmonary damage that we measured were respiratory rate, BAL fluid albumin concentration, and dynamic lung compliance (a previous study showed that dynamic lung compliance correlates well with arterial oxygen partial pressure as an indicator of impaired lung function during Pneumocystis infections [74]). As seen in the other models of impaired neutrophil function, indicators of pulmonary damage in Pneumocystis infection with and without neutrophil depletion were not significantly different: both respiratory rates and BAL fluid albumin concentrations were similar in mice depleted of CD4+ lymphocytes and neutrophils and mice depleted of CD4+ lymphocytes only (Fig. 9), and Pneumocystis infection resulted in similar decreases in dynamic lung compliance in mice depleted of CD4+ lymphocytes and neutrophils and mice depleted of CD4+ lymphocytes only (Fig. 10).
FIG. 10.
Indicators of lung damage in BALB/c mice in which circulating neutrophils were depleted with the antibody RB6 (RB6) and BALB/c wild-type mice (WT) both depleted of CD4+ lymphocytes and inoculated with 107 Pneumocystis organisms, as well as those in nondepleted, noninfected BALB/c control mice (CON). (Upper panel) Respiratory rates expressed in breaths per minute. (Center panel) Dynamic lung compliance (CDYN) determined for anesthetized, intubated mice. (Bottom panel) Albumin concentrations in BAL fluid in milligrams per milliliter. Values are means ± SD. n = 4.
DISCUSSION
In humans with Pneumocystis pneumonia, the degree of clinical impairment, and in fact a poorer prognosis, correlates with the finding of increasing numbers of neutrophils in the BAL fluids (4, 39, 42, 61). Just as noted in humans, we show that in mice pulmonary damage, as indicated by the decrease in dynamic lung compliance, correlates with the numbers of neutrophils found in BAL fluids. Although we confirmed the association between elevated levels of pulmonary neutrophils and a poorer outcome from Pneumocystis pneumonia, we wished to establish whether neutrophils were actually responsible for the pulmonary damage seen in Pneumocystis pneumonia in mice. In this report, we utilized four separate mouse models of impaired neutrophil function to examine the role of neutrophil mechanisms in host tissue damage in Pneumocystis pneumonia. Surprisingly, in all cases, the levels of lung damage seen during Pneumocystis infection were the same in wild-type mice and neutrophil-impaired knockout mice.
Neutrophil host defense mechanisms are categorized as oxidative and nonoxidative. Oxidative mechanisms rely upon the production of superoxide, primarily by the multisubunit enzyme NADPH oxidase (73). The importance of this enzyme in host defense against certain pathogens is demonstrated by the serious infections common in individuals with chronic granulomatous disease, in which one of the NADPH oxidase components is missing (52). However, excessive ROS production in certain pulmonary states can also have deleterious consequences. Direct peroxidation of membrane lipids by ROS and formation of nitrotyrosine-protein adducts by peroxynitrite (which is itself formed by the reaction of superoxide with NO) can directly disrupt the function of molecules on host cells (41). ROS are also implicated in the activation of transcriptional factors (NF-κB and activator protein 1) leading to the transcription of genes that accentuate the inflammatory process (41, 49).
The implication of these neutrophil mechanisms in host damage is most evident in controlled studies using environmental irritants, such as cigarette smoke (reviewed in references 21 and 41) and oil fly ash (26). There is also considerable evidence that neutrophil oxidants are directly involved with the pathology found in acute respiratory distress syndrome (5, 30). However, the direct role of neutrophil oxidants in host tissue damage observed during acute infectious diseases is less clear, although there is some circumstantial evidence. In a mouse model of pneumococcal pneumonia, occurrence of high levels of malondialdehyde, a marker of lipid peroxidation, in lung lavage fluid coincides with the appearance of profound pulmonary histopathology (12). A similar response is seen in mice infected with various doses of Pseudomonas aeruginosa in that markers of oxidative stress, such as lipid peroxidation, increase in conjunction with the levels of biochemical markers of pulmonary damage (63). Biochemical indices of oxidative damage to pulmonary tissue also occur in virus-induced pneumonia in mice (1, 44), although the source of the oxidants in this model may not be entirely the phagocyte NADPH oxidase. The availability of mouse strains in which a protein subunit of the NADPH oxidase has been genetically deleted has allowed for in vivo investigations into the importance of neutrophil ROS production in pulmonary damage. In a study in which mice were challenged with Escherichia coli to produce sepsis, the NADPH oxidase knockout mice had increased migration of neutrophils into the lung but at the same time had reduced lung microvascular injury, suggesting that superoxide production was important in the development of the pulmonary injury (24). In contrast, in a model of complement-induced lung injury, the absence of a functional NADPH oxidase did not result in less pulmonary damage, as assessed by accumulation of extravascular albumin (35).
Our results indicate that in Pneumocystis-infected mice, the absence of superoxide produced by NADPH oxidase does not ameliorate the damage that occurs during the disease process. This does not, however, totally rule out a role for phagocyte-derived oxidants in the pulmonary damage. RNS, most notably NO produced by iNOS, can be produced by phagocytes, predominately macrophages (13). NO is known to have a variety of important roles in host defense (reviewed in reference 18). NO is also implicated in host tissue damage, including that of the lung, because of its reaction with superoxide to form the highly reactive oxidant peroxynitrite (60, 66). A recent report indicates that ROS production and RNS production can compensate for each other in host defense such that mice with knockouts of either gp91phox or iNOS by themselves are not susceptible to certain pathogens but mice deficient in both gp91phox and iNOS are highly susceptible to infection with the same pathogens (57). As a parallel to this effect on host defense, there also appear to be compensatory adjustments in these pathways that account for similar levels of pulmonary damage when one pathway is deleted. For example, when gp91phox knockout animals are challenged with cobra venom factor to produce complement-induced lung injury, the damage is the same as that seen in wild-type animals; however, NG-methyl-l-arginine, an inhibitor of iNOS, is protective in the gp91phox knockout animals but not in wild-type animals according to a previous study (35). That study and others (e.g., reference 1) suggest that peroxynitrite can still be formed in gp91phox knockout animals by combining NO produced by iNOS and superoxide produced by xanthine oxidase and that this compound is what causes the oxidative damage. Therefore, we elected to compare levels of pulmonary damage in mice that have both the gp91phox and iNOS pathways disrupted. Like the gp91phox single knockouts, these double knockout mice exhibited little difference from wild-type mice in levels of Pneumocystis-related pulmonary damage (although increased BAL fluid LDH concentrations could be indicative of a trend towards greater damage), in spite of the elevated numbers of neutrophils in the alveolar compartments. Together, the results from the gp91phox-KO and phox/iNOS-KO mouse models strongly suggest that oxidative mechanisms of neutrophil function (and assumedly phagocytes other than neutrophils) do not play a major role in the pathogenesis seen in Pneumocystis infections in mice.
Neutrophils also possess many nonoxidative effector mechanisms, including proteolytic enzymes (elastase and cathepsin G) and cationic proteins with direct antimicrobial actions (α-defensins and cathelicidins) (14, 58). A great deal of in vitro work has implicated these proteins in pulmonary pathology (34, 45, 46, 67). Recently, there have also been several in vivo studies on resistance to infection and damage to pulmonary tissue with the use of models with knockouts of neutrophil proteins such as elastase, cathepsin G, and matrix metalloproteinase type 9. Results from these studies indicate a complex role for these proteins: defects in these proteins do not impair transendothelial migration of neutrophils (2), which is contrary to the accepted role for these proteins (55). In spite of this finding, an important role for neutrophil elastase in pathogen resistance is evident from studies that show that mice deficient in elastase have greater susceptibility to high doses of several gram-negative pathogens (11) and Candida albicans (50). In addition, mice doubly deficient in elastase and cathepsin G are more susceptible to Aspergillus (65) and Staphylococcus aureus (50). With regard to host tissue damage, deficiency of neutrophil elastase is protective against the induction of emphysema by cigarette smoke in mice (56) and also against fibrosis induced by bleomycin in mice (20). Mice that are doubly deficient in elastase and cathepsin G are resistant to some of the damaging effects on pulmonary tissue during endotoxic shock (65).
However, because there appear to be coordinate expression and redundant function of the neutrophil proteases (40), determining whether any one of these individual proteins is responsible for host damage in an infectious disease model is not straightforward. Furthermore, neutrophil elastase has an important role in the recruitment of macrophages into the lung (56), and so effects on host tissue damage in animals with knockouts of this protein may reflect the role of macrophages as much as that of neutrophils. For these reasons, the question of whether nonoxidative neutrophil effector functions are involved in pulmonary pathology in Pneumocystis infections in mice did not seem approachable using the limited applicability of in vivo models of knockouts of those proteins. However, we reasoned that it was unlikely that these functions were a major factor in the damage that we observed since in the gp91phox-KO and phox/iNOS-KO mice, even with four- to sixfold more neutrophils in the alveolar spaces, measurements of lung damage were the same or marginally greater than those in wild-type mice. To further address the question of whether the presence of neutrophils, acting through either nonoxidative or oxidative mechanisms, in the alveoli is instrumental in Pneumocystis-related pulmonary damage, we employed the CXCR2-KO mouse model, in which recruitment of neutrophils to the site of infection was greatly diminished. In CXCR2-KO animals, in spite of the fact that neutrophil accumulation in the alveolar compartments was only 5 to 10% of that seen in wild-type mice similarly infected, there were no significant differences in pulmonary pathology. Therefore, as a whole, our findings point to the simple but somewhat surprising conclusion that in spite of their accumulation in the lung during later stages of Pneumocystis infections, neutrophils do not play a major role, either through oxidative or nonoxidative mechanisms, in the inducement of the pulmonary damage that occurs during this time.
It should be stated, however, that these studies do not rule out indirect neutrophil involvement in Pneumocystis-related host tissue damage. Although neutrophils are normally thought of as effector cells, there is some evidence that they may also have an immunoregulatory role, through release of cytokines or other regulatory substances (32, 51, 64). If so, then neutrophils may be able to exert some effects on lung tissue responses, even without entering the alveoli. Experiments to completely deplete circulating neutrophils have been performed using other disease models (59, 72). We performed similar experiments with the antineutrophil antibody RB6; the results were consistent with other data reported here in that measurements of pulmonary damage were the same between wild-type and neutrophil-depleted mice, which suggests that neutrophils have neither a local nor distant role in Pneumocystis infections.
This study also confirms previous observations which suggested that neutrophils do not have a substantial role in the clearance of Pneumocystis, at least not when CD4+ lymphocytes are absent (16, 33). We found no significant differences in the levels of Pneumocystis between wild-type animals and any of the three sets of neutrophil function knockout mice that were inoculated at the same time. However, our histological observations do seem to suggest that these neutrophil functions have some effect on the pattern of Pneumocystis growth in the lung. This was most evident in the phox/iNOS-KO mice, in which the Pneumocystis grew in denser clumps but in fewer alveoli than it did in the wild-type mice (even though the numbers of Pneumocystis organisms in the lungs were the same in both groups). While it is tempting to speculate that the Pneumocystis organisms are aided in their dispersion through the lung by the oxidative functions of the neutrophils, we have no supporting evidence for this possibility at this time.
The apparent lack of a significant causative role for neutrophils in the induction of Pneumocystis-induced lung damage raises some interesting questions about the major mechanisms behind this type of pulmonary damage. In susceptible animals, Pneumocystis will grow in the alveolar compartments, with some degree of direct attachment to the type I epithelial cells (22, 48, 76). Although the direct attachment may result in alteration of pulmonary barrier function and subsequent dysfunction (reviewed in reference 62), it appears that most pulmonary pathophysiology coincides with the appearance of inflammatory cells in the alveoli (27, 74). Several cell types may be involved in this process. Alveolar macrophages have potent microbicidal mechanisms, including production of ROS and RNS, and they increase in both number and level of activation in the lung during Pneumocystis infection (7, 38). However, the fact that gp91phox and gp91phox-iNOS knockouts also affect aspects of macrophage function makes any conclusions about the role of macrophages difficult at this time. CD8+ lymphocytes accumulate in high numbers in the alveoli of Pneumocystis-infected animals, and there is strong evidence that these cells are responsible for much of the inflammatory-derived damage to host tissue (3, 8, 74). However, while the implication of CD8+ lymphocytes in host tissue damage is more compelling than we have shown here for neutrophils, the mechanisms by which these cells cause this damage is still unclear. Further studies are necessary to elucidate what mechanisms of cells other than neutrophils are involved in Pneumocystis-related pulmonary damage.
Acknowledgments
We are indebted to the expert assistance of G. Callis, A. Harmsen, S. Han, M. Higgins, Q. King, L. McNamee, N. Meissner, S. Sheldon, M. Tighe, and J. Wiley.
This work was supported by National Institutes of Health grants HL 55002, HL 71659, and HL 064559 and the Montana Agricultural Experimental Station.
Editor: T. R. Kozel
REFERENCES
- 1.Akaike, T., Y. Noguchi, S. Ijiri, K. Setoguchi, M. Suga, Y. M. Zheng, B. Dietzschold, and H. Maeda. 1996. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc. Natl. Acad. Sci. USA 93:2448-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allport, J. R., Y. C. Lim, J. M. Shipley, R. M. Senior, S. D. Shapiro, N. Matsuyoshi, D. Vestweber, and F. W. Luscinskas. 2002. Neutrophils from MMP-9- or neutrophil elastase-deficient mice show no defect in transendothelial migration under flow in vitro. J. Leukoc. Biol. 71:821-828. [PubMed] [Google Scholar]
- 3.An, C. L., X. P. Su, and A. G. Harmsen. 2000. The role of CD8+ T cells in the pathogenesis of Pneumocystis carinii pneumonia in mice depleted of CD4+ T cells. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 18:207-212. [PubMed]
- 4.Azoulay, E., A. Parrot, A. Flahault, D. Cesari, I. Lecomte, P. Roux, F. Saidi, M. Fartoukh, J. F. Bernaudin, J. Cadranel, and C. Mayaud. 1999. AIDS-related Pneumocystis carinii pneumonia in the era of adjunctive steroids: implication of BAL neutrophilia. Am. J. Respir. Crit. Care Med. 160:493-499. [DOI] [PubMed] [Google Scholar]
- 5.Babior, B. M. 2000. Phagocytes and oxidative stress. Am. J. Med. 109:33-44. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett, M. S., W. L. Current, A. Orazi, N. L. Bauer, R. S. Neiman, S. F. Queener, and J. W. Smith. 1994. Comparison of corticosteroid- and L3T4+ antibody-immunosuppressed mouse models of Pneumocystis carinii pneumonia for evaluation of drugs and leukocytes. Clin. Diagn. Lab. Immunol. 1:511-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck, J. M., H. D. Liggitt, E. N. Brunette, H. J. Fuchs, J. E. Shellito, and R. J. Debs. 1991. Reduction in intensity of Pneumocystis carinii pneumonia in mice by aerosol administration of gamma interferon. Infect. Immun. 59:3859-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck, J. M., R. L. Newbury, B. E. Palmer, M. L. Warnock, P. K. Byrd, and H. B. Kaltreider. 1996. Role of CD8+ lymphocytes in host defense against Pneumocystis carinii in mice. J. Lab. Clin. Med. 128:477-487. [DOI] [PubMed] [Google Scholar]
- 9.Beck, J. M., M. L. Warnock, J. L. Curtis, M. J. Sniezek, S. M. Arraj-Peffer, H. B. Kaltreider, and J. E. Shellito. 1991. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am. J. Respir. Cell Mol. Biol. 5:186-197. [DOI] [PubMed] [Google Scholar]
- 10.Beck, J. M., M. L. Warnock, H. B. Kaltreider, and J. E. Shellito. 1993. Host defenses against Pneumocystis carinii in mice selectively depleted of CD4+ lymphocytes. Chest 103:116S-118S. [DOI] [PubMed] [Google Scholar]
- 11.Belaaouaj, A., R. McCarthy, M. Baumann, Z. Gao, T. J. Ley, S. N. Abraham, and S. D. Shapiro. 1998. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat. Med. 4:615-618. [DOI] [PubMed] [Google Scholar]
- 12.Bergeron, Y., N. Ouellet, A. M. Deslauriers, M. Simard, M. Olivier, and M. G. Bergeron. 1998. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect. Immun. 66:912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12:64-76. [DOI] [PubMed] [Google Scholar]
- 14.Borregaard, N., and J. B. Cowland. 1997. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89:3503-3521. [PubMed] [Google Scholar]
- 15.Cacalano, G., J. Lee, K. Kikly, A. M. Ryan, S. Pitts-Meek, B. Hultgren, W. I. Wood, and M. W. Moore. 1994. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science 265:682-684. [DOI] [PubMed] [Google Scholar]
- 16.Chen, W., E. A. Havell, and A. G. Harmsen. 1992. Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis carinii infection. Infect. Immun. 60:1279-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, W., J. W. Mills, and A. G. Harmsen. 1992. Development and resolution of Pneumocystis carinii pneumonia in severe combined immunodeficient mice: a morphological study of host inflammatory responses. Int. J. Exp. Pathol. 73:709-720. [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman, J. W. 2001. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 1:1397-1406. [DOI] [PubMed] [Google Scholar]
- 19.Dallaire, F., N. Ouellet, Y. Bergeron, V. Turmel, M. C. Gauthier, M. Simard, and M. G. Bergeron. 2001. Microbiological and inflammatory factors associated with the development of pneumococcal pneumonia. J. Infect. Dis. 184:292-300. [DOI] [PubMed] [Google Scholar]
- 20.Dunsmore, S. E., J. Roes, F. J. Chua, A. W. Segal, S. E. Mutsaers, and G. J. Laurent. 2001. Evidence that neutrophil elastase-deficient mice are resistant to bleomycin-induced fibrosis. Chest 120:35S-36S. [DOI] [PubMed] [Google Scholar]
- 21.Evans, M. D., and W. A. Pryor. 1994. Cigarette smoking, emphysema, and damage to alpha 1-proteinase inhibitor. Am. J. Physiol. 266:L593-L611. [DOI] [PubMed] [Google Scholar]
- 22.Filice, G., G. Carnevale, P. Lanzarini, F. Castelli, P. Olliaro, P. Orsolini, G. Carosi, and E. G. Rondanelli. 1985. Life cycle of P. carinii in the lung of immuno-compromised hosts: an ultrastructural study. Microbiologica 8:319-328. [PubMed] [Google Scholar]
- 23.Furuta, T., T. Nagata, T. Kikuchi, and H. Kikutani. 2001. Fatal spontaneous pneumocystosis in CD40 knockout mice. J. Eukaryot. Microbiol. Suppl.:129S-130S. [DOI] [PubMed] [Google Scholar]
- 24.Gao, X. P., T. J. Standiford, A. Rahman, M. Newstead, S. M. Holland, M. C. Dinauer, Q. H. Liu, and A. B. Malik. 2002. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J. Immunol. 168:3974-3982. [DOI] [PubMed] [Google Scholar]
- 25.Garvy, B. A., and A. G. Harmsen. 1996. The role of T cells in infection-driven interstitial pneumonia after bone marrow transplantation in mice. Transplantation 62:517-525. [DOI] [PubMed] [Google Scholar]
- 26.Ghio, A. J., H. B. Suliman, J. D. Carter, A. M. Abushamaa, and R. J. Folz. 2002. Overexpression of extracellular superoxide dismutase decreases lung injury after exposure to oil fly ash. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L211-L218. [DOI] [PubMed] [Google Scholar]
- 27.Hahn, P. Y., S. E. Evans, T. J. Kottom, J. E. Standing, R. E. Pagano, and A. H. Limper. 2003. Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J. Biol. Chem. 278:2043-2050. [DOI] [PubMed] [Google Scholar]
- 28.Harmsen, A. G. 1988. Role of alveolar macrophages in lipopolysaccharide-induced neutrophil accumulation. Infect. Immun. 56:1858-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harmsen, A. G., and M. Stankiewicz. 1990. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J. Exp. Med. 172:937-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasleton, P. S., and T. E. Roberts. 1999. Adult respiratory distress syndrome—an update. Histopathology 34:285-294. [DOI] [PubMed] [Google Scholar]
- 31.Heitmann, S., T. Tschernig, M. Larbig, I. Steinmetz, H. J. Hedrich, and R. Pabst. 1999. Immunohistological characterization of leukocytes in the lungs of healthy mice and after bacterial intratracheal infection. Lab. Anim. 33:288-294. [DOI] [PubMed] [Google Scholar]
- 32.Holmes, M. C., P. Zhang, S. Nelson, W. R. Summer, and G. J. Bagby. 2002. Neutrophil modulation of the pulmonary chemokine response to lipopolysaccharide. Shock 18:555-560. [DOI] [PubMed] [Google Scholar]
- 33.Ieki, R., T. Furuta, S. Asano, S. Mori, S. Kudoh, H. Kimura, and F. Takaku. 1989. Effect of recombinant human granulocyte colony-stimulating factor on Pneumocystis carinii infection in nude mice. Jpn. J. Exp. Med. 59:51-58. [PubMed] [Google Scholar]
- 34.Kawabata, K., T. Hagio, and S. Matsuoka. 2002. The role of neutrophil elastase in acute lung injury. Eur. J. Pharmacol. 451:1-10. [DOI] [PubMed] [Google Scholar]
- 35.Kubo, H., D. Morgenstern, W. M. Quinian, P. A. Ward, M. C. Dinauer, and C. M. Doerschuk. 1996. Preservation of complement-induced lung injury in mice with deficiency of NADPH oxidase. J. Clin. Investig. 97:2680-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latge, J. P. 2001. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 9:382-389. [DOI] [PubMed] [Google Scholar]
- 37.Lee, J., G. Cacalano, T. Camerato, K. Toy, M. W. Moore, and W. I. Wood. 1995. Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 155:2158-2164. [PubMed] [Google Scholar]
- 38.Limper, A. H. 1998. Alveolar macrophage and glycoprotein responses to Pneumocystis carinii. Semin. Respir. Infect. 13:339-347. [PubMed] [Google Scholar]
- 39.Limper, A. H., K. P. Offord, T. F. Smith, and W. J. Martin II. 1989. Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am. Rev. Respir. Dis. 140:1204-1209. [DOI] [PubMed] [Google Scholar]
- 40.MacIvor, D. M., S. D. Shapiro, C. T. Pham, A. Belaaouaj, S. N. Abraham, and T. J. Ley. 1999. Normal neutrophil function in cathepsin G-deficient mice. Blood 94:4282-4293. [PubMed] [Google Scholar]
- 41.MacNee, W. 2001. Oxidative stress and lung inflammation in airways disease. Eur. J. Pharmacol. 429:195-207. [DOI] [PubMed] [Google Scholar]
- 42.Mason, G. R., C. H. Hashimoto, P. S. Dickman, L. F. Foutty, and C. J. Cobb. 1989. Prognostic implications of bronchoalveolar lavage neutrophilia in patients with Pneumocystis carinii pneumonia and AIDS. Am. Rev. Respir. Dis. 139:1336-1342. [DOI] [PubMed] [Google Scholar]
- 43.Miller, R. 1996. HIV-associated respiratory diseases. Lancet 348:307-312. [DOI] [PubMed] [Google Scholar]
- 44.Oda, T., T. Akaike, T. Hamamoto, F. Suzuki, T. Hirano, and H. Maeda. 1989. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science 244:974-976. [DOI] [PubMed] [Google Scholar]
- 45.Palmgren, M. S., R. D. deShazo, R. M. Carter, M. L. Zimny, and S. V. Shah. 1992. Mechanisms of neutrophil damage to human alveolar extracellular matrix: the role of serine and metalloproteases. J. Allergy Clin. Immunol. 89:905-915. [DOI] [PubMed] [Google Scholar]
- 46.Peterson, M. W., P. Stone, and D. M. Shasby. 1987. Cationic neutrophil proteins increase transendothelial albumin movement. J. Appl. Physiol. 62:1521-1530. [DOI] [PubMed] [Google Scholar]
- 47.Pollock, J. D., D. A. Williams, M. A. Gifford, L. L. Li, X. Du, J. Fisherman, S. H. Orkin, C. M. Doerschuk, and M. C. Dinauer. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9:202-209. [DOI] [PubMed] [Google Scholar]
- 48.Pottratz, S. T. 1998. Pneumocystis carinii interactions with respiratory epithelium. Semin. Respir. Infect. 13:323-329. [PubMed] [Google Scholar]
- 49.Rahman, I. 2003. Oxidative stress, chromatin remodeling and gene transcription in inflammation and chronic lung diseases. J. Biochem. Mol. Biol. 36:95-109. [DOI] [PubMed] [Google Scholar]
- 50.Reeves, E. P., H. Lu, H. L. Jacobs, C. G. Messina, S. Bolsover, G. Gabella, E. O. Potma, A. Warley, J. Roes, and A. W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291-297. [DOI] [PubMed] [Google Scholar]
- 51.Romani, L., A. Mencacci, E. Cenci, G. Del Sero, F. Bistoni, and P. Puccetti. 1997. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J. Immunol. 158:2356-2362. [PubMed] [Google Scholar]
- 52.Roos, D., R. van Bruggen, and C. Meischl. 2003. Oxidative killing of microbes by neutrophils. Microbes Infect. 5:1307-1315. [DOI] [PubMed] [Google Scholar]
- 53.Roths, J. B., J. D. Marshall, R. D. Allen, G. A. Carlson, and C. L. Sidman. 1990. Spontaneous Pneumocystis carinii pneumonia in immunodeficient mutant scid mice. Natural history and pathobiology. Am. J. Pathol. 136:1173-1186. [PMC free article] [PubMed] [Google Scholar]
- 54.Schneider, R. F., and M. J. Rosen. 1996. Respiratory infections in patients with HIV infection. Curr. Opin. Pulm. Med. 2:246-252. [DOI] [PubMed] [Google Scholar]
- 55.Shapiro, S. D. 2002. Neutrophil elastase: path clearer, pathogen killer, or just pathologic? Am. J. Respir. Cell Mol. Biol. 26:266-268. [DOI] [PubMed] [Google Scholar]
- 56.Shapiro, S. D., N. M. Goldstein, A. M. Houghton, D. K. Kobayashi, D. Kelley, and A. Belaaouaj. 2003. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am. J. Pathol. 163:2329-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shiloh, M. U., J. D. MacMicking, S. Nicholson, J. E. Brause, S. Potter, M. Marino, F. Fang, M. Dinauer, and C. Nathan. 1999. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10:29-38. [DOI] [PubMed] [Google Scholar]
- 58.Sibille, Y., and F. X. Marchandise. 1993. Pulmonary immune cells in health and disease: polymorphonuclear neutrophils. Eur. Respir. J. 6:1529-1543. [PubMed] [Google Scholar]
- 59.Sitia, G., M. Isogawa, K. Kakimi, S. F. Wieland, F. V. Chisari, and L. G. Guidotti. 2002. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 99:13717-13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sittipunt, C., K. P. Steinberg, J. T. Ruzinski, C. Myles, S. Zhu, R. B. Goodman, L. D. Hudson, S. Matalon, and T. R. Martin. 2001. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 163:503-510. [DOI] [PubMed] [Google Scholar]
- 61.Smith, R. L., W. M. el-Sadr, and M. L. Lewis. 1988. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest 93:60-64. [DOI] [PubMed] [Google Scholar]
- 62.Su, T. H., and W. J. Martin II. 1994. Pathogenesis and host response in Pneumocystis carinii pneumonia. Annu. Rev. Med. 45:261-272. [DOI] [PubMed] [Google Scholar]
- 63.Suntres, Z. E., A. Omri, and P. N. Shek. 2002. Pseudomonas aeruginosa-induced lung injury: role of oxidative stress. Microb. Pathog. 32:27-34. [DOI] [PubMed] [Google Scholar]
- 64.Tateda, K., T. A. Moore, J. C. Deng, M. W. Newstead, X. Zeng, A. Matsukawa, M. S. Swanson, K. Yamaguchi, and T. J. Standiford. 2001. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 166:3355-3361. [DOI] [PubMed] [Google Scholar]
- 65.Tkalcevic, J., M. Novelli, M. Phylactides, J. P. Iredale, A. W. Segal, and J. Roes. 2000. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity 12:201-210. [DOI] [PubMed] [Google Scholar]
- 66.van der Vliet, A., J. P. Eiserich, M. K. Shigenaga, and C. E. Cross. 1999. Reactive nitrogen species and tyrosine nitration in the respiratory tract: epiphenomena or a pathobiologic mechanism of disease? Am. J. Respir. Crit. Care Med. 160:1-9. [DOI] [PubMed] [Google Scholar]
- 67.Van Wetering, S., S. P. Mannesse-Lazeroms, J. H. Dijkman, and P. S. Hiemstra. 1997. Effect of neutrophil serine proteinases and defensins on lung epithelial cells: modulation of cytotoxicity and IL-8 production. J. Leukoc. Biol. 62:217-226. [DOI] [PubMed] [Google Scholar]
- 68.Vassallo, R., J. E. Standing, and A. H. Limper. 2000. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J. Immunol. 164:3755-3763. [DOI] [PubMed] [Google Scholar]
- 69.Walzer, P. D. 1999. Immunological features of Pneumocystis carinii infection in humans. Clin. Diagn. Lab. Immunol. 6:149-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walzer, P. D., R. D. Powell, Jr., and K. Yoneda. 1979. Experimental Pneumocystis carinii pneumonia in different strains of cortisonized mice. Infect. Immun. 24:939-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wiley, J. A., and A. G. Harmsen. 1995. CD40 ligand is required for resolution of Pneumocystis carinii pneumonia in mice. J. Immunol. 155:3525-3529. [PubMed] [Google Scholar]
- 72.Wipke, B. T., and P. M. Allen. 2001. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol. 167:1601-1608. [DOI] [PubMed] [Google Scholar]
- 73.Witko-Sarsat, V., P. Rieu, B. Descamps-Latscha, P. Lesavre, and L. Halbwachs-Mecarelli. 2000. Neutrophils: molecules, functions and pathophysiological aspects. Lab. Investig. 80:617-653. [DOI] [PubMed] [Google Scholar]
- 74.Wright, T. W., F. Gigliotti, J. N. Finkelstein, J. T. McBride, C. L. An, and A. G. Harmsen. 1999. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J. Clin. Investig. 104:1307-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wright, T. W., G. S. Pryhuber, P. R. Chess, Z. Wang, R. H. Notter, and F. Gigliotti. 2004. TNF receptor signaling contributes to chemokine secretion, inflammation, and respiratory deficits during Pneumocystis pneumonia. J. Immunol. 172:2511-2521. [DOI] [PubMed] [Google Scholar]
- 76.Yoneda, K., and P. D. Walzer. 1980. Interaction of Pneumocystis carinii with host lungs: an ultrastructural study. Infect. Immun. 29:692-703. [DOI] [PMC free article] [PubMed] [Google Scholar]