Patients coinfected with human immunodeficiency virus (HIV) and Mycobacterium tuberculosis (Mtb; the causative agent of TB) have dramatically increased rates of mortality and morbidity compared with singly infected patients. Evidence supporting various mechanisms of synergism has been published: HIV replication at sites of Mtb infection leads to reduced bacterial containment and facilitated HIV spread, HIV inhibits macrophage killing of Mtb, HIV reduces CD4 T cells within granulomas, thereby allowing activation of Mtb, and HIV impairs Mtb-specific T cells.2
Although macrophages are susceptible to infection by both HIV and Mtb, the concept that coinfection on the cellular level could contribute to the particularly poor prognosis of coinfected individuals has received little consideration. To our knowledge, the existence of HIV/Mtb coinfected cells has never been demonstrated directly. Here we report that HIV and Mtb can, in fact, productively infect the same macrophage (Fig. 1).
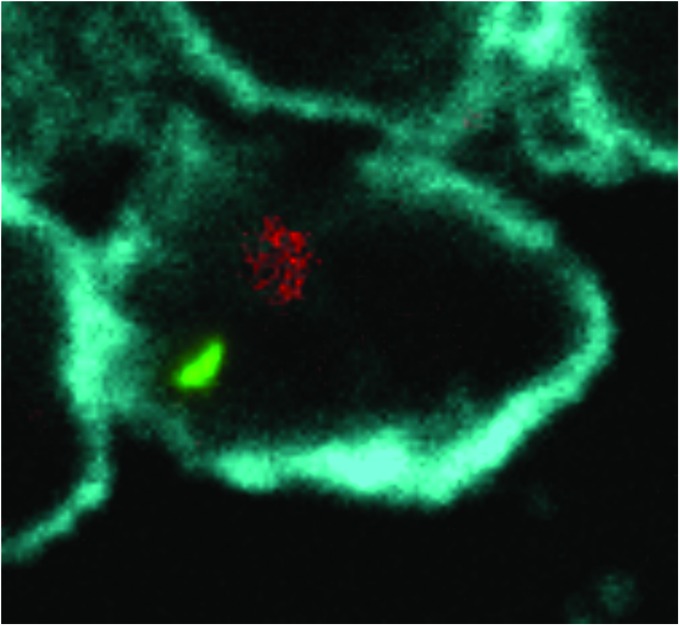
FIG. 1.
THP-1, a macrophage cell line, coinfected with DHIV-mCherry and H37Ra gfp. THP-1 cells were differentiated with PMA (20 ng/ml) for 24 h before coinfection. DHIV-mCherry (red; ∼5 × 106 TU/ml, determined by flow cytometry) and H37Ra gfp (green; MOI 1:1) were coincubated for 16 h. Cells were fixed in 1% formaldehyde and actin was stained with Abnova™ Fluorescent Dye 405-I Phalloidin (cyan). The image was acquired on a Nikon A1 confocal microscope using a 60 × oil lens and processed using Fiji1. It is an average intensity projection of 6 z-stacks, spaced 0.5 μm apart. MOI, multiplicity of infection; PMA, phorbol-12-myristate-13-acetate. Color images available online at www.liebertpub.com/aid
The monocytic leukemia macrophage cell line, THP-1, is known to phagocytose Mtb in vitro. We confirmed this using simple coculture of H37Ra gfp,3 an attenuated GFP-expressing Mtb strain, with THP-1 cells (1:1 MOI) for 4 h or longer and flow cytometry to quantify infection, showing a GFP-positive subset within the viable THP-1 cell population (LIVE/DEAD® Fixable Violet Stain). We showed that H37Ra gfp was likely phagocytosed by THP-1 cells in this culture system because the percentage infected THP-1 cells increased after phorbol-12-myristate-13-acetate (PMA) activation (not shown). Furthermore, we observed that Rab20, a marker of early-to-late stages of phagosomal/endosomal maturation, was upregulated in H37Ra gfp-containing THP-1 cells and that Rab20+/GFP+ populations increased, indicating internalization (not shown).
To investigate whether THP-1 macrophages could harbor HIV and Mtb concurrently, we used the mentioned system and coincubated THP-1 overnight (16 h) with DHIV-mCherry and H37Ra gfp. DHIV is a “defective” HIV strain that contains a short deletion within the env gene, thereby preventing more than one round of infection. The strain we used expresses mCherry in substitution for the nef gene, which allows for quantitation of infection by flow cytometry or cell imaging when the mCherry protein is synthesized during viral replication. DHIV-mCherry was constructed by replacing the GFP gene with that for mCherry in the DHIV-GFP construct previously described.4 Although the H37Ra gfp is probably located within the THP-1 phago/endosome, we hypothesize that the concentrated regions of mCherry apparent in the image most likely indicate the pooling of Env proteins in the trans-Golgi network during the early stages of the viral life cycle. In any case, the image indicates viral entry and progression through its life cycle occurs in the presence of intracellular H37Ra.
This is the first image to capture replication of HIV within an Mtb-infected macrophage. This productive cohabitation (producing both GFP and mCherry-Env) within the same cell in vitro suggests that cellular coinfection of macrophages, or we speculate T cells or syncytia, may be common in coinfected individuals. If this observation is confirmed in patients, it is possible that coinfected cells contribute to the remarkable pathogenic synergism observed in coinfected individuals.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Schindelin J, Arganda-Carreras I, Frise E, et al. : Fiji: An open-source platform for biological-image analysis. Nat Methods 2012;9:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diedrich CR, Flynn JL: HIV-1/Mycobacterium tuberculosis coinfection immunology: How does HIV-1 exacerbate tuberculosis? Infect Immun 2011;79:1407–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins LA, Torrero MN, Franzblau SG: Green fluorescent protein reporter microplate assay for high-throughput screening of compounds against mycobacterium tuberculosis. Antimicrob Agents Chemother 1998;42:344–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosque A, Planelles V: Induction of HIV-1 latency and reactivation in primary memory CD4 T cells. Blood 2009;113:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]