Abstract
A patient is reported with resistance to thyroid hormone beta caused by a novel THRB gene mutation and coexisting pituitary microadenoma. A 41-year-old Thai woman presented with elevated serum thyroid hormone levels and non-suppressed thyrotropin (TSH). Magnetic resonance imaging showed a 4 mm × 2 mm pituitary adenoma. Five of her relatives had similar thyroid tests abnormalities, but a sister had Graves' disease. Thyroperoxidase and thyroglobulin antibodies were positive in all affected family members, except for the proband's 4.5-year-old niece. Lack of thyrotoxic symptoms and TSH suppression by triiodothyronine indicated incidentaloma rather than a TSH-secreting pituitary adenoma. Genetic analysis revealed a THRB gene mutation (c.1037G>T), resulting in p.G251V.
Keywords: : autoimmune diseases, inappropriate TSH secretion syndrome, mutation, pituitary tumor, thyroid hormone receptor, thyroid hormone resistance syndrome
Introduction
Non-suppressed serum thyrotropin (TSH) in the presence of elevated serum thyroid hormone levels is due to either TSH-secreting pituitary adenomas (TSHoma) or resistance to thyroid hormone beta (RTHβ). Establishing the correct diagnosis is important. A patient is reported with RTHβ caused by a novel THRB gene mutation with an incidental pituitary microadenoma. Moreover, the family showed an association of RTHβ with autoimmune thyroid disease (AITD).
Patient
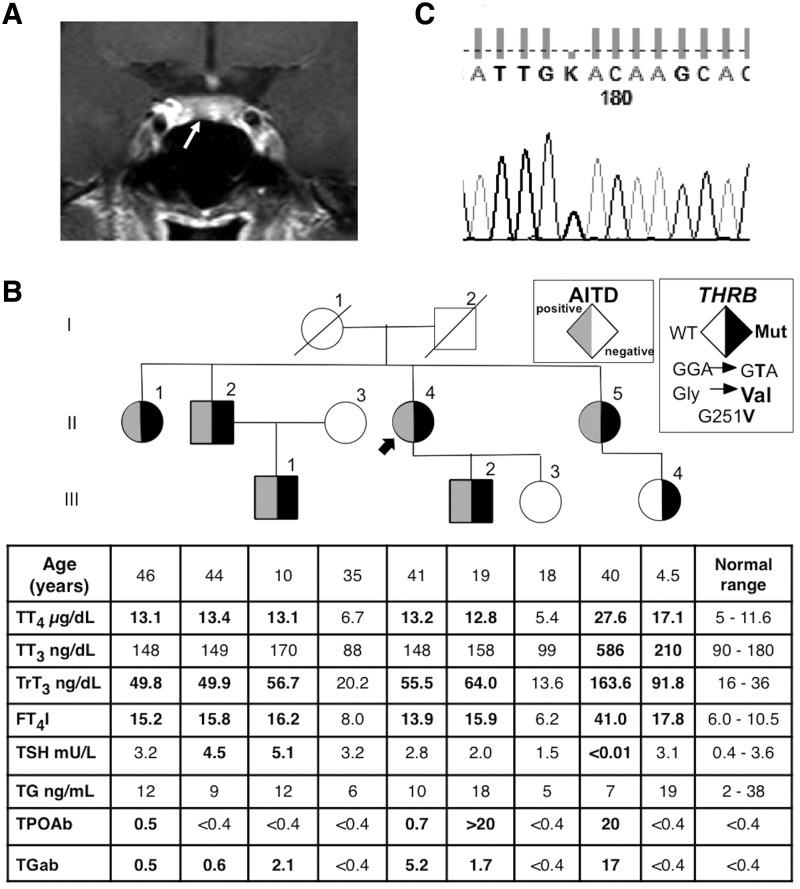
A 41-year-old Thai woman sought medical attention because her younger sister had a relapse of Graves' disease. She has no goiter or tachycardia. Thyroid function tests (TFTs) showed a high total thyroxine (TT4; 13.2 μg/dL; normal range 5–11 μg/dL), free T4 index (fT4I; 13.9; normal range 6–11.5), and reverse triiodothyronine (rT3; 55.5 ng/dL; normal range 16–36), a normal total T3 (TT3; 148 ng/dL; normal range 90–180 ng/dL), and a non-suppressed TSH (2.8 mIU/L; normal range 0.4–3.6 mIU/L). TFTs were repeated using different platforms (Elecsys, Roche; Architect, Abbott) and showed similar results.
These TFTs were compatible with inappropriate TSH secretion. Serum ferritin and sex hormone-binding globulin were 13.7 ng/mL (normal range 10–130 ng/mL) and 89.8 nmol/L (normal range 18–114 nmol/L), respectively. Magnetic resonance imaging (MRI) of the pituitary gland showed a 4 mm × 2 mm nodule in the anterior pituitary gland (Fig. 1A). Anterior pituitary hormone assessment was within normal limits. Her older sister (II-1), her brother (II-2), her fraternal nephew (III-1), her son (III-2), and her younger sister's daughter (III-4) also had similar TFTs abnormalities without clinical signs and symptoms (Fig. 1B). Her younger sister (II-5) had much higher serum TT4, TT3, fT4I, and rT3 levels with suppressed TSH levels, which, together with positive thyroid autoantibodies, were compatible with Graves' disease. There was no history of consanguinity. Thyroperoxidase (TPO) and thyroglobulin (Tg) antibodies were positive in affected family members who had abnormal TFTs, except for her 4.5-year-old niece (Fig. 1B). Her older sister (II-1) lost three children. Two died one day after birth, and one child with developmental defect died at two years of age. There was no history of miscarriages or early deaths in other members of the family.
FIG. 1.
(A) A coronal view of a dynamic gadolinium-enhanced fat-suppressed T1-weighted section of magnetic resonance imaging of the pituitary gland demonstrated a 4 mm × 2 mm nodule (arrow) in the anterior pituitary gland. (B) Pedigree of the family with thyroid function test results aligned with each symbol representing a family member. Abnormal values are in bold numbers. An arrow indicates the index case. Squares indicate males, circles indicate females, Roman numerals to the left of the pedigree indicate the generation, and numerals to the right of each symbol indicate individual family members. Symbols filled in gray indicate individuals with autoimmune thyroid disease. Symbols filled in black indicate subjects heterozygous for the novel thyroid hormone receptor beta (THRB) gene mutation as indicated in the legend. AITD, autoimmune thyroid disease; T3, triiodothyronine; rT3, reverse T3; T4, thyroxine; TT3, total T3; TrT3, total rT3; TT4, total T4; fT4I, free T4 index; TSH, thyrotropin; Tg, thyroglobulin; TPO, thyroperoxidase; ab, autoantibodies. (C) Chromatogram showing the monoallelic substitution c.1037G>T (p.G251V) in affected members of the family.
The serum TSH of the proband was suppressed from 2.94 to 0.09 mIU/L (97% reduction) after oral administration of 100 μg of T3/day for five days.
Written consent to perform genetic testing was obtained from the patient and family members. Genomic DNA extracted from peripheral blood leucocytes was amplified by polymerase chain reaction. Direct sequencing of exon 7 through 10 of the THRB gene revealed a novel single nucleotide substitution (c.1037G>T; Fig. 1C), resulting in the replacement of the normal glycine at position 251 with a valine (p.G251V), in the proband and all affected family members, including her sister with Graves' disease (II-1, II-2, III-1, III-2, and III-4; Fig. 1B). Spouses of II-4 and II-5 were not tested.
Discussion
This report emphasizes the importance of careful investigations to distinguish between RTHβ and TSHoma, especially given that their coexistence has been previously reported (1). Based on a lack of symptoms of thyrotoxicosis, the apparent inheritance of the non-suppressed TSH, the magnitude of T3-mediated suppressed TSH, and the absence of pituitary function abnormalities indicated that the MRI finding represented an incidental nonfunctioning microadenoma, which, in the absence of an alternative diagnosis, would have required a longer follow-up to exclude a clinically silent TSHoma. A TSH releasing hormone test and two-month administration of long-acting somatostatin analogs could have been performed as alternative tests to differentiate between these two conditions (2). An elevated serum glycoprotein hormone alpha-subunit (αSU) level/TSH molar ratio is found in most patients with TSHoma. However, the interpretation must take other hormones such as luteinizing hormone and follicle stimulating hormone into consideration because this ratio can be raised, especially in postmenopausal women.
While this particular mutation in codon 251 (GTA) has not been previously reported, other mutations of the same codon (GAA and AGA), resulting in p.G251E and p.G251R, respectively, have been previously reported by Macchia et al. and Shiwa et al., and have been shown to be functionally impaired (3,4). The additional aspect in this family is that with exception of the youngest family member, all affected individuals have AITD, based on the presence of Tg and/or TPO antibodies. The AITD of the youngest sister with RTHβ (II-5) resulted in Graves' disease. One first-degree relative (III-3) without the THRB gene mutation also had no evidence of AITD. This association has been reported among the RTHβ cohort but not to the extent observed in this family (5). The mechanism underlying the association of AITD in subjects with RTHβ remains unclear. Chronic TSH stimulation in RTHβ may activate intrathyroidal lymphocytes and increase the likelihood of AITD. In addition, chronic elevation of thyroid hormone levels may stimulate the immune system. As patients with AITD are prone to developing hyperthyroidism or hypothyroidism, monitoring clinical symptoms and an annual TSH determination is recommended.
Conclusions
A novel THRB gene mutation is reported. Clinical, laboratory, and genetic evaluations are required to distinguish between a TSHoma from an incidental pituitary adenoma in an individual with RTHβ. The association between RTHβ and AITD is also demonstrated.
Acknowledgments
This study was supported in part by grant R37DK15070 from the National Institutes of Health and Seymour J. Abrams fund for thyroid research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. The abstract of this manuscript was presented at the Endocrine Society's 98th Annual Meeting and Expo.
Author Disclosure Statement
The authors have no competing financial interests exist.
References
- 1.Teng X, Jin T, Brent GA, Wu A, Teng W, Shan Z. 2015. A patient with a thyrotropin-secreting microadenoma and resistance to thyroid hormone (P453T). J Clin Endocrinol Metab 100:2511–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mannavola D, Persani L, Vannucchi G, Zanardelli M, Fugazzola L, Verga U, Facchetti M, Beck-Peccoz P. 2005. Different responses to chronic somatostatin analogues in patients with central hyperthyroidism. Clin Endocrinol 62:176–181 [DOI] [PubMed] [Google Scholar]
- 3.Macchia E, Agostini M, Sarkissian G, Giorgilli G, Canale D, Scartabelli G, Margotat A, Torresani J, Pinchera A. 1998. Detection of a new de novo mutation at codon 251 of exon 8 of thyroid hormone receptor beta gene in an Italian kindred with resistance to thyroid hormone. J Endocrinol Invest 21:226–233 [DOI] [PubMed] [Google Scholar]
- 4.Shiwa T, Oki K, Awaya T, Nakanishi S, Yamane K. 2011. Resistance to thyroid hormone accompanied by Graves' disease. Intern Med 50:1977–1980 [DOI] [PubMed] [Google Scholar]
- 5.Barkoff MS, Kocherginsky M, Anselmo J, Weiss RE, Refetoff S. 2010. Autoimmunity in patients with resistance to thyroid hormone. J Clin Endocrinol Metab 95:3189–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]