Abstract
Background: Major regulation of thyroid gland function is mediated by thyrotropin (TSH) activating the TSH receptor (TSHR) and inducing upregulation of genes involved in thyroid hormone synthesis. Evidence suggests that the insulin-like growth factor 1 (IGF-1) receptor (IGF-1R) may play a role in regulating TSHR functional effects. This study examined the potential role of TSHR/IGF-1R crosstalk in primary cultures of human thyrocytes.
Results: TSH/IGF-1 co-treatment elicited additive effects on thyroglobulin (TG), thyroperoxidase (TPO), and deiodinase type 2 (DIO2) mRNA levels but synergistic effects on sodium–iodide symporter (NIS) mRNA. Similar cooperativity was seen on the level of TG protein secretion (additive) and NIS protein expression (synergistic). The IGF-1R tyrosine kinase inhibitor linsitinib inhibited TSH-stimulated upregulation of NIS but not TG, indicating that NIS regulation is in part IGF-1R dependent and occurs via receptor crosstalk. Cooperativity was not seen at the level of cAMP/protein kinase A (PKA) signaling, IGF-1R phosphorylation, or Akt activation. However, TSH and IGF-1 synergistically activated ERK1/2. Pharmacological inhibition of ERK1/2 by the MEK1/2 inhibitor U0126 and of Akt by MK-2206 virtually abolished NIS stimulation by TSH and the synergistic effect of IGF-1.
Conclusion: As linsitinib inhibited upregulation of NIS stimulated by TSH alone, it is concluded that crosstalk between TSHR and IGF-1R, without agonist activation of IGF-1R, plays a role in NIS regulation in human thyrocytes via a mechanism involving ERK1/2 and/or Akt. Fully understanding the nature of this crosstalk has clinical implications for the treatment of thyroid diseases, including thyroid cancer.
Keywords: : cAMP, sodium–iodide symporter, TSHR, IGF-1R
Introduction
The thyrotropin (TSH) receptor (TSHR) is a G-protein coupled receptor (GPCR) that serves as the main control mechanism for the development and function of the thyroid gland. TSHR is capable of coupling to all four G protein families (1), though the Gαs/cAMP and Gαq/calcium pathways appear to be the most physiologically relevant (2,3). There is also growing appreciation of the role of β-arrestins (βARRs), with βARR1 contributing to TSH-mediated signaling and βARR2 facilitating homologous desensitization of the TSHR (4–7). Regardless of the specific signaling mechanisms, TSH acting through TSHR stimulates the expression of various thyroid-specific genes, including thyroglobulin (TG), thyroperoxidase (TPO), sodium–iodide symporter (NIS), and type II iodothyronine deiodinase (DIO2). Together these and other factors lead to the production and secretion of thyroid hormones into the circulation.
The insulin-like growth factor 1 (IGF-1) receptor (IGF-1R) has recently gained renewed attention in the field of thyroid biology due to its apparent effect on thyroid function and possible contribution to thyroid-related diseases. IGF-1R is a transmembrane tyrosine kinase receptor most notably involved in growth and development. More recently, the IGF-1R has been ascribed several novel signaling properties, including the ability to co-opt aspects of canonically GPCR-based signaling: G proteins, βARRs, and G protein-coupled receptor kinases (GRKs) (8–10). Given the ability of the IGF-1R to utilize and participate in canonically GPCR-mediated processes such as those employed by the TSHR, it is not surprising that the relationship between these two types of receptors is of great interest. In fact, cooperativity between GPCRs and receptor tyrosine kinases (RTKs) is a well-established phenomenon (8,11), leading to cell proliferation or differentiation depending on context (12–14).
The phenomenon has recently been described of TSHR/IGF-1R crosstalk in fibroblasts/pre-adipocytes derived from the retro-orbital space of patients with Graves' ophthalmopathy (GO) undergoing decompression surgery (GOFs) by demonstrating that TSH and IGF-1 synergistically increase hyaluronan (HA) secretion in this disease model, and that 30% of the TSH response is via a IGF-1R-dependent pathway (15). It has also been shown that inhibition of the IGF-1R partially inhibits the stimulation of HA by TSHR-targeting antibodies derived from patients with GO (16). The main distinguishing feature of this phenomenon is that modulation of the TSHR response by IGF-1R is not dependent on IGF-1R activation by an IGF-1R-binding agonist.
Several lines of evidence suggest cooperativity and even possibly receptor crosstalk may also occur and have functional significance in the thyroid. TSHR and IGF-1R both colocalize in and coimmunoprecipitate from primary human thyrocytes and GOFs (17), suggesting a close spatial relationship and possibly a functional complex. Combination treatment of various thyroid-derived cell lines (FRTL-5, WRT, and PC Cl3), as well as primary cultures of dog thyrocytes, with TSH and IGF-1 has been reported to promote DNA synthesis synergistically versus either agonist alone (reviewed in Kimura et al.) (18). However, whether TSH stimulates proliferation of human thyroid cells remains controversial (17–19). Lung fibroblasts derived from Tshr knockout mice exhibited reduced Igf-1r surface expression, as well as altered subcellular localization and signaling (19). Additionally, mice overexpressing both Igf-1 and Igf-1r show a reduced requirement for TSH to maintain normal thyroid function (20).
The goals of the current study were to investigate TSHR/IGF-1R cooperativity and potential crosstalk in the context of human thyrocytes and to understand the mechanism by which IGF-1R regulates the effects of TSHR signaling.
Materials and Methods
Materials
TSH from bovine pituitary was purchased from Sigma-Aldrich (St. Louis, MO). Recombinant human IGF-1 was purchased from R&D Systems (Minneapolis, MN). IGF-1R tyrosine kinase inhibitor linsitinib (LINS) was purchased from Selleckchem (Houston, TX). IGF-1R tyrosine kinase inhibitor PQ-401 was purchased from Sigma-Aldrich. The antibody to human NIS was kindly provided by Dr. Nancy Carrasco (Department of Cellular and Molecular Physiology, Yale School of Medicine, New Haven, CT). Antibodies to phospho-IGF-1Rβ (Y1135), phospho-p44/42 MAPK (phospho-ERK1/2, T202/Y204), and phospho-Akt (S473) were from Cell Signaling Technology (Danvers, MA). The antibody to β-actin was from Sigma-Aldrich. The antibody to GAPDH was from Abcam (Cambridge, MA). RDye 680 or 800 secondary antibodies were from LiCOR (Lincoln, NE). The MEK1/2 inhibitor U0126 and the Akt inhibitor MK-2206 were both purchased from Selleckchem.
Isolation and culture of primary human thyrocytes
Primary cultures of human thyrocytes were established as described previously (21). Thyroid tissue samples were collected from normal thyroid tissue from patients undergoing total thyroidectomy for thyroid cancer at the National Institutes of Health Clinical Center. Patients provided informed consent on an Institutional Review Board–approved protocol, and materials were received anonymously with approval of research activity through the Office of Human Subjects Research, National Institutes of Health.
Specimens were maintained in Hanks' balanced salt solution (HBSS) on ice, and isolation of cells was initiated within 4 h after surgery. All manipulations were performed under sterile conditions. Tissue samples were minced into small pieces with fine surgical forceps and scissors in a 10 cm dish with a small volume of HBSS. Tissue pieces were transferred to a 15 mL tube (Falcon) and washed at least three times with HBSS. Afterward, tissue pieces were incubated with HBSS containing 3 mg/mL Collagenase Type IV (Gibco™/Thermo Fisher Scientific, Waltham, MA). Enzymatic digestion proceeded for 30 min or longer with constant shaking at 37°C until a suspension of isolated cells was obtained. After centrifugation for 5 min at 150 g the supernatant was removed, and cells were resuspended in 10 mL of Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS). Cells were plated in 10 cm tissue culture dishes and incubated at 37°C in a humidified 5% CO2 incubator. After 24 h, the supernatant containing non-adherent cells was removed. The primary cultures of thyroid cells formed a confluent monolayer within 5–7 days.
Cells were propagated/maintained in DMEM supplemented with 10% FBS, 100 IU/mL of penicillin, and 10 μg/mL of streptomycin (Life Technologies Corp., Carlsbad, CA) at 37°C in a humidified 5% CO2 incubator. Typical doubling time following the first passage was approximately 72–96 h. If at any time proliferation slowed significantly (doubling time >120 h), the culture was discarded. Once established, cultures were also verified for TG, TPO, NIS, DIO2, and TSHR expression via quantitative reverse transcription polymerase chain reaction (RT-PCR). Baseline (unstimulated) values for the genes studied are listed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/thy). Cultures were discarded if (i) initial expression of these genes fell outside the expected range, (ii) at any time the TSHR mRNA level fell significantly (10-fold reduction from the original level), or (iii) at any time cultures failed to respond to stimulation with TSH (either by cAMP generation or gene upregulation). Cultures were generally viable for at least nine passages (approximately six weeks following tissue isolation). Experiments were performed with early passage cells when possible, with the vast majority of experiments utilizing third- to sixth-passage cells. Each study was conducted using multiple donors each in independent experiments, with n indicating the number of unique donors utilized.
Quantitative RT-PCR
Thyrocytes (1 × 105 cells/well) were seeded into 12-well plates in DMEM containing 10% FBS. The media was changed 24 h prior to the experiment to 0.1% bovine serum albumin (BSA)-containing DMEM. Cells were stimulated with TSH (0–300 mIU/mL) alone or in combination with 100 ng/mL of IGF-1 for 48 h. Total RNA was purified using RNeasy Micro kits (Qiagen, Hilden, Germany). First-strand cDNA was prepared using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Quantitative RT-PCR was performed in 25 μL reactions using the prepared cDNA and iTaq™ Universal Probes Supermix (Bio-Rad Laboratories, Hercules, CA). Primers and probes were Assay-on-Demand (Applied Biosystems). Quantitative RT-PCR results were normalized to GAPDH to correct for differences in RNA input. Results were calculated using the 2–ΔΔCT method, then normalized to each individual donor's maximal TSH response, which varied by gene and donor.
Secreted TG assay
Conditioned media from cells designated for PCR or Western blot were collected and assayed using a human thyroglobulin enzyme-linked immunosorbent assay (RAB0458; Sigma-Aldrich) as per the manufacturer's instructions. The standard curve was adjusted to cover the range 0.3–20 ng/well to cover the range of conditions better observed in the cells.
Immunoblotting
Thyrocytes (1 × 105 cells/well) were seeded into 12-well plates in DMEM containing 10% FBS. The media was changed 24 h prior to the experiment to 0.1% BSA-containing DMEM. Cells were stimulated with TSH (0–300 mIU/mL) alone or in combination with 100 ng/mL of IGF-1 for 7 days. Cells were washed once with cold phosphate-buffered saline and lysed by addition of 1% SDS sample buffer. Lysates were boiled for 10 min, and 40 μL of cell lysate was electrophoresed on a standard 10% SDS-polyacrylamide gel (Bio-Rad Laboratories). After electrophoresis, proteins were transferred to nitrocellulose membranes (Bio-Rad Laboratories) and subsequently probed with the indicated primary antibodies and appropriate secondary antibodies conjugated with infrared fluorophores for detection and quantification on the Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE).
Measurement of IGF-1R phosphorylation
Thyrocytes (1 × 105 cells/well) were seeded into 12-well plates in DMEM containing 10% FBS. The media was changed 24 h prior to the experiment to 0.1% BSA-containing DMEM. Cells were pretreated with DMSO (0.1%) or LINS (10 μM) for 1 h, then incubated for 10 min with TSH (100 mIU/mL), IGF-1 (100 ng/mL), or a combination. Lysates were prepared using the Bio-Plex Pro cell signaling kit (catalog number 171-304006M; Bio-Rad Laboratories) according to the manufacturer's directions. Phosphorylated IGF-1R levels were measured with a Bio-Plex MAGPIX multiplex reader (Bio-Rad Laboratories) using the Phospho-IGF-1R (Tyr1131) set (catalog number 171-V50009M; Bio-Rad Laboratories) according to the manufacturer's directions. Data were normalized by concurrent measurement of GAPDH (catalog number 171-V60019M).
Determination of intracellular cAMP accumulation
Thyrocytes (0.8 × 105 cells/well) were seeded into 24-well plates in DMEM containing 10% FBS. The media was changed 24 h prior to the experiment to 0.1% BSA-containing DMEM. Cells were equilibrated for 30 min in HBSS/10 mM HEPES, pH 7.4, prior to stimulation. To determine the immediate effects of TSH stimulation, cAMP production was measured in cells incubated for 2 h at 37°C in a humidified incubator in HBSS/HEPES containing 1 mM of 3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich) in the presence or absence of TSH alone (0–300 mIU/mL) or in combination with IGF-1 (100 ng/mL) or LINS (1 nM to 10 μM) as described previously (21). Incubations were stopped, and the cells were lysed by adding 125 μL of lysis buffer from the cAMP-Screen Direct™ System (Applied Biosystems). The cAMP content of the cell lysate was determined using the method described in the manufacturer's protocol. The chemiluminescence signal was measured in a VICTOR3™ V 1420 Multilabel Counter (Perkin Elmer, Waltham, MA).
Statistical analysis
Statistical analysis was performed by GraphPad Prism v6.04 for Windows (GraphPad Software, La Jolla, CA). Data are presented as mean ± standard error values from n experiments, in which each experiment was performed using a different culture derived from a unique donor. Statistically significant differences among groups were assessed either by analysis of variance with Fisher's post hoc analysis, t-test, or by t-test for paired samples (as appropriate) with p < 0.05 sufficient to reject the null hypothesis.
Results
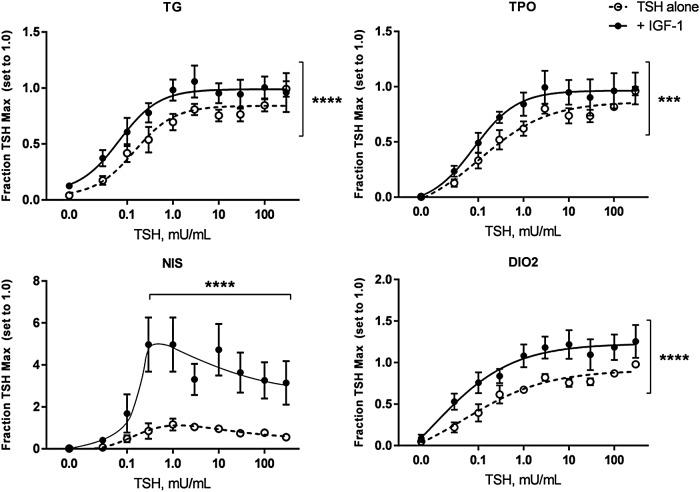
Treatment of cells with TSH led to upregulation of mRNA for all four thyroid-specific genes examined: TG, TPO, NIS, and DIO2 (Fig. 1). IGF-1 alone upregulated TG mRNA (p < 0.01) but had no significant effect on the other genes. Concomitant addition of 100 ng/mL of IGF-1 with TSH elicited additive effects on TG, TPO, and DIO2, but synergistically induced NIS mRNA. Of note and in contrast to the other genes, the TSH dose–response of NIS mRNA exhibited a biphasic response, with less upregulation at doses >1 mIU/mL. While TSH did elicit a dose-dependent increase in TSHR mRNA (up to 8.8 ± 1.8-fold at 300 mIU/mL), there was no additional effect with IGF-1 (data not shown). IGF-1R mRNA was not significantly affected by any treatment (data not shown).
FIG. 1.
Insulin-like growth factor 1 (IGF-1) has additive to synergistic effects on thyrotropin (TSH) stimulation of thyroid-specific genes, particularly the sodium–iodide symporter (NIS). Human thyrocytes were stimulated with the indicated doses of TSH alone or in combination with 100 ng/mL of IGF-1. RNA was isolated after 48 h, cDNA synthesized, and the indicated genes measured by quantitative polymerase chain reaction (PCR). Data are expressed as the mean ± standard error of the mean (SEM), n = 6. ***p < 0.001, ****p < 0.0001, TSH + IGF-1 versus TSH alone curve for thyroglobulin (TG), thyroperoxidase (TPO), and deiodinase type 2 (DIO2), at indicated doses for NIS.
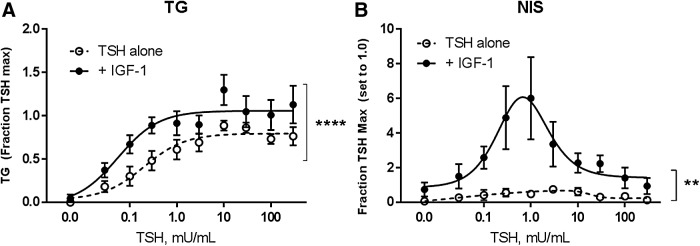
Focusing on TG and NIS as potentially being regulated by different mechanisms, the study then examined whether the cooperativity between TSHR and IGF-1R seen at the mRNA level could be replicated with measurements of protein. Thyrocytes were treated as before with various doses of TSH alone or in the presence of IGF-1. Conditioned media was collected from the cells and assayed for secreted TG protein. In this case, there was no effect of IGF-1 alone, but TSH/IGF-1 co-treatment appeared to induce TG secretion modestly above that observed with TSH alone (Fig. 2A). Western blotting for NIS protein showed a similar pattern to that seen at the mRNA level, with TSH alone inducing a low level of NIS, peaking at the 1 mIU/mL dose and decreasing at higher doses (Fig. 2B). Concomitant addition of IGF-1 synergistically upregulated NIS protein, particularly at TSH doses of 0.3–1.0 mIU/mL.
FIG. 2.
IGF-1 has synergistic effects on TSH stimulation of NIS protein expression. Human thyrocytes were stimulated with the indicated doses of TSH alone or in combination with 100 ng/mL of IGF-1. (A) Conditioned media was collected from wells after 48 h. TG secretion was measured using enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± SEM, n = 4. ****p < 0.0001 TSH + IGF-1 versus TSH alone curve. (B) Cells were lysed after 7 days and NIS protein expression measured by immunoblotting. Data are expressed as mean ± SEM, n = 3. **p < 0.01 paired t-test of +IGF-1 versus TSH alone.
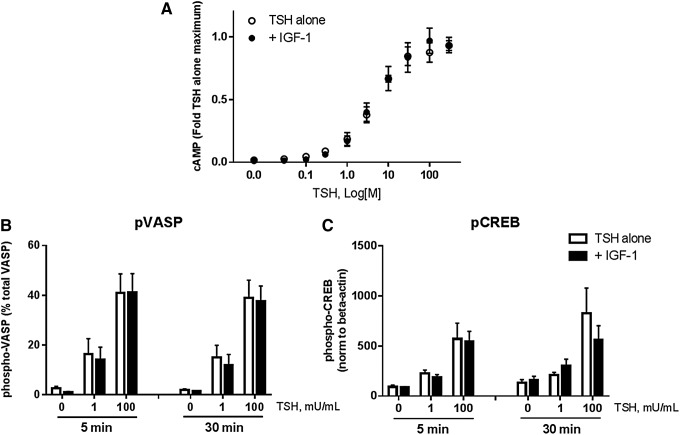
As a potential mechanism for the cooperativity, the study examined whether IGF-1 affected cAMP/protein kinase A (PKA) signaling, widely accepted as the dominant signaling pathway for TSHR. IGF-1 had no effect on cAMP accumulation at any dose of TSH (Fig. 3A). To confirm that the cAMP/PKA pathway was not enhanced by co-treatment with TSH and IGF-1, the study measured phosphorylation of vasodilator-stimulated phosphoprotein (VASP), which undergoes phosphorylation by PKA at Ser-157 to cause a mobility shift (46–50 kDa). This has been used previously as a marker for PKA activity (22–26). The phosphorylation of cAMP response element-binding protein (CREB), a PKA-responsive transcription factor, was also examined. While both proteins were robustly phosphorylated by TSH, there was no additional effect of IGF-1 treatment (Fig. 3B and C). Thus, global cAMP accumulation and PKA activity were also not able to account for the combined effects of TSH and IGF-1.
FIG. 3.
TSHR/IGF-1R cooperativity does not occur via enhancement of the cAMP/PKA pathway. (A) Cells were stimulated with the indicated doses of TSH alone or in combination with 100 ng/mL of IGF-1 in the presence of 1 mM of IBMX for 2 h and cAMP measured by ELISA. The EC50 for cAMP accumulation mediated by TSH in these cells was 79 nM (approx. 1.5 mIU/mL). Data are expressed as mean ± SEM, n = 4. (B) and (C) Cells were treated for 5 or 30 min with the indicated doses of TSH, then washed and lysed for immunoblotting of vasodilator-stimulated phosphoprotein (VASP, a PKA target) and cAMP response element-binding protein (CREB, a PKA-activated transcription factor). Data are expressed as mean ± SEM, n = 4.
As IGF-1 stimulation was clearly able to augment the TSH-induced upregulation of thyroid-specific genes, next the study investigated whether this phenomenon constituted crosstalk. Linsitinib (LINS), a small molecule inhibitor of the IGF-1R tyrosine kinase activity (IC50 = 35 nM in a cell-free assay), had no significant effect by itself on the mRNA levels of TG and NIS (Fig. 4A). When added concomitantly with TSH, LINS had no effect on the TSH-mediated induction of TG mRNA but dose-dependently inhibited the TSH effect on NIS mRNA expression (IC50 = 360 nM; Fig. 4A). Of note, TSH-mediated cAMP was not altered by any dose of LINS (data not shown). The effect of LINS on gene expression was replicated with another IGF-1R kinase inhibitor, PQ401 (IC50 = 12 μM in vitro), which did not affect TG levels but did significantly inhibit TSH-upregulation of NIS (by 48 ± 6%; Fig. 4B). PQ-401 showed significant toxicity at doses >1 μM, and thus a definitive IC50 for this inhibitor could not be determined.
FIG. 4.
IGF-1R tyrosine kinase inhibition by Linsitinib (LINS) or PQ-401 mitigates the ability of TSH to stimulate NIS gene expression. (A) Human thyrocytes were treated with the indicated dose of LINS or (B) PQ-401 alone or in combination with 100 mIU/mL of TSH. RNA was isolated after 48 h, cDNA synthesized, and the indicated genes measured by quantitative PCR. Data are expressed as mean ± SEM, n = 4. *p < 0.05, TSH + indicated LINS dose versus TSH alone; ****p < 0.0001, TSH + PQ-401 versus TSH alone. No significant effect of LINS or PQ-401 alone was observed on either gene.
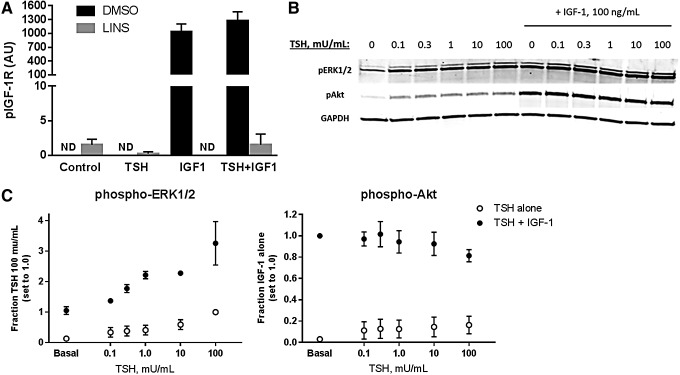
Having established that the TSHR/IGF-1R interaction regulating NIS constitutes crosstalk, the study examined whether TSH was capable of initiating or enhancing IGF-1R phosphorylation, the first step in the canonical signaling pathway of this receptor. This mechanism has been suggested by previous studies using FRTL-5 and transformed human thyrocytes (27,28). However, in primary human thyrocytes, TSH alone did not trigger IGF-1R phosphorylation, nor was it able to augment IGF-1-stimulated receptor phosphorylation (Fig. 5A). Thus, the observed synergism did not appear to occur at the initial canonical step in IGF-1R activation. As it has also been suggested that enhancement of IGF-1R signaling can be mediated at the level of downstream kinases (29), activation of ERK1/2 and Akt was examined for possible evidence of synergy. TSH induced phosphorylation of ERK1/2 in a dose-dependent manner, and IGF-1 alone also stimulated this phosphorylation (Fig. 5B and C). Combination TSH and IGF-1 treatment led to synergistic activation of ERK1/2. Akt was also phosphorylated by TSH, but this signal was small compared to that caused by IGF-1, and thus there was no additive effect of co-treatment.
FIG. 5.
TSH does not induce phosphorylation of the IGF-1R or augment IGF-1-induced activation, nor are synergistic effects seen on activation of ERK1/2 and Akt. (A) Cells were pretreated with 0.1% DMSO or 10 μM of LINS for 1 h, and then stimulated with 100 mIU/mL of TSH, 100 ng/mL of IGF-1, or a combination for 10 min. IGF-1R phosphorylation (Tyr1131) was measured using the Bio-Plex MAGPIX multiplex system. Phospho-IGF-1R signal was normalized to GAPDH measured simultaneously. ND, no signal detected. (B, C) Cells were treated with the indicated doses of TSH ± IGF-1 (100 ng/mL) for 10 min. Cells were then lysed and phosphorylation of kinases ERK1/2 and Akt determined by immunoblotting. Data are expressed as mean ± SEM, n = 3. IGF-1R phosphorylation with TSH + IGF-1 was not statistically different from that seen with IGF-1 alone.
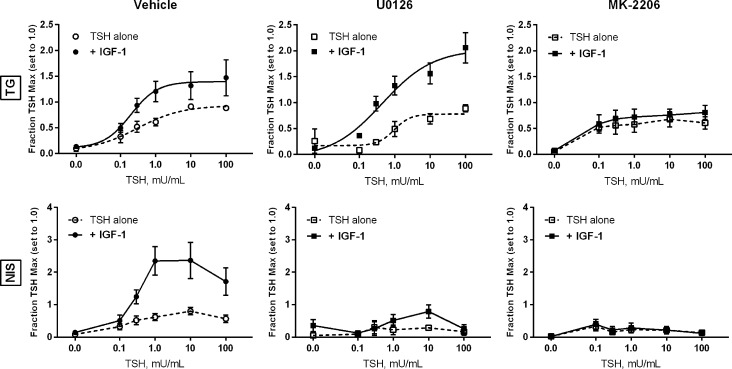
Using the pharmacological inhibitors U0126 (targeting MEK1/2, the MAPKKs upstream of ERK1/2) and MK-2206 (targeting Akt), the study investigated whether these kinases were involved in crosstalk between TSHR and IGF-1R. Both U0126 and MK-2206 were able to inhibit their respective kinases selectively (and not affect IGF-1R phosphorylation) in the cells (Supplementary Fig. S1). U0126 had no effect on the induction of TG mRNA by TSH alone or in combination with IGF-1 (Fig. 6). Akt inhibition by MK-2206 did not alter the TSH-mediated induction of TG, but it did abolish the additive effect of IGF-1. Pharmacological inhibition of either ERK1/2 (via inhibition of the upstream activators MEK1/2) or Akt greatly reduced the ability of TSH to induce NIS mRNA, and completely blocked any additional or synergistic effect of IGF-1.
FIG. 6.
Pharmacological inhibition of ERK1/2 or Akt blocks the synergistic induction of NIS by TSH + IGF-1. Human thyrocytes were pretreated with the MEK1/2 (ERK1/2) inhibitor U0126 (final concentration 1 μM) or the Akt inhibitor MK-2206 (final concentration 5 μM) for 30 min, then treated with the indicated dose of TSH alone or in combination with 100 ng/mL of IGF-1. RNA was isolated after 48 hours, cDNA synthesized, and the indicated genes measured by quantitative PCR. Data are expressed as mean ± SEM, n = 4.
Discussion
This study examined the cooperative effects of TSH and IGF-1 on the differentiated function of cultured primary human thyrocytes. Numerous studies have reported that IGF-1 has weak effects by itself but clearly potentiates the effects of other hormones, including but not limited to TSH (27,30–32). Indeed, the concept that TSHR and IGF-1R cooperate to promote thyrocyte function has been widely investigated and is consistent with in vitro and in vivo data. Numerous studies have established a priming effect of TSH/cAMP on IGF-1-mediated effects in FRTL-5 cells including [3H]-thymidine incorporation and iodine uptake (33). Transgenic mice overexpressing IGF-1 and its receptor were observed to have significantly higher thyroid gland weight compared with wild-type controls or mice with only the ligand or receptor overexpressed, due to expanded colloid volume attributed to increased TG synthesis (20). These findings support the hypothesis that the cooperative effects of TSHR and IGF-1R regulate thyrocyte function.
This study began by examining the ability of TSH and IGF-1 to regulate cooperatively genes involved in thyroid hormonogenesis in primary human thyrocytes, as had been suggested by previous studies using other thyroid cell models (14,27,34–37). Here, the positive regulatory effects of IGF-1 on TSH-mediated mRNA and protein induction are clearly shown, with the synergistic effects on NIS being particularly striking and suggesting a potentially unique regulatory mechanism (Figs. 1 and 2).
One proposed mechanism for this cooperativity is enhancement of cAMP/PKA-based signaling, the major pathway attributed to TSHR. In FRTL-5 cells, IGF-1 was reportedly shown to augment the stimulation of cAMP by TSH doses as low as 100 pM (approximately 0.005 mIU/mL) (27). Other studies indicated that IGF-1 did not alter TSH-induced cAMP accumulation using this same cell line (31,37,38). IGF-1 had no effect on cAMP accumulation in primary human thyrocytes at any dose of TSH, nor was there any augmentation of PKA activity as shown by VASP and CREB phosphorylation (Fig. 3). While it is possible and even likely that the primary induction of all four genes (TG, TPO, NIS, DIO2) by TSH may be dependent on cAMP, it is clear from the studies that the TSHR/IGF-1R crosstalk examined here in mature human thyrocytes does not occur via quantitative modulation of this second messenger or subsequent induction of global PKA activity. One caveat to this conclusion is the recent appreciation that cAMP accumulation within the cytoplasm is restricted rather than freely diffusible (39). Compartmentalization of cAMP and/or PKA may be altered with concomitant IGF-1R stimulation, resulting in the additive/synergistic effects seen, despite no change in global cAMP-derived readouts. Unfortunately, no studies to date have studied the roll of cAMP/PKA compartmentalization with regards to TSHR signaling, and thus any role of this mechanism has not been examined at this time.
As canonical IGF-1R signaling is initiated by receptor autophosphorylation, enhanced IGF-1R phosphorylation has often been investigated as a potential mechanism for cooperation between TSHR and IGF-1R. Treatment of N-thy-ori 3-1 cells (normal human differentiated thyrocytes immortalized by SV40 large-T-antigen) with TSH alone has been reported to cause IGF-1R phosphorylation (28). In FRTL-5 cells, TSH augmented IGF-1-mediated receptor autophosphorylation and the resultant kinase activity toward the endogenous IGF-1R substrate p175 (40). In contrast, another study reported that pretreatment of FRTL-5 cells with TSH for 24 h had no effect on IGF-1 induced autophosphorylation, nor did it affect more direct measurements of IGF-1R kinase activity (41). In human thyrocytes, it is clearly shown that TSH alone does not induce IGF-1R phosphorylation, nor does it significantly augment that induced by IGF-1 itself (Fig. 5A). While TSHR and IGF-1 unquestionably cooperate to enhance downstream gene expression, this does not appear to occur at the very proximal step of IGF-1R phosphorylation. Similar findings were observed in GOFs (15,16). However, the possibility cannot be excluded that IGF-1R signaling is amplified further downstream via an autophosphorylation-independent mechanism.
ERK1/2 and Akt activities have been proposed as more distal sites of TSHR/IGF-1R cooperation. Synergistic activation (phosphorylation) of ERK1/2 was seen in FRTL-5 cells pretreated with cAMP or TSH and then stimulated with IGF-1 (29). The MEK inhibitor PD98059 partially inhibited this synergistic effect on [3H]-thymidine incorporation, and inhibition of P13K (an upstream activator of Akt) by LY294002 virtually abolished these effects (29). In primary human thyrocytes, a synergistic effect of TSH and IGF-1 on ERK1/2 phosphorylation was also observed, but no effect of TSH above that seen with IGF-1 alone on Akt (Fig. 4B). However, pharmacological inhibition of either ERK1/2 (via inhibition of the upstream activators MEK1/2) or Akt greatly reduced the ability of TSH to induce NIS mRNA, and completely abolished any additional or synergistic effect of IGF-1 on this message. This suggests that ERK1/2 and/or Akt are important mediators of NIS regulation by TSH, in addition to showing that these factors are critical for the synergistic upregulation seen with IGF-1. Interestingly, Akt inhibition also seems to have abolished the additive effect of IGF-1 on TG induction by TSH, though it did not significantly alter the TSH effect itself. This suggests that the additive effects of TSH and IGF-1 on TG are from a combination of classical (cAMP for TSH and Akt for IGF-1) signaling paradigms. Together, these findings suggest that TSH utilizes a variety of signaling mechanisms to achieve its functional effects. Clearly, regulation of TG and NIS by TSH, alone or in combination with IGF-1, occurs through distinct and possibly independent mechanisms.
While ERK1/2 and Akt are implicated in the process of TSHR/IGF-1R cooperation, the precise location of this cooperativity or the mechanism through which it occurs has not been pinpointed. As TSH-mediated cAMP/PKA activity in primary human thyrocytes is not affected by IGF-1, several potential non-cAMP mechanisms could be responsible for the synergistic effects seen here on NIS. TSH may utilize another family of G proteins (Gαq/11, Gαi/o, Gα12/13) to facilitate receptor cooperativity. To this end, additive or synergistic effects may be detectable on signaling components such as calcium, downstream phosphokinases (aside from ERK1/2 and Akt), or transcription factors/co-factors other than CREB. Signaling through βARRs, of which the importance in TSHR signaling has only recently been identified and defined (4,5,7), could also play a role, especially in light of emerging evidence for βARRs in IGF-1R function (10,42). Additionally, there is emerging evidence that IGF-1R signaling may occur in the absence of receptor phosphorylation (42,43). This idea, coupled with the recent understanding that IGF-1R can co-opt various signaling components usually attributed to GPCRs (10), suggests that some alternative mechanism may link the two receptors. Finally, the key to cooperation may lie in the assembly of a functional signaling complex involving either direct (heterodimers/multimers) or indirect (via other scaffolding molecules) association of TSHR and IGF-1R, as suggested by the coimmunoprecipitation studies in thyrocytes and GOFs (7). These possibilities need to be addressed in future studies.
The clear discrepancies between rodent-derived cell lines (i.e., FRTL-5) and primary human thyrocytes should be highlighted, particularly with regard to cAMP generation and IGF-1R phosphorylation status. While many findings using FRTL-5 cells do and will invariably hold true for human cells, clearly this is not always the case. In fact, studies using these cell lines have sometimes contradicted each other, as discussed previously. The ability of cell type and context to modulate the functional consequences of a common signaling pathway is not novel with regard to TSHR or thyroid-like cells. Differences have already been demonstrated with regard to NIS expression in FRTL-5 cells compared to the non-thyroidal MCF-7 breast cancer cell line. In MCF-7 cells, all-trans retinoic acid (tRA) markedly upregulates NIS mRNA and iodine uptake (44), while tRA inhibits NIS expression in FRTL5 cells (45,46). Thus, caution is needed when extrapolating the results of cell models, even those derived from primary cells of other species, to human cells.
Finally, the difference should be emphasized between cooperation of the TSHR and IGF-1R, occurring in the presence of agonists acting at both receptors, and receptor crosstalk, when the effects of activation of TSHR by an exogenously added agonist such as TSH are, at least in part, mediated by IGF-1R signaling that is independent of an exogenous IGF-1R agonist. Cooperativity—that is, additivity or synergy—was seen for regulation of TG and NIS; only NIS appeared to be regulated by receptor crosstalk. It is believed that crosstalk between TSHR and IGF-1R occurs in human thyrocytes (and in human GOFs) (15,16), as has been shown for other GPCR activation of other receptor tyrosine kinases (for a review, see Delcourt et al.) (11).
In conclusion, this study has established that cooperativity between the TSHR and IGF-1R promotes the upregulation of thyroid-specific functions of human thyrocytes in primary culture. In the case of NIS, co-regulation by TSHR and IGF-1R occurs through receptor crosstalk. This interaction, which has previously been demonstrated in the extrathyroidal tissue of GOFs, appears to be independent of the cAMP pathway and IGF-1R activation, even though the interaction clearly involves activation of the downstream kinases ERK1/2 and Akt.
Supplementary Material
Acknowledgments
We thank Monica Skarulis and Brent Abel from the Diabetes, Endocrinology, and Obesity Branch for providing human thyroid tissue. This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (Z01 DK011006).
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Laugwitz KL, Allgeier A, Offermanns S, Spicher K, Van Sande J, Dumont JE, Schultz G. 1996. The human thyrotropin receptor: a heptahelical receptor capable of stimulating members of all four G protein families. Proc Natl Acad Sci U S A 93:116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laurent E, Mockel J, Van Sande J, Graff I, Dumont JE. 1987. Dual activation by thyrotropin of the phospholipase C and cyclic AMP cascades in human thyroid. Mol Cell Endocrinol 52:273–278 [DOI] [PubMed] [Google Scholar]
- 3.Raspé E, Laurent E, Andry G, Dumont JE. 1991. ATP, bradykinin, TRH and TSH activate the Ca(2+)-phosphatidylinositol cascade of human thyrocytes in primary culture. Mol Cell Endocrinol 81:175–183 [DOI] [PubMed] [Google Scholar]
- 4.Boutin A, Eliseeva E, Gershengorn MC, Neumann S. 2014. β-Arrestin-1 mediates thyrotropin-enhanced osteoblast differentiation. FASEB J 28:3446–3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutin A, Neumann S, Gershengorn MC. Multiple transduction pathways mediate thyrotropin receptor signaling in preosteoblast-like cells. Endocrinology 157:2173–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frenzel R, Voigt C, Paschke R. 2006. The human thyrotropin receptor is predominantly internalized by β-arrestin 2. Endocrinology 147:3114–3122 [DOI] [PubMed] [Google Scholar]
- 7.Neumann S, Geras-Raaka E, Marcus-Samuels B, Gershengorn MC. 2010. Persistent cAMP signaling by thyrotropin (TSH) receptors is not dependent on internalization. FASEB J 24:3992–3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyne NJ, Pyne S. 2011. Receptor tyrosine kinase–G-protein-coupled receptor signalling platforms: out of the shadow? Trends Pharmacol Sci 32:443–450 [DOI] [PubMed] [Google Scholar]
- 9.Biesen Tv, Luttrell LM, Hawes BE, Lefkowitz RJ. 1996. Mitogenic signaling via G protein-coupled receptors. Endocr Rev 17:698–714 [DOI] [PubMed] [Google Scholar]
- 10.Girnita L, Worrall C, Takahashi S-I, Seregard S, Girnita A. 2014. Something old, something new and something borrowed: emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cell Mol Life Sci 71:2403–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delcourt N, Bockaert J, Marin P. 2007. GPCR-jacking: from a new route in RTK signalling to a new concept in GPCR activation. Trends Pharmacol Sci 28:602–607 [DOI] [PubMed] [Google Scholar]
- 12.Rozengurt E, Sinnett-Smith J, Kisfalvi K. 2010. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res 16:2505–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tramontano D, Cushing GW, Moses AC, Ingbar SH. 1986. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves'-IgG. Endocrinology 119:940–942 [DOI] [PubMed] [Google Scholar]
- 14.Santisteban P, Kohn LD, Di Lauro R. 1987. Thyroglobulin gene expression is regulated by insulin and insulin-like growth factor I, as well as thyrotropin, in FRTL-5 thyroid cells. J Biol Chem 262:4048–4052 [PubMed] [Google Scholar]
- 15.Krieger CC, Neumann S, Place RF, Marcus-Samuels B, Gershengorn MC. 2015. Bidirectional TSH and IGF-1 receptor cross talk mediates stimulation of hyaluronan secretion by Graves' disease immunoglobins. J Clin Endocrinol Metab 100:1071–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieger CC, Place RF, Bevilacqua C, Marcus-Samuels B, Abel BS, Skarulis MC, Kahaly GJ, Neumann S, Gershengorn MC. 2016. TSH/IGF-1 receptor cross talk in Graves' ophthalmopathy pathogenesis. J Clin Endocrinol Metab 101:2340–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, Smith TJ. 2008. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol 181:4397–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura T, Keymeulen AV, Golstein J, Fusco A, Dumont JE, Roger PP. 2001. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev 22:631–656 [DOI] [PubMed] [Google Scholar]
- 19.Atkins SJ, Lentz SI, Fernando R, Smith TJ. 2015. Disrupted TSH receptor expression in female mouse lung fibroblasts alters subcellular IGF-1 receptor distribution. Endocrinology 156:4731–4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clément S, Refetoff S, Robaye B, Dumont JE, Schurmans S. 2001. Low TSH requirement and goiter in transgenic mice overexpressing IGF-I and IGF-I receptor in the thyroid gland. Endocrinology 142:5131–5139 [DOI] [PubMed] [Google Scholar]
- 21.Neumann S, Huang W, Titus S, Krause G, Kleinau G, Alberobello AT, Zheng W, Southall NT, Inglese J, Austin CP, Celi FS, Gavrilova O, Thomas CJ, Raaka BM, Gershengorn MC. 2009. Small-molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc Natl Acad Sci 106:12471–12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan SJ, Deshpande DA, Tiegs BC, Misior AM, Yan H, Hershfeld AV, Rich TC, Panettieri RA, An SS, Penn RB. 2014. β-Agonist-mediated relaxation of airway smooth muscle is protein kinase A-dependent. J Biol Chem 289:23065–23074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo M, Pascual RM, Wang S, Fontana MF, Valancius CA, Panettieri RA, Tilley SL, Penn RB. 2005. Cytokines regulate β-2-adrenergic receptor responsiveness in airway smooth muscle via multiple PKA- and EP2 receptor-dependent mechanisms. Biochemistry 44:13771–13782 [DOI] [PubMed] [Google Scholar]
- 24.Yan H, Deshpande DA, Misior AM, Miles MC, Saxena H, Riemer EC, Pascual RM, Panettieri RA, Penn RB. 2011. Anti-mitogenic effects of β-agonists and PGE2 on airway smooth muscle are PKA dependent. FASEB J 25:389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. 1994. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem 269:14509–14517 [PubMed] [Google Scholar]
- 26.Horstrup K, Jablonka B, Hönig-Liedl P, Just M, Kochsiek K, Walter U. 1994. Phosphorylation of focal adhesion vasodilator-stimulated phosphoprotein at Ser157 in intact human platelets correlates with fibrinogen receptor inhibition. Eur J Biochem 225:21–27 [DOI] [PubMed] [Google Scholar]
- 27.Koide T, Ono Y, Ito Y, Akahori M, Nedachi T, Hakuno F, Takenaka A, Takahashi S-I, Noguchi T. 1998. Insulin-like growth factor-1 potentiates protein synthesis induced by thyrotropin in FRTL-5 cells: comparison of induction of protein synthesis and DNA synthesis. Endocr J 45:151–163 [DOI] [PubMed] [Google Scholar]
- 28.Ock S, Ahn J, Lee SH, Kang H, Offermanns S, Ahn HY, Jo YS, Shong M, Cho BY, Jo D, Abel ED, Lee TJ, Park WJ, Lee I-K, Kim J. 2013. IGF-1 receptor deficiency in thyrocytes impairs thyroid hormone secretion and completely inhibits TSH-stimulated goiter. FASEB J 27:4899–4908 [DOI] [PubMed] [Google Scholar]
- 29.Ariga M, Nedachi T, Akahori M, Sakamoto H, Ito Y, Hakuno F, Takahashi S. 2000. Signalling pathways of insulin-like growth factor-I that are augmented by cAMP in FRTL-5 cells. Biochem J 348:409–416 [PMC free article] [PubMed] [Google Scholar]
- 30.Kurtz A, Härtl W, Jelkmann W, Zapf J, Bauer C. 1985. Activity in fetal bovine serum that stimulates erythroid colony formation in fetal mouse livers is insulinlike growth factor I. J Clin Invest 76:1643–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tramontano D, Moses AC, Veneziani BM, Ingbar SH. 1988. Adenosine 3′,5′-monophosphate mediates both the mitogenic effect of thyrotropin and its ability to amplify the response to insulin-like growth factor I in FRTL5 cells. Endocrinology 122:127–132 [DOI] [PubMed] [Google Scholar]
- 32.Cara JF, Rosenfield RL. 1988. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology 123:733–739 [DOI] [PubMed] [Google Scholar]
- 33.Lombardi A, Veneziani BM, Tramontano D, Ingbar SH. 1988. Independent and interactive effects of tetradecanoyl phorbol acetate on growth and differentiated functions of FRTL5 cells. Endocrinology 123:1544–1552 [DOI] [PubMed] [Google Scholar]
- 34.Pohl V, Roger PP, Christophe D, Pattyn G, Vassart G, Dumont JE. 1990. Differentiation expression during proliferative activity induced through different pathways: in situ hybridization study of thyroglobulin gene expression in thyroid epithelial cells. J Cell Biol 111:663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fayet G, Hovsépian S. 2004. Normal human thyroid cells from the ARAMIS line follow the general concept of growth or differentiation: a study with thyroglobulin as a marker. Thyroid 14:571–579 [DOI] [PubMed] [Google Scholar]
- 36.Zarrilli R, Formisano S, Jeso BD. 1990. Hormonal regulation of thyroid peroxidase in normal and transformed rat thyroid cells. Mol Endocrinol 4:39–45 [DOI] [PubMed] [Google Scholar]
- 37.García B, Santisteban P. 2002. PI3K is involved in the IGF-I inhibition of TSH-induced sodium/iodide symporter gene expression. Mol Endocrinol 16:342–352 [DOI] [PubMed] [Google Scholar]
- 38.Saji M, Kohn LD. 1991. Insulin and insulin-like growth factor-I inhibit thyrotropin-increased iodide transport in serum-depleted FRTL-5 rat thyroid cells: modulation of adenosine 3′,5′-monophosphate signal action. Endocrinology 128:1136–1143 [DOI] [PubMed] [Google Scholar]
- 39.Zaccolo M, Pozzan T. 2002. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295:1711–1715 [DOI] [PubMed] [Google Scholar]
- 40.Condorelli G, Formisano P, Miele C, Beguinot F. 1992. Thyrotropin regulates autophosphorylation and kinase activity in both the insulin and the insulin-like growth factor-I receptors in FRTL5 cells. Endocrinology 130:1615–1625 [DOI] [PubMed] [Google Scholar]
- 41.Takahashi S, Conti M, Prokop C, Van Wyk JJ, Earp HS. 1991. Thyrotropin and insulin-like growth factor I regulation of tyrosine phosphorylation in FRTL-5 cells. Interaction between cAMP-dependent and growth factor-dependent signal transduction. J Biol Chem 266:7834–7841 [PubMed] [Google Scholar]
- 42.Zheng H, Worrall C, Shen H, Issad T, Seregard S, Girnita A, Girnita L. 2012. Selective recruitment of G protein-coupled receptor kinases (GRKs) controls signaling of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci U S A 109:7055–7060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girnita L, Shenoy SK, Sehat B, Vasilcanu R, Vasilcanu D, Girnita A, Lefkowitz RJ, Larsson O. 2007. β-Arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. J Biol Chem 282:11329–11338 [DOI] [PubMed] [Google Scholar]
- 44.Kogai T, Ohashi E, Jacobs MS, Sajid-Crockett S, Fisher ML, Kanamoto Y, Brent GA. 2008. Retinoic acid stimulation of the sodium/iodide symporter in MCF-7 breast cancer cells is meditated by the insulin growth factor-I/phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase signaling pathways. J Clin Endocrinol Metab 93:1884–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kogai T, Schultz JJ, Johnson LS, Huang M, Brent GA. 2000. Retinoic acid induces sodium/iodide symporter gene expression and radioiodide uptake in the MCF-7 breast cancer cell line. Proc Natl Acad Sci U S A 97:8519–8524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmutzler C, Winzer R, Meissner-Weigl J, Köhrle J. 1997. Retinoic acid increases sodium/iodide symporter mRNA levels in human thyroid cancer cell lines and suppresses expression of functional symporter in nontransformed FRTL-5 rat thyroid cells. Biochem Biophys Res Commun 240:832–838 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.