Abstract
Although sarcopenia, age-associated loss of muscle mass and strength, is neither accelerated nor exacerbated by depletion of muscle stem cells, satellite cells, we hypothesized that adaptation in sarcopenic muscle would be compromised. To test this hypothesis, we depleted satellite cells with tamoxifen treatment of Pax7CreER-DTA mice at 4 months of age, and 20 months later subjected the plantaris muscle to 2 weeks of mechanical overload. We found myofiber hypertrophy was impaired in aged mice regardless of satellite cell content. Even in the absence of growth, vehicle-treated mice mounted a regenerative response, not apparent in tamoxifen-treated mice. Further, myonuclear accretion occurred in the absence of growth, which was prevented by satellite cell depletion, demonstrating that myonuclear addition is insufficient to drive myofiber hypertrophy. Satellite cell depletion increased extracellular matrix content of aged muscle that was exacerbated by overload, potentially limiting myofiber growth. These results support the idea that satellite cells regulate the muscle environment, and that their loss during aging may contribute to fibrosis, particularly during periods of remodeling. Overload induced a fiber-type composition improvement, independent of satellite cells, suggesting that aged muscle is very responsive to exercise-induced enhancement in oxidative capacity, even with an impaired hypertrophic response.
Key Words: Satellite, cells, Sarcopenia, Regeneration, Muscle, overload, Fibrosis.
Sarcopenia, age-associated loss of muscle mass and strength, contributes to weakness and frailty (1). Frailty limits performance of activities of daily living and is the leading risk factor for falls in the elderly adult. In fact, as life expectancy continues to increase, loss of muscle mass and strength is the primary determinant in one’s ability to live an independent life (2). Currently, resistance exercise training is the most effective countermeasure to combat sarcopenia, as it preserves and in some cases enhances muscle mass (3,4). In response to resistance exercise, muscle stem cells, satellite cells, fuse with myofibers to increase myonuclear number as myofiber cytoplasmic volume increases. Satellite cell abundance and myonuclear accretion correlate with increases in myofiber size in response to resistance exercise training in humans (5) and mechanical overload in rodents (6,7). Thus, age-associated reduction in satellite cells (8–10) and satellite cell activity (11–13) have been assumed to negatively impact the adaptability of aged muscle to respond to growth stimuli, contributing to an impaired (14–16) or completely abolished (17) growth response. However, although satellite cells normally fuse into growing myofibers, they are not necessary for effective myofiber hypertrophy (18). Using the Pax7CreER-DTA mouse that allows for the specific and conditional depletion of satellite cells in adult muscle through tamoxifen-dependent Cre recombination, we showed that although regeneration was significantly blunted following satellite cell depletion, robust increases in muscle mass occurred in response to hypertrophic stimuli, suggesting that a compensatory mechanism exists in adult muscle that enables growth independent of satellite cell fusion. These results support the idea that myonuclear accretion is not requisite for muscle growth; existing myonuclei can support a larger volume of cytoplasm in response to a growth stimulus (18). Similarly, muscle regrowth after atrophy does not require satellite cells in young adult mice (19,20). We found that satellite cell-depleted mice showed normal recovery of muscle mass and function after atrophy in a hind-limb suspension and reloading paradigm (20). These results clearly demonstrate that myofiber size adaptation to different activity levels is not dependent on myonuclear number.
More recently, we reported that following tamoxifen treatment of Pax7CreER-DTA mice, satellite cells do not recover even after 20 months (21). A detailed analysis of multiple muscles revealed that the life-long reduction of satellite cells did not accelerate nor exacerbate sarcopenia; however, we hypothesized that the muscle hypertrophic response, which is impaired with age (reviewed in ref. 22), would be further compromised by satellite cell depletion. Thus, the purpose of this study was to determine if the plasticity observed in young adult muscle in response to overload in the absence of satellite cell activity is maintained in old age.
Methods
Mouse Model
The Pax7CreER/+;Rosa26DTA/+ (here referred to as Pax7CreER-DTA) strain was generated by crossing Pax7CreER/CreER and Rosa26DTA/DTA strains. Both parental strains are on a 129/C57BL6 background. The Pax7CreER-DTA genetic mouse model allows for the specific and inducible depletion of satellite cells upon tamoxifen treatment, through activation of the diphtheria toxin A gene only in Pax7-expressing cells (18). All animal procedures were conducted in accordance with institutional guidelines, as approved by the institutional animal care and use committee of the University of Kentucky. Mice were housed in a temperature- and humidity-controlled, but nonpathogen free room, and maintained on a 14:10-hour light:dark cycle with food and water ad libitum.
Experimental Design
Adult male Pax7CreER-DTA mice were administered by intraperitoneal injection either vehicle (15% ethanol in sunflower seed oil) or tamoxifen (2.0mg/day) for five consecutive days at 4 months of age, 2 hours prior to lights out. Following 20 months (at 24 months of age), vehicle- and tamoxifen-treated mice were randomly divided into sham or synergist ablation (SA) groups (n = 4–8 mice/group).
SA Surgery
Following a 20-month recovery period after vehicle or tamoxifen treatment, mice were subjected to either sham or SA surgery to induce hypertrophy of the plantaris muscle as described in detail (18). Briefly, following anesthetization (95% oxygen and 5% isoflurane gas), the gastrocnemius and soleus muscles were surgically removed through an incision in the hind limb. Sham surgeries involved similar procedures without gastrocnemius and soleus muscle excision. After 2 weeks, mice were anesthetized and the plantaris muscles excised followed by euthanization via cervical dislocation.
Histochemistry/immunohistochemistry
Muscles were mounted in freezing medium and frozen in liquid nitrogen-cooled isopentane and stored at −80°C prior to cryosectioning (7 µm). For Pax7 (satellite cells), muscle sections were fixed in 4% paraformaldehyde (PFA) followed by epitope retrieval using sodium citrate (10mM, pH 6.5) at 92°C for 20 minutes. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in phosphate-buffered saline for 7 minutes followed by an additional Mouse-on-Mouse Blocking Reagent (Vector Laboratories, Burlingame, CA) step. Incubation with Pax7 antibody (Developmental Studies Hybridoma Bank, Iowa City, IA) was followed by a biotin-conjugated secondary antibody and streptavidin–horseradish peroxidase from a Tyramide Signal Amplification kit (Invitrogen, Carlsbad, CA). Tyramide Signal Amplification-Alexa Fluor 488 was used to visualize antibody binding. Tissue was incubated for 10 minutes with 4ʹ,6ʹ-diamidino-2-phenylindole (DAPI) (10 nM; Invitrogen), washed, and mounted.
For fiber cross-sectional area determination and myonuclei counting, a dystrophin antibody (Vector) was applied to fresh frozen sections followed by Texas Red-conjugated goat anti-mouse secondary antibody (#601-109-121; Rockland Immunochemicals Inc., Gilbertsville, PA) and DAPI staining. For identification of myonuclei that had undergone DNA replication, following dystrophin detection, sections were fixed in absolute methanol, treated with 2N HCl to denature DNA and neutralized with 0.1-borate buffer (BORAX), pH 8.5. 2-Bromo-deoxyuridine antibody incubation was followed by biotin-conjugated goat anti-mouse secondary antibody and streptavidin–fluorescein isothiocyanate (FITC). Sections were postfixed in 4% PFA and stained with DAPI. For extracellular matrix (ECM) accumulation, muscle sections were pre-fixed in 4% PFA, and then incubated with Texas-Red-conjugated α-wheat germ agglutinin (1 mg/mL; Invitrogen W21405).
For fiber typing, unfixed sections were incubated in antibodies against myosin heavy chain (MyHC) types 1, 2a, and 2b (type 1: BA.D5; 2a: SC.71; and 2b: BF.F3, Developmental Studies Hybridoma Bank) in addition to dystrophin (Vector). MyHC type 2x expression was assumed from unstained fibers. Fluorescent-conjugated secondary antibodies against various mouse immunoglobulin subtypes were applied to visualize MyHC expression (Gt anti-Ms IgG2b AF647 1:250 #A21242, Gt anti-Ms IgG1 AF488 1:500 #A21121, Gt anti-Ms IgM AF555 1:250 #A21426; Invitrogen) and dystrophin (Texas-Red-conjugated goat anti-mouse; Rockland). Sections were postfixed in 4% PFA prior to mounting, unless 2-bromo-deoxyuridine detection was performed.
Image Acquisition and Quantification
Images were captured with an upright microscope (AxioImager M1; Zeiss, Göttingen, Germany). Myofiber frequency distribution, cross-sectional area, and fiber type were quantified using a newly developed image segmentation algorithm (23,24). All other images were quantified with Zeiss Axiovision rel. software (v4.8). Satellite cell abundance was assessed using Pax7 staining and only those cells that were Pax7+ and DAPI+ were counted. Wheat germ agglutinin staining was quantified using the threshold intensity programs within the Zeiss Axiovision imaging software.
Myonuclear Accretion on Single Isolated Fibers
Plantaris muscles were fixed in situ at resting length in 4% PFA for 48 hours. Single myofibers were isolated by 40% NaOH digestion, as previously described (18). Single myofibers were stained with DAPI and nuclei from 15–25 myofibers per animal (n = 4–8 mice/group) within a given segment were counted by z-stack analysis using the AxioImager M1 microscope. Axiovision software was used to measure myofiber dimensions and myonuclear number per myofiber segment.
Statistics
All data were analyzed with SigmaPlot v12.0 software (Systat Software, San Jose, CA) via a two-factor analysis of variance (eg, vehicle/tamoxifen × sham/surgery) or where appropriate a Student’s t-test, for each dependent variable under consideration. If a significant interaction was detected, Bonferroni post hoc analysis was employed to determine the source of the significance. Statistical significance was accepted at p ≤ .05. Data are reported as mean ± standard error of the mean.
Results
The Age-Attenuated Growth Response to Overload Is Further Impaired by Satellite Cell Depletion
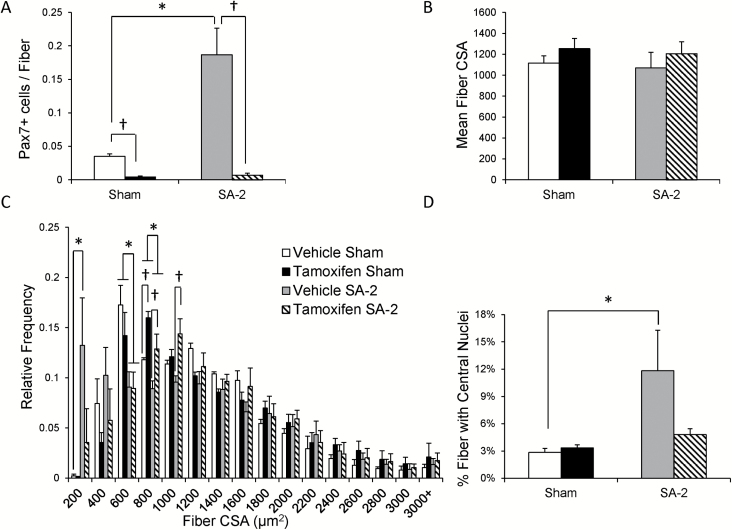
To evaluate the role of satellite cells in aged muscle growth adaptation, Pax7CreER-DTA mice were treated at 4 months of age with vehicle or tamoxifen and 20 months later subjected to either sham or synergist ablation (SA) surgery, removing the gastrocnemius and soleus muscles thereby overloading the plantaris, for 2 weeks (SA-2). The plantaris muscles from the aged mice were sarcopenic, with muscles from the sham group weighing approximately 20% less than those from young adult, 5-month-old Pax7CreER-DTA mice (21). Immunohistochemistry (IHC) with an antibody against Pax7 showed effective satellite cell depletion was maintained in the 24-month-old mice and that the large increase in satellite cell abundance in response to 2 weeks of overload, apparent in vehicle-treated mice, was abolished (Figure 1A, representative images are shown in Supplementary Figure 1A and B). IHC with an antibody against dystrophin showed no increase in mean myofiber cross-sectional area in response to overload in either vehicle- or tamoxifen-treated mice (Figure 1B, representative images are shown in Supplementary Figure 1C and D). Figure 1C shows in aged mice, regardless of treatment, an absence in the characteristic rightward shift in fiber size distribution that occurs in adult mice following 2 weeks of overload and results in increased frequency of fibers in the 1,200-2,000 um2 range (18,25). Overload did cause a significant increase in the number of very small fibers (<200 µm2) preferentially in muscles with their full complement of satellite cells (Figure 1C). The increased frequency of small fibers in vehicle SA-2 plantaris muscles likely contributes to the different relative fiber frequencies between vehicle- and tamoxifen-treated mice in the 800–1,199 µm2 range following overload. In addition to increased abundance of small fibers with overload, the abundance of centrally nucleated fibers increased only in vehicle-treated mice (Figure 1D). These observations support the conclusion that overload induces a muscle regenerative response that is dependent on satellite cells. Although satellite cell proliferation and fusion appear to occur in aged mice, this is not associated with myofiber hypertrophy.
Figure 1.
Attenuated growth response in aged animals is altered with satellite cell depletion. (A) Quantification of satellite cells in the plantaris was evaluated with Pax7 immunohistochemistry and demonstrated robust depletion in both sham and overloaded (SA-2) tamoxifen-treated Pax7CreER-DTA mice. Two weeks of overload induced a significant increase (400%) only in vehicle-treated Pax7CreER-DTA mice. †Significant difference between vehicle and tamoxifen treatment, p < .05. *Significant difference between sham and SA-2, p < .05. (B) No increase in mean myofiber cross-sectional area (CSA) following overload in vehicle- and tamoxifen-treated Pax7CreER-DTA mice. (C) Increased frequency of small fibers (<200 μm2) in vehicle-treated SA-2 Pax7CreER-DTA mice. No hypertrophic response to overload occurred in both vehicle- and tamoxifen-treated SA-2 Pax7CreER-DTA mice as evidenced by a lack of a rightward shift in the myofiber CSA distribution. †Significant difference between vehicle and tamoxifen treatment, p < .05. *Significant difference between sham and SA-2, p < .05. (D) Increased frequency of fibers containing one or more central nuclei following overload occurs only in vehicle-treated Pax7CreER-DTA mice. *Significant difference between sham and SA-2, p < .05.
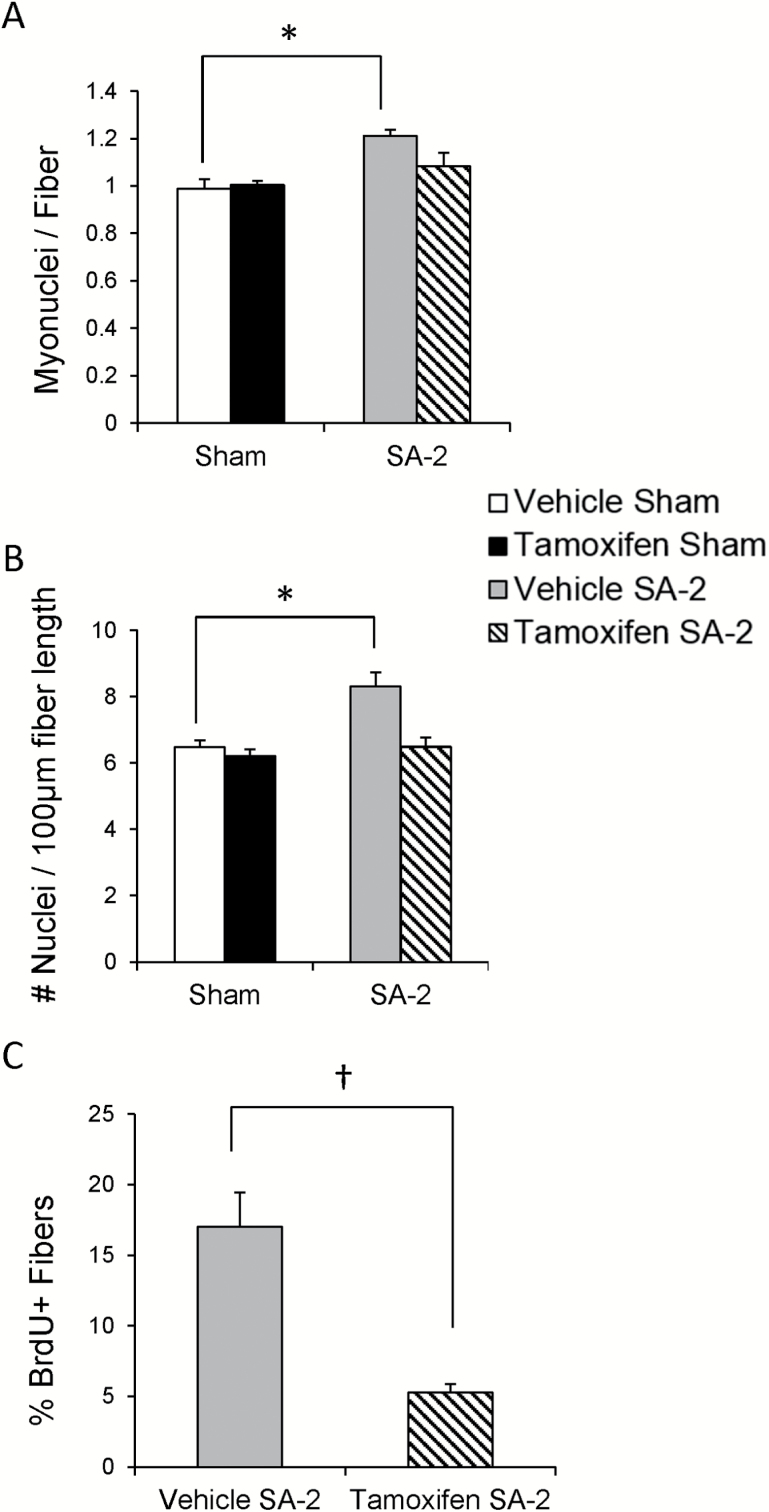
Overload-Induced Myonuclear Accretion Is Absent in Satellite Cell-Depleted Aged Muscle
Mechanical overload of muscle is normally associated with myonuclear accretion to maintain a constant myonuclear domain as the myofiber hypertrophies. Although myofiber growth was impaired in the aged mice, we wanted to determine if myonuclear addition still occurred. Myonuclei were counted using two methods: on cross sections following dystrophin IHC and DAPI staining (Supplementary Figure 1C and D), and following DAPI staining of isolated, fixed myofibers (Supplementary Figure 1E and F). Overload increased the number of myonuclei on cross sections in vehicle-treated mice, whereas no myonuclear accretion was apparent in tamoxifen-treated mice (Figure 2A). These results were confirmed counting nuclei on single fibers (Figure 2B). Further, a subset of mice were provided 2-bromo-deoxyuridine in their drinking water during the 2 weeks of overload and the vehicle-treated mice showed a significantly higher number of myofibers with 2-bromo-deoxyuridine-labeled nuclei (Figure 2C), indicating that the nuclei had undergone DNA replication and subsequently fused into the fiber. Thus, the lack of growth response in the aged mice is not due to an inability of satellite cells to provide new myonuclei.
Figure 2.
Overload-induced myonuclear accretion is absent in satellite cell-depleted aged muscle. (A) Myonuclear accretion occurs following overload only in vehicle-treated Pax7CreER-DTA mice as measured with immunohistochemistry with an antibody against dystrophin and DAPI co-stain on plantaris cross sections. (B) Myonuclear accretion occurs following overload only in vehicle-treated Pax7CreER-DTA mice as measured on fixed, single myofibers stained with DAPI. *Significant difference between sham and SA-2, p < .05. *Significant difference between sham and SA-2, p < .05. (C) Incorporation of myonuclei positive for 2-bromo-deoxyuridine is attenuated in satellite cell-depleted plantaris muscle following overload. †Significant difference between vehicle and tamoxifen treatment, p < .05.
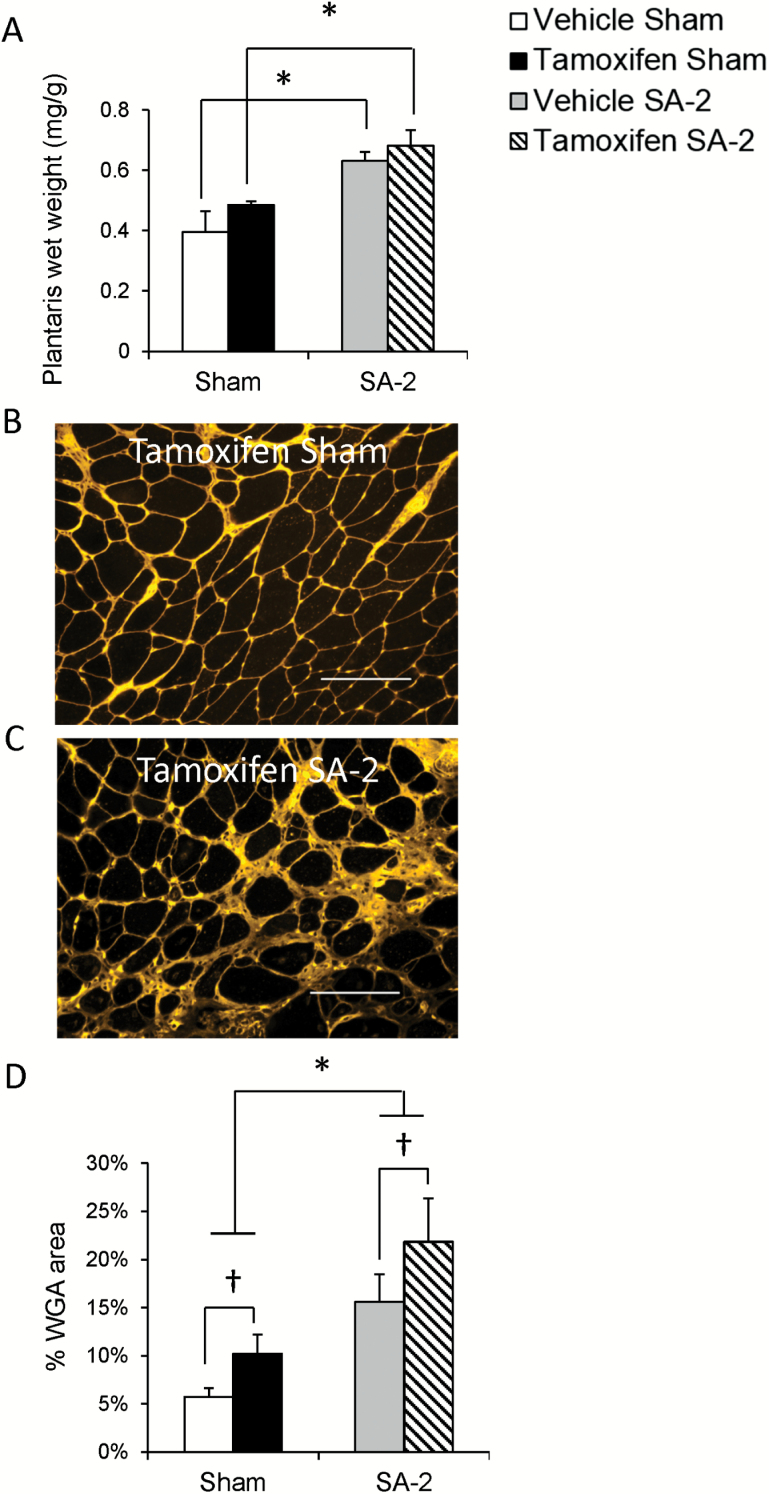
Life-Long Satellite Cell Depletion Contributes to Extracellular Matrix Accumulation That Is Exacerbated by Overload
Although we did not see an overall increase in myofiber CSA in aged mice following 2 weeks of overload, we did observe an approximate 20% increase in whole muscle mass in both vehicle- and tamoxifen-treated SA-2 mice (Figure 3A). This is significantly lower than the doubling in muscle mass we routinely observe in young adult mice (18,25). Staining with α-wheat germ agglutinin showed that overload significantly increased ECM accumulation in both satellite cell-depleted (Figure 3B and C, quantified in D) and vehicle-treated mice (Figure 3D). Tamoxifen treatment and overload appeared additive so that the overall ECM accumulation was highest in satellite cell-depleted, overloaded muscle (Figure 3D).
Figure 3.
Life-long satellite cell depletion contributes to extracellular matrix (ECM) accumulation that is exacerbated by overload. (A) Plantaris muscle wet weight increased in both vehicle- and tamoxifen-treated Pax7CreER-DTA mice following 2 weeks of overload. *Significant difference between sham and SA-2, p < .05. (B and C) Representative plantaris images of tamoxifen-treated sham (B) and SA-2 (C) Pax7CreER-DTA mice stained with wheat germ agglutinin, which binds N-acetyl-glucosamine found in the ECM. Scale bar = 100 μm. (D) Quantification of wheat germ agglutinin staining demonstrates increased ECM content following life-long satellite cell depletion that is enhanced following 2 weeks of overload. †Significant difference between vehicle and tamoxifen treatment, p < .05. *Significant difference between sham and SA-2, p < .05.
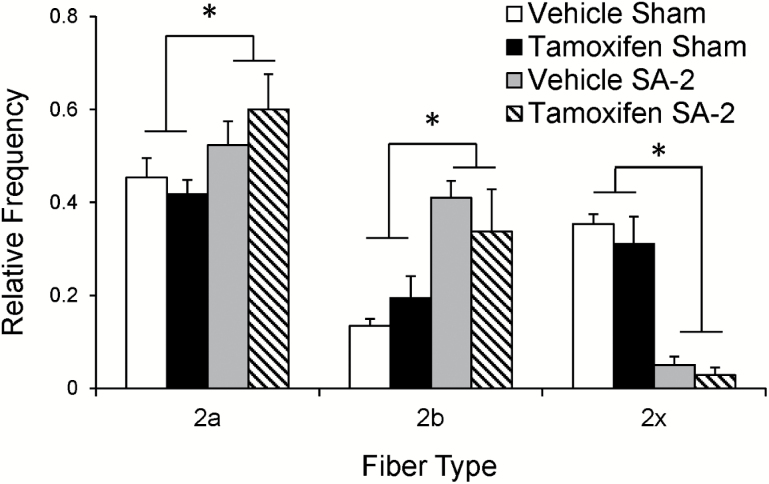
Overload-Induced Fiber-Type Changes are Independent of Satellite Cells
Although aged muscle did not mount a hypertrophic response to overload, the fiber-type composition adapted to increased demand, regardless of satellite cell number. A representative image of a plantaris muscle cross section following IHC with isoform-specific MyHC antibodies shows the distribution of type 2a, 2b, and 2x fibers (Supplementary Figure 2). Overload resulted in an increase in type 2b fiber frequency with a corresponding decrease in relative abundance of 2x fibers, irrespective of satellite cell number. In addition, type 2a fibers increased in abundance (Figure 4). Thus, aged muscle adapts to 2 weeks of overload by altering fiber-type composition that is not affected by satellite cell content.
Figure 4.
Overload induces a shift in fiber-type distribution independent of satellite cells. Antibodies against myosin heavy chain isoforms were used to determine the fiber-type distribution of plantaris cross sections. Two weeks of overload induced a similar shift in plantaris fiber-type distribution in both vehicle- and tamoxifen-treated SA-2 Pax7CreER-DTA mice. *Significant difference between sham and SA-2, p < .05.
Discussion
The old mice in this study failed to increase myofiber size in response to overload, independent of satellite cell number, suggesting that satellite cell loss over time is not causing the impaired growth response associated with aging. This is in contrast to recent work showing impaired but significant hypertrophy in response to overload in mice of a comparable age, correlated to reduced satellite cell abundance (16). This may be due to the different surgical techniques to induce compensatory overload, a greater duration of time (6 weeks) the plantaris muscle was mechanically overloaded, and also the degree of sarcopenia observed prior to overload in that study. Ballak and colleagues utilized the synergist denervation model of hypertrophy, an approach that minimizes the inflammatory response to surgery, relative to SA, and has a unique growth time course as a result (26). In spite of the lack of growth observed in our study, results support several conclusions regarding the role of satellite cells in aged muscle adaptation: (a) overload induced by SA causes a regenerative response in aged muscle that is dependent on satellite cells, but independent of hypertrophy; (b) myonuclear accretion occurs in response to overload, even in the absence of growth, which is prevented by satellite cell depletion; (c) satellite cell depletion exacerbates overload-induced ECM accumulation in aged muscle; and (d) aged muscle fiber-type adaptation is responsive to overload and not contingent on satellite cells. Implications of each conclusion are discussed in the following sections.
Aged Muscle Hypertrophy and Regeneration Responses to Overload are Distinct
It has been firmly established in adult mice that muscle regeneration is dependent on satellite cells (18,27–29). Overload of the plantaris muscle by SA elicits a regenerative response in adult mice (18), which may be more severe in aged mice, as they appear more sensitive to contraction-induced injury (30). Vehicle-treated mice in this study showed evidence of muscle regeneration, indicated by an increase in very small fibers, likely due to de novo fiber formation, as well as an increase in centrally nucleated fibers. Overload was also associated with a nearly 4-fold increase in satellite cell number, comparable with the increase observed in young adult mice in response to 2 weeks of overload (18,25). However, the increase in satellite cell abundance and the regenerative response did not contribute to increased myofiber size in vehicle-treated mice, supporting the idea that regeneration and hypertrophy are different processes mechanistically. Both the increase in satellite cell abundance and markers of muscle regeneration were abrogated in tamoxifen-treated mice in response to overload, without significant effect on mean fiber cross-sectional area. Thus, 24-month-old mice were unable to mount a muscle growth response regardless of satellite cell abundance and independent of muscle regeneration.
Myonuclear Accretion Occurs Independently of Muscle Growth
Satellite cell proliferation in response to overload of the plantaris muscle is normally associated with fusion into growing myofibers to maintain a fairly constant myonuclear domain (18). However, we previously demonstrated that considerable growth can occur in the absence of myonuclear accretion and the original intent of this study was to determine if aged muscle maintained the ability to undergo compensatory growth in the absence of satellite cells. However, myofiber CSA did not increase in response to overload in either treatment group, in spite of the fact that myonuclear accretion occurred in the vehicle-treated group. In muscle with the normal satellite cell content, the hypertrophic stimulus activated satellite cells, and induced proliferation and fusion, with no increase in myofiber size. Thus, satellite cells are required to add a myonucleus in response to overload, presumably to increase the biosynthetic capacity of the myofiber, but this is insufficient to drive growth in aged muscle. Together, these data support the idea that the myonuclear domain is plastic and plays little role in controlling myofiber size, and suggest that satellite cell therapy geared toward increasing myonuclei may fail to halt sarcopenia or to augment a growth response in aged muscle.
Life-Long Satellite Cell Depletion Exacerbates Overload-Induced Increases in ECM Accumulation
We previously demonstrated that depletion of satellite cells early in adulthood results in elevated ECM accumulation in the plantaris muscle as the mice age (21). In this study, we demonstrate that 2 weeks of overload in aged mice exacerbates ECM deposition, particularly in satellite cell-depleted muscle. By contrast, in 4-month-old mice, longer periods of overload (4–8 weeks) are required to elicit a fibrotic response following satellite cell depletion (25). Results from in vitro experiments suggest that a secreted factor from satellite cells inhibits ECM gene expression in fibroblasts (25). We hypothesize that the excessive ECM accumulation may contribute, at least in part, to the small observed increase in plantaris whole muscle wet weight in response to overload, while at the same time, restricting myofiber hypertrophy in aged muscle. This is consistent with the observation in young adult satellite cell-depleted muscle that growth plateaus with chronic overload, coincident with excessive ECM accumulation (25). These results show that satellite cells regulate the myofiber extracellular environment, especially during periods of remodeling, and that loss of satellite cells during aging may contribute to muscle fibrosis. Thus, reduction in the satellite cell pool over time in degenerative diseases such as muscular dystrophies may contribute not only to impaired regenerative capacity but also directly to fibrosis (31,32).
Overload Restores Age-Associated Fiber-Type Composition
Previous plantaris analyses demonstrated an age-associated shift of glycolytic fast-twitch fiber types: a loss of 2b and increase in 2x MyHC isoform-expressing fibers (21). In humans, increased MyHC 2x fiber frequency is characteristic of poor metabolic health and physical inactivity (33). Two weeks of overload restored the muscle to a fiber-type distribution more closely resembling that of young animals, regardless of satellite cell content. The increase in type 2b fiber frequency is opposite to the work of Ballak and colleagues (16), who report a decrease in 2b fibers with overload in aged mice. This discrepancy may be due to the fact that the muscle in that study was overloaded for 6 weeks. We identified type 2x myofibers by absence of staining with type 1-, 2a-, or 2b-specific antibodies, so it is likely underestimating the degree of adaptation due to the possibility of hybrid 2x-expressing fibers. We also observed an increase in the relative frequency of MyHC 2a fibers, the most oxidative-type 2 fiber type. That these dramatic changes in fiber type occur following only 2 weeks of overload in aged mice is remarkable given that fiber-type shifts (ie, increased 2a abundance) in young adult mice do not occur by 2 weeks, but are observed following 8 weeks of overload (25). It is possible that with a longer period of overload, type 2b fiber frequency would begin to decline (16), associated with a further increase in type 2a oxidative fibers. Taken together, these results suggest that fiber type in aged mice is highly responsive to exercise, particularly the largest myofibers expressing fast-twitch isoforms most affected by age-associated fiber atrophy (34).
In summary, although age blunts the hypertrophic response to overload, fiber-type composition in aged muscle is highly responsive to loading, irrespective of satellite cell abundance. Thus, improvements in oxidative capacity and endurance, independent of growth, may be derived from resistance training in older adults. A major function of increased satellite cell abundance with resistance training may be to limit fibrosis, thereby promoting growth and remodeling.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by the Ellison Medical Foundation/American Federation of Aging Research Fellow (EPD 12102) to J.D.L.; Jeane B. Kempner Postdoctoral Scholar Award and the National Institutes of Health (NIH) grants (AR065337) to C.S.F.; NIH grants AG34453 to C.A.P. and AR60701 to C.A.P. and J.J.M.; and the National Center for Advancing Translational Sciences (UL1TR000117). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or American Federation of Aging Research.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors would like to thank Catherine Starnes for her biostatistics expertise; Thomas Chaillou, Margo Ubele, Rod Erfani, Jake Beggs, Marilyn Campbell, Tyler Kmiec, Robin Anglin, and Zakkary Hardyniec for technical assistance, image acquisition, and quantification.
References
- 1. Bortz WM., II A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57:M283–M288. [DOI] [PubMed] [Google Scholar]
- 2. Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. [DOI] [PubMed] [Google Scholar]
- 3. Candow DG, Forbes SC, Little JP, Cornish SM, Pinkoski C, Chilibeck PD. Effect of nutritional interventions and resistance exercise on aging muscle mass and strength. Biogerontology. 2012;13:345–358. [DOI] [PubMed] [Google Scholar]
- 4. Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. [DOI] [PubMed] [Google Scholar]
- 5. Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol. 2008;104:1736–1742. [DOI] [PubMed] [Google Scholar]
- 6. Akiho M, Nakashima H, Sakata M, Yamasa Y, Yamaguchi A, Sakuma K. Expression profile of Notch-1 in mechanically overloaded plantaris muscle of mice. Life Sci. 2010;86:59–65. [DOI] [PubMed] [Google Scholar]
- 7. Ishido M, Uda M, Kasuga N, Masuhara M. The expression patterns of Pax7 in satellite cells during overload-induced rat adult skeletal muscle hypertrophy. Acta Physiol (Oxf). 2009;195:459–469. [DOI] [PubMed] [Google Scholar]
- 8. Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. [DOI] [PubMed] [Google Scholar]
- 10. Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sousa-Victor P, Gutarra S, García-Prat L, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. [DOI] [PubMed] [Google Scholar]
- 12. Cosgrove BD, Gilbert PM, Porpiglia E, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, Olwin BB. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med. 2014;20:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Degens H, Alway SE. Skeletal muscle function and hypertrophy are diminished in old age. Muscle Nerve. 2003;27:339–347. [DOI] [PubMed] [Google Scholar]
- 15. Thomson DM, Gordon SE. Diminished overload-induced hypertrophy in aged fast-twitch skeletal muscle is associated with AMPK hyperphosphorylation. J Appl Physiol. 2005;98:557–564. [DOI] [PubMed] [Google Scholar]
- 16. Ballak S, Jaspers RT, Deldicque L, et al. Blunted hypertrophic response in old mouse muscle is associated with a lower satellite cell density and is not alleviated by resveratrol. Exp Gerontol. 2015;62:23–31. [DOI] [PubMed] [Google Scholar]
- 17. Blough ER, Linderman JK. Lack of skeletal muscle hypertrophy in very aged male Fischer 344 x Brown Norway rats. J Appl Physiol. 2000;88:1265–1270. [DOI] [PubMed] [Google Scholar]
- 18. McCarthy JJ, Mula J, Miyazaki M, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bruusgaard JC, Egner IM, Larsen TK, Dupre-Aucouturier S, Desplanches D, Gundersen K. No change in myonuclear number during muscle unloading and reloading. J Appl Physiol. 2012;113:290–296. [DOI] [PubMed] [Google Scholar]
- 20. Jackson JR, Mula J, Kirby TJ, et al. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol. 2012;303:C854–C861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fry CS, Lee JD, Mula J, et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med. 2014;21:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. 2013;41:169–173. [DOI] [PubMed] [Google Scholar]
- 23. Liu F, Fry CS, Mula J. et al. Automated fiber-type-specific cross-sectional area assessment and myonuclei counting in skeletal muscle. J Appl Physiol. 2013;115:1714–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mula J, Lee JD, Liu F, Yang L, Peterson CA. Automated image analysis of skeletal muscle fiber cross-sectional area. J Appl Physiol. 2013;114:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fry CS, Lee JD, Jackson JR, et al. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J. 2014;28:1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lowe DA, Alway SE. Animal models for inducing muscle hypertrophy: are they relevant for clinical applications in humans? J Orthop Sports Phys Ther. 2002;32:36–43. [DOI] [PubMed] [Google Scholar]
- 27. Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sambasivan R, Yao R, Kissenpfennig A, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. [DOI] [PubMed] [Google Scholar]
- 30. Koh TJ, Brooks SV. Lengthening contractions are not required to induce protection from contraction-induced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2001;281:R155–R161. [DOI] [PubMed] [Google Scholar]
- 31. Dayanidhi S, Lieber RL. Skeletal muscle satellite cells: mediators of muscle growth during development and implications for developmental disorders. Muscle Nerve. 2014;50:723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Serrano AL, Mann CJ, Vidal B, Ardite E, Perdiguero E, Muñoz-Cánoves P. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr Top Dev Biol. 2011;96:167–201. [DOI] [PubMed] [Google Scholar]
- 33. Fry CS, Noehren B, Mula J, et al. Fibre type-specific satellite cell response to aerobic training in sedentary adults. J Physiol. 2014;592:2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Larsson L. Motor units: remodeling in aged animals. J Gerontol A Biol Sci Med Sci. 1995;50:91–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.