Abstract
In this study, we examined the combined effect of aging and myocardial infarction on left ventricular remodeling, focusing on matrix metalloproteinase (MMP)-9-dependent mechanisms. We enrolled 55 C57BL/6J wild type (WT) and 85 MMP-9 Null (Null) mice of both sexes at 11–36 months of age and evaluated their response at Day 7 post–myocardial infarction. Plasma MMP-9 levels positively linked to age in WT mice (r = .46, p = .001). MMP-9 deletion improved survival (76% for WT vs 88% for Null, p = .021). Post–myocardial infarction, there was a progressive increase in left ventricular dilation with age in WT but not in Null mice. By inflammatory gene array analysis, WT mice showed linear age-dependent increases in three different proinflammatory genes (C3, CCl4, and CX3CL1; all p < .05), whereas Null mice showed increases in three proinflammatory genes (CCL5, CCL9, and CXCL4; all p < .05) and seven anti-inflammatory genes (CCL1, CCL6, CCR1, IL11, IL1r2, IL8rb, and Mif; all p < .05). Compared with WT, macrophages isolated from Null left ventricle infarct demonstrated enhanced expression of anti-inflammatory M2 markers CD163, MRC1, TGF-β1, and YM1 (all p < .05), without affecting proinflammatory M1 markers. In conclusion, MMP-9 deletion stimulated anti-inflammatory polarization of macrophages to attenuate left ventricle dysfunction in the aging post–myocardial infarction.
Key Words: Aging, Cardiac remodeling, Macrophage polarization, M2 phenotype.
Every year, nearly 1 million people are diagnosed with myocardial infarction (MI) in the United States. The average age for MI is 65 years for men and 72 years for women (1). MI develops as a result of disruption of the blood supply to myocardium, which leads to remodeling of the left ventricle (LV). The post-MI response includes cardiomyocyte death, initiation of a robust inflammatory reaction, reorganization of extracellular matrix (ECM), and formation of a stable scar. Matrix metalloproteinases (MMPs) are a group of proteolytic enzymes that degrade ECM and contribute to LV remodeling. The effect of aging on post-MI LV remodeling has not been extensively studied in particular in the setting of MMP-9 deletion.
Recent advancements in MI therapy have concentrated on early interventions to restore blood supply to the ischemic myocardium. Currently, primary coronary stenting is a primary initial strategy to acutely reperfuse the ischemic myocardium (2). However, access to reperfusion therapies varies significantly (3). Additionally, 20% of patients have contraindications for coronary stenting that include bleeding risk, no chest pain, or no ST segment elevation (4). Until recently, advanced age was also a contraindication for thrombolytic therapy (5). Thus, up to 40% of post-MI patients will continue to remodel and will progress to heart failure, and about 4% of reperfused patients will still develop heart failure (6,7). Thus, there is a need to better understand the post-MI remodeling process, particularly in an aging cohort.
MMP-9 is highly expressed in macrophages and has been implicated in LV remodeling processes (8). Previously, we reported that aging in mice is associated with increased MMP-9 expression and a subtle yet significant decline in LV function (9). LV functional changes occurred in mice over 18 months of age and were associated with an increase in ECM gene and protein levels (9). We also reported that the MMP-9 deletion attenuates age-associated desensitization of cardiac tissue to proangiogenic stimulation, which results in improved blood vessel numbers and decreased vascular permeability (10). In the post-MI setting, the effects of MMP-9 have been studied in young but not aging mice. MMP-9 deletion in young mice reduces LV rupture rates compared with wild type (WT) mice and attenuates LV dilation and collagen deposition post-MI (11). MMP-9 deletion also improves LV function post-MI by increasing neovascularization in the remodeling myocardium (12).
Aging is a major risk factor for MI, and MMP-9 levels increase with age. Therefore, it is important to investigate the effects of MMP-9 deletion post-MI in an aging environment. In this study, we hypothesized that the MMP-9 deletion would improve LV remodeling post-MI in aged mice.
Methods
Mice
All animal procedures were performed based on the “Guide for the Care and Use of Laboratory Animals” and were approved by the Institutional Animal Care and Use Committees at the University of Texas Health Science Center at San Antonio and the University of Mississippi Medical Center. Adult C57BL/6J WT (n = 55, 27 males and 28 females) and MMP-9 Null (Null; n = 85, 33 males and 52 females) 11- to 36-month-old mice were compared at Day 7 post-MI. An additional 12 WT (4 males and 8 females) and 11 Null (4 males and 7 females) 3- to 6-month-old mice were used as reference young MI groups. For macrophage isolation, we used 12 WT (6 males and 6 females) and 12 Null (6 males and 6 females) 15- to 24-month-old mice. A total of 187 mice were used for this study. All MMP-9 Null mice were genotyped prior to experimental use and showed no MMP-9 gene expression.
Echocardiography
Mice were anesthetized with 1%–2% isoflurane in an oxygen mix. Heart rate, respiratory rate, and body temperature were continuously monitored throughout the procedure to ensure an adequate depth of anesthesia. Transthoracic echocardiography measurements were acquired using the Vevo 2100 system (VisualSonics). Measurements were acquired from the LV parasternal long axis (B-mode). For each variable, images from three cardiac cycles were analyzed and averaged for each mouse.
Myocardial Infarction Surgery
Before surgery, mice were given buprenorphine (0.05–0.1mg/kg, i.p.). Mice were anesthetized with 2% isoflurane in an oxygen mix and intubated. After skin incision, pectoralis muscles were retracted and an intercostal incision was made. The left descending coronary artery was permanently ligated with 8-0 prolene suture. The ribs were closed using 6-0 prolene suture, muscles were placed back, and skin was closed using 6-0 prolene suture. Mice were recovered under an oxygen mix until mobile. Mice were monitored daily for the event of death, and autopsy was performed when needed.
Tissue Collection
At Day 7 post-MI, mice were euthanized under isoflurane anesthesia. At sacrifice, heparin (4 µg/g body weight, i.p.) was injected, and after 5 min, blood was collected from the common carotid artery and centrifuged for plasma MMP-9 measurements. The heart was removed from the chest cavity and flushed with cardioplegic solution to arrest the heart in diastole. The right ventricle was dissected, and the LV was cut into three sections: base, middle section, and apex. The right ventricle and the three LV sections were stained with 1% 2,3,5-triphenyl-tetrazolium chloride for 5min and photographed for infarct area measurements. The middle section was fixed in 10% zinc formalin, and the base and apex were snap frozen for RNA extraction.
MMP-9 Measurements
Plasma samples (100 µL) were analyzed for MMP-9. Concentrations were measured by a clinical laboratory improvement amendments–certified biomarker testing laboratory using an immunoassay.
RT2 ProfileTM PCR Array
The infarct area was separated from the noninfarct region, and only the infarct area was analyzed. RNA was extracted using TRIzol Reagent (Invitrogen, 15596). Commercially available inflammatory cytokines and receptors (Qiagen, PAMM-013E) and ECM (Qiagen, PAMM-011E) arrays were used to measure the expression of inflammatory cytokines and kinetics in ECM. All data are reported as 2−ΔCt values normalized results to Hprt1 housekeeping gene. The experiments were performed according to the minimum information for publication of quantitative real-time polymerase chain reaction (RT-PCR) experiments guidelines with one exception. Hprt1 was the only reference gene showing no change in expression (GusB, Hsp90ab1, Actb, and Gapdh were all significantly changed post-MI).
Macrophage Isolation and Phenotyping
The LV tissue was dissociated using 600U/mL collagenase type 2 (Worthington Biochemicals, CLS-2) and 60U/mL DNase1 (AppliChem, A3778.0500). Cells were washed and resuspended in cold phosphate buffered saline with 0.5% bovine serum albumin and 2mM ethylenediaminetetraacetic acid. Macrophages were isolated using CD11b microbeads (Miltenyi Biotec, 130-049-601). RNA extraction was performed using TRIzol Reagent (Invitrogen, 15596), and cDNA was synthesized using the High Capacity RNA to cDNA Kit (Applied Biosystems, 4387406). Taqman gene expression assays (Applied Biosystems) were used to evaluate mRNA expression of M1 (Ccl3, Mm00441259_g1; IFN-γ, Mm01168134_m1; IL-1β, Mm01336189_m1; IL-6, Mm00446190_m1; and TNF-α, Mm00443258_m1) and M2 (Arginase-1, Mm00443258_m1; CD163, Mm00474091_m1; Mannose receptor 1, Mm00485148_m1; TGF-β1, Mm01178820_m1; and Ym1, Mm00657889_mH) macrophage markers. Hprt1 served as the housekeeping control gene.
Histology and Immunohistology
LV sections were deparaffinazed in citrisolv and ethanol. Sections were stained with picrosirius red to visualize collagen content. Immunohistochemistry was performed with the use of a Vectastain ABC Kit (Vector Laboratories). HistoMark Black (KPL 54-75-00) was used to evaluate positive staining. Eosin was used to counterstain. An antibody specific for macrophages (anti-Mac-3, Cedarlane, 1:100 dilution) was used to selectively detect macrophages.
Statistical Analyses
All analyses were performed blinded to groups, and data are presented as mean ± SEM. Comparisons between groups of WT and Null mice were made using Student’s t test for continuous measurements and Fisher’s Exact Test for numbers of cardiac rupture rates. Survival curves were estimated by Kaplan–Meier survival analysis and compared by the log-rank test. Scatterplots for plasma MMP-9 levels, end-systolic volume, end-diastolic volume, ejection fraction, and scar collagen content were analyzed using linear regression by age. Analyses were done using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com); a p value of <.05 was considered statistically significant in all analyses. For the heatmap analysis, WT and Null mice were grouped into three age groups (11–15, 16–24, and >24 months of age) and analyzed by analysis of variance. Heatmaps were generated using Matlab R2011 and R 3.0.1.
Results
Post-MI MMP-9 Levels Increased With Age
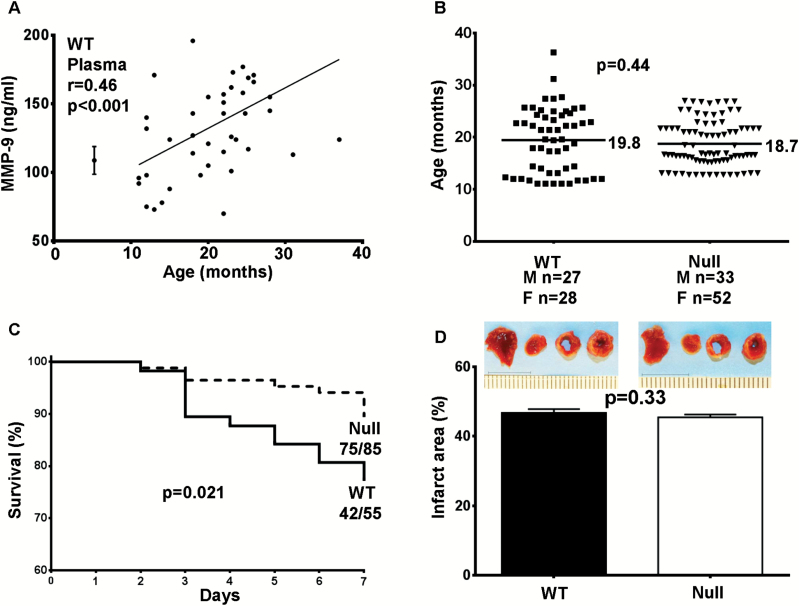
We have shown that MMP-9 expression increases with aging, in both plasma and LV (9,10). Post-MI in young mice, MMP-9 levels peak at Days 2–4, and subsequently decrease to baseline levels by Day 14 (13). As shown in Figure 1A, post-MI plasma MMP-9 concentrations were increased over young reference concentrations, and there was a positive linear correlation between Day 7 post-MI plasma concentrations and age (r = .46, p < .001).
Figure 1.
(A) In WT mice, matrix metalloproteinase (MMP)-9 plasma levels positively correlate with age at Day 7 post–myocardial infarction (MI, r = .46, p = .001). The young 3- to 6-month old reference controls are shown on the left plotted as mean ± SEM concentrations. MMP-9 plasma levels in Null mice were below detection. (B) We enrolled 27 WT males and 28 females and 33 Null males and 52 females. The average age for WT was 19.8 months of age and 18.7 months of age for Null. (C) MMP-9 deletion resulted in significantly improved survival at Day 7 post-MI (76% for WT vs 88% for Null, p = .021). (D) The infarct area was similar between WT and Null mice at Day 7 post-MI (47% ± 7% for WT, n = 42 and 46% ± 6% for Null, n = 75; p = .33).
MMP-9 Deletion Improved Survival in Aged Mice Post-MI
In humans, higher post-MI plasma MMP-9 concentrations correlate with increased mortality (14). In young 3- to 6-month-old post-MI mice, MMP-9 deletion improves survival (15). The average age for the mice enrolled in this study was 19.8 months of age for WT mice and 18.7 months of age for Null mice (Figure 1B, p = .44). By Kaplan–Meier test, the survival rates were not different between sexes (p = not significant). For this reason, survival is shown combined for males and females. Out of 55 WT mice, 42 (76%) survived to Day 7 post-MI. Null mice showed significantly improved survival (75 out of 85 mice, 88%) over 7 days post-MI (Figure 1C, p = .021). Infarct areas were similar between WT and Null mice (Figure 1D, 47% ± 7% for WT and 46% ± 6% for Null; p = .33), indicating that the MMP-9 deletion had no effect on infarct expansion. WT and Null mice showed comparable rupture rates post-MI (4 out of 13 [30%] WT ruptured and 1 out of 10 [10%] Null ruptured; p = .36). These data indicated that MMP-9 deletion improved survival post-MI in aged mice beyond effects on infarct rupture.
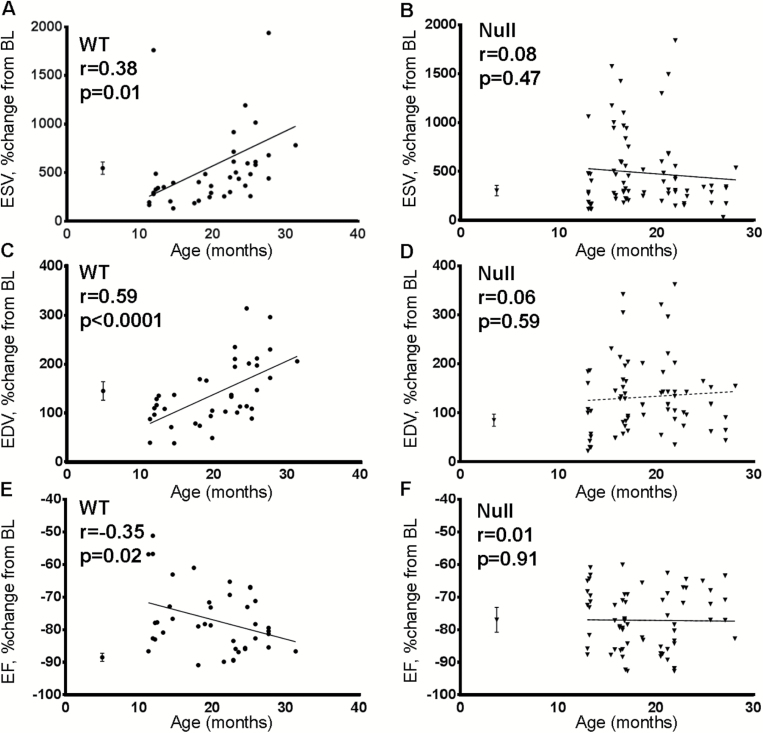
MMP-9 Deletion Attenuated Age-Dependent Increase in LV Dilation and Decrease in LV Function at Day 7 Post-MI
By regression analysis, end-systolic volume and end-diastolic volume showed a strong age-dependent post-MI increase in WT but not Null mice (Figure 2A–D). LV systolic function by ejection fraction decreased with age in WT mice, whereas Null mice showed similar ejection fraction in all ages (Figure 2E and F). These effects were not due to potential differences in heart rate, as all age groups had similar heart rate (465±10 bpm for WT, 485±9 bpm for Null, p = .19).
Figure 2.
(A) and (C) WT mice showed linear age-related increase in the extent of post–myocardial infarction (MI) left ventricle (LV) dilation, as evidenced by increases in the percent change from baseline of end-systolic volume (ESV) and end-diastolic volume (EDV) with age (n = 42). (B) and (D) Matrix metalloproteinase (MMP)-9 deletion abolished the age relationship (n = 75). (E) WT mice showed age-associated decrease in ejection fraction, and (F) MMP-9 deletion removed this effect. The young 3- to 6-month old reference controls are shown on the left of each graph, plotted as mean ± SEM values. BL—baseline.
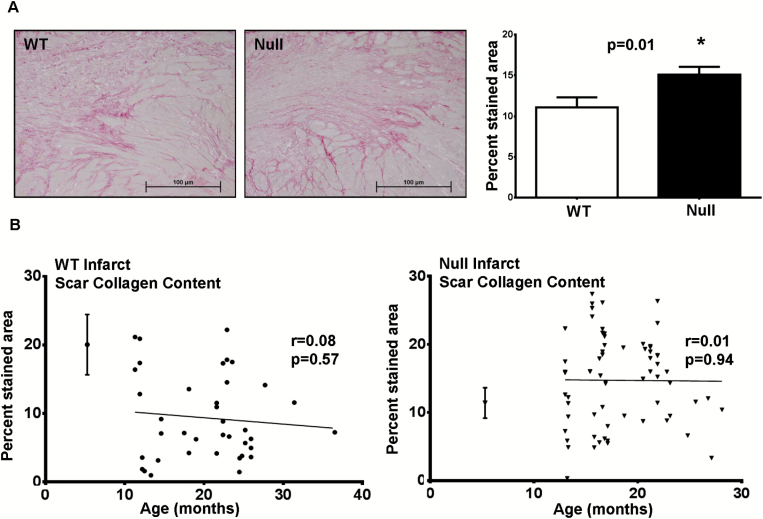
MMP-9 Deletion Improved Scar Formation Through Increased Collagen Deposition
Post-MI
Aging is associated with increased collagen deposition, and MMP-9 deletion attenuates this effect (9). Interesting, post-MI Null mice showed significantly higher collagen content post-MI compared with WT mice (Figure 3A, p = .01), and we did not observe an age-associated relationship in collagen deposition in the WT or Null mice post-MI (Figure 3B).
Figure 3.
(A) At Day 7 post–myocardial infarction (MI), matrix metalloproteinase (MMP)-9 deletion resulted in increased collagen accumulation. Representative photomicrographs of picrosirius red stained border zone areas are shown on the left. Images were randomly acquired from the border zone through the infarct area to the other border zone. Quantification is shown on the right for WT (n = 42) and Null (n = 75) left ventricle (LV). (B) WT and Null mice did not show age-dependent effects on collagen deposition (r = .08 for WT and r = .01 for Null), but levels were higher at all ages for the Null. The young reference controls are shown on the left of each graph, plotted as mean ± SEM values.
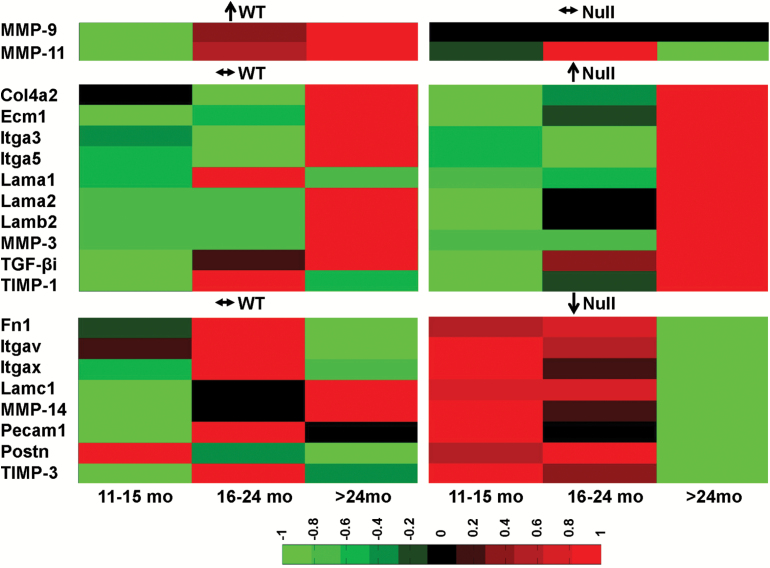
To further investigate molecular mechanisms, we performed expression analysis for 84 ECM and adhesion molecules genes (Figure 4). By ECM array analysis of post-MI infarct tissue, only two genes in the WT (MMP-9 and MMP-11) and 18 genes in the Null showed age dependence. Both MMP-9 and MMP-11 positively correlated with age in the WT mice. In the Null mice, 10 genes (collagen type 4 alpha 2, ECM protein 1, integrin alpha [Itga] 3, Itga5, laminin [Lam] alpha 1, Lam alpha 2, Lam beta 2, MMP-3, TGF-β-induced, and tissue inhibitor of matrix metalloproteinase [TIMP]-1) positively associated with age and eight genes (fibronectin 1, ItgaV, ItgaX, Lam gamma 1, MMP-14, platelet endothelial cell adhesion molecule 1 (Pecam1), periostin, and TIMP-3) negatively associated with age. These genes are responsible for angiogenesis and scar formation, indicating that MMP-9 deletion mediates LV remodeling by modifying these specific aspects of the wound healing response.
Figure 4.
Matrix metalloproteinase (MMP)-9 and -11 mRNA increased with age in the WT infarct. Collagen type 4 alpha 2 (Col4a2), extracellular matrix protein 1 (Ecm1), integrin alpha (Itga) 3, Itga5, laminin (Lam) alpha 1, Lam alpha 2, Lam beta 2 (Lamb2), MMP-3, TGF-β-induced (TGF-βi), and tissue inhibitor of matrix metalloproteinase (TIMP)-1 mRNA increased with age in the Null infarct. Null mice also showed age-dependent decrease in fibronectin 1 (Fn1), ItgaV, ItgaX, Lam gamma 1, MMP-14, platelet endothelial cell adhesion molecule 1 (Pecam1), periostin (Postn), and TIMP-3 mRNA. Sample sizes are n = 22 for WT and n = 35 for Null. ↑: gene expression increased with age (by regression analysis); ↓: gene expression decreased with age (by regression analysis); ↔: gene expression did not change with age (by regression analysis).
MMP-9 Deletion Reduced Inflammation in the Post-MI LV Infarct
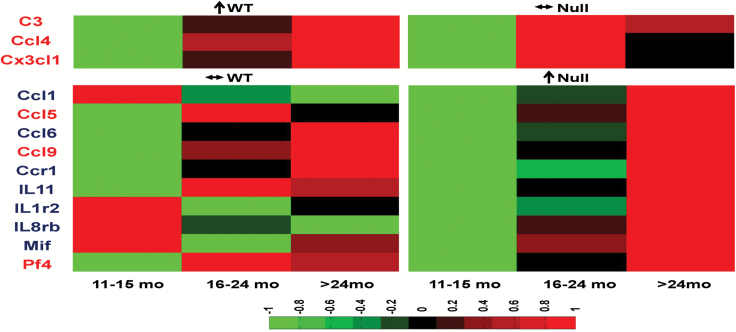
Because aging and MI are associated with increased inflammation (16), we investigated the effects of MI superimposed on aging on inflammation (Figure 5). By inflammatory array analysis of infarct tissue, 3 of 84 genes increased in an age-dependent manner in the WT (complement component 3, Ccl4, and chemokine [C-X3-C motif] ligand 1 [Cx3cl1]). All three genes are proinflammatory mediators. A total of 10 genes increased in an age-dependent manner in the Null (Ccl1, Ccl5, Ccl6, Ccl9, CC chemokine receptor 1, interleukin [IL]11, IL1 receptor 2, IL8 receptor beta, macrophage inhibitory factor [Mif], and platelet factor 4 [Pf4]). Of these, three are proinflammatory (Ccl5, Ccl9, and Pf4) and seven are anti-inflammatory (Ccl1, Ccl6, Ccr1, IL11, IL1 receptor 2, IL8 receptor beta, and Mif). All of the 13 genes showed distinct patterns between WT and Null mice. The altered genes are all associated with macrophage functions, which indicated that macrophage infiltration or polarization may be MMP-9 dependent.
Figure 5.
WT mice showed age-dependent increased expression of complement component 3 (C3), CC chemokine ligand (Ccl) Ccl4, and chemokine (C-X3-C motif) ligand 1 (Cx3cl1). Null mice showed age-dependent increased expression of Ccl1, Ccl5, Ccl6, Ccl9, CC chemokine receptor 1 (Ccr1), interleukin (IL)-1, IL-1 receptor 2, IL8 receptor beta, macrophage inhibitory factor (Mif), and platelet factor 4 (Pf4). Genes in red font are proinflammatory, and genes in blue font are anti-inflammatory. Sample sizes are n = 22 for WT and n = 35 for Null. ↑: gene expression increased with age (by regression analysis); ↔: gene expression did not change with age (by regression analysis).
MMP-9 Deletion Promoted Anti-Inflammatory M2 Polarization in Macrophages at Day 7 Post-MI
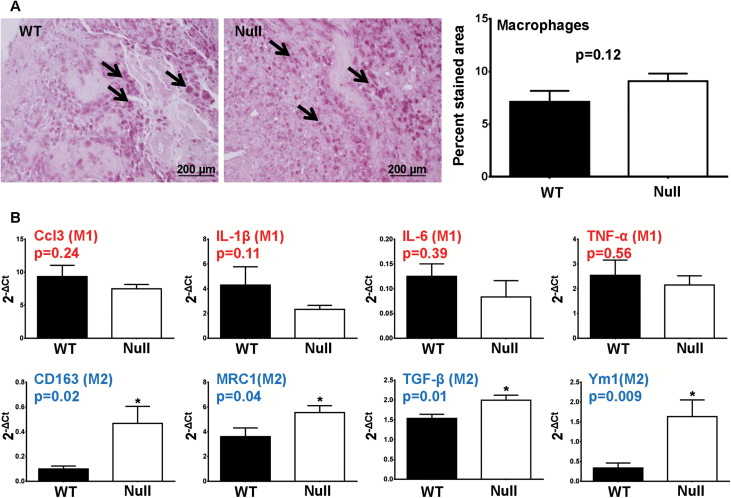
We investigated the effect of MMP-9 deletion on post-MI macrophage infiltration and polarization. Mac-3 immunohistochemical staining showed that WT and Null mice had similar numbers of macrophages at Day 7 post-MI (Figure 6A, p = .12), indicating that macrophage infiltration was not altered by MMP-9 deletion. To investigate the effect of MMP-9 deletion on macrophage polarization, we isolated macrophages from the infarcts of WT and Null mice at Day 7 post-MI. Our quantitative RT-PCR data showed that at Day 7 post-MI, MMP-9 deletion had no effect on any of the five M1 markers (Figure 6B, top panel, p > .05) but increased four of the five M2 markers (CD163, mannose receptor 1, TGF-β1, and YM1; Figure 6B, bottom panel, all p < .05). The M2 marker CD163 was not changed. At Day 7 post-MI, CD11b positive cell numbers were similar between WT and Null mice (p = .29), which was consistent with our histological measurements that showed no difference in macrophage numbers. Based on M2 marker expression (MRC1 and TGF-β1 in particular), the number of M2 macrophages in the Null mice was 15% higher than in WT mice. These results revealed that although MMP-9 deletion in aged mice at Day 7 post-MI had no effect on M1 polarization, it promoted M2 macrophage polarization.
Figure 6.
(A) Representative photomicrographs for macrophage (Mac-3) immunohistochemistry in WT and Null mice at Day 7 post–myocardial infarction (MI; left, arrows show positively stained macrophages). WT (n = 42) and Null (n = 75) mice showed similar numbers of macrophages in the infarct (right, p = .12). (B) Matrix metalloproteinase (MMP)-9 deletion did not affect the expression of M1 markers in isolated macrophages from the left ventricle (LV) infarcts at Day 7 post-MI (upper panel) but have promoted the M2 polarization (lower panel). The red font indicates M1 markers, and the blue font indicates M2 makers. Samples sizes are n = 12 for WT and n = 12 for Null.
Discussion
In this study, we investigated whether MMP-9 deletion improved LV remodeling post-MI in aged mice. The major findings were (a) plasma MMP-9 concentrations increased post-MI in an age-dependent manner, (b) MMP-9 deletion attenuated the age-dependent increase in LV dilation and decrease in LV function at Day 7 post-MI, and (c) MMP-9 deletion attenuated age-associated inflammation post-MI by stimulating M2 anti-inflammatory macrophage polarization rather than inhibiting M1 proinflammatory macrophage polarization. Combined, our results reveal MMP-9-dependent mechanisms through which aging impairs wound healing in the post-MI LV (Figure 7).
Figure 7.
Mechanistic figure showing the effects of matrix metalloproteinase (MMP-9) deletion on myocardial infarction (MI) superimposed in an aging environment. Aging and MI lead to an increased MMP-9 production and increased inflammation to impair scar formation and amplify dysfunction. MMP-9 deletion attenuated inflammation through M2 macrophage polarization to improve scar formation and attenuate left ventricle (LV) dysfunction post-MI.
Aging and MI are associated with an increase in MMP-9 expression (9). In humans, plasma MMP-9 levels positively correlate with severity of coronary artery lesions, the progression of left ventricular dilation, and survival rate (17). Our data extend previous findings by demonstrating that MMP-9 effects are both MI and age dependent. Similar to young mice, MMP-9 deletion improved post-MI survival in the aged group, indicating that therapies targeting MMP-9 would likely have a uniform effect across the age spectrum, if not a more pronounced effect in aged subjects (12). Functional analysis of the LV revealed that post-MI WT mice showed an age-dependent increase in LV volumes and decrease in ejection fraction, an effect attenuated by MMP-9 deletion. These findings correlate to human data, in which older patients are particularly prone to developing heart failure post-MI (18,19).
We previously reported that aging alone is associated with an increase in collagen deposition, and MMP-9 deletion attenuates this effect (9). Although the Day 7 infarct in both WT and Null mice showed no age-dependent correlation with collagen deposition, the amount of collagen deposition was higher in the Null mice at every age. This is opposite of what we and others have reported previously for the young MI mice (11). These findings could be explained by delayed collagen synthesis in older fibroblasts from post-MI WT mice due to a decreased responsiveness to TGF-β stimulation (20). Recently, Zhu and coworkers (21) have reported a decrease in collagen synthesis in senescent cardiac fibroblasts post-MI, consistent with this theory. Out of eight collagen genes measured (collagens 1α1, 2α1, 3α1, 4α1, 4α2, 4α3, 5α1, and 6α1), the Null mice showed an increase in collagen 4α2. TGF-β-induced protein gene expression was also increased, and both genes link to the increased total collagen content in the Null mice (22). Fibronectin 1 and periostin mediate cell adhesion, motility, cell-mediated matrix assembly, and collagen type I deposition aspects of ECM remodeling, and a decrease in the expression of these genes at Day 7 post-MI in aged Null mice indicates that a stable scar has already formed (23,24). Increased collagen synthesis at Day 7 post-MI can contribute to a stiffer infarct, which would attenuate LV dilation and may in part explain the better survival and improved LV function post-MI in the Null mice.
In addition to the age-dependent increase in MMP-9 post-MI, WT mice showed an age-dependent increase in MMP-11. Unlike the majority of other MMPs, MMP-11 is released to the ECM in an active form, which may contribute to increased proteolytic activity, unstable scar, and enhanced inflammation (17). We observed a compensatory increase in MMP-3 and decrease in MMP-14 in the Null mice. Although the role of MMP-3 in LV remodeling is unknown, MMP-14 positively associates with mortality post-MI, thus, lower MMP-14 gene levels in the Null mice provide a mechanism for improved survival (25–27). Additionally, the well-established profibrotic marker TIMP-1 was also increased in the Null mice at Day 7 post-MI, which correlated with attenuated LV dilation (28).
TIMP-3 is important for angiogenesis, as TIMP-3 inhibition blunts capillary morphogenesis in vitro and angiogenesis in vivo (29). Decreased TIMP-3 expression in the Null mice may explain the better angiogenesis response previously reported for the Null mice (10). The Null mice also showed increased expression of Ecm1, Itga3, Itga5, Lama1, Lama2, and Lamb2 genes, which are responsible for angiogenic processes and neovascularization (30–32). Pecam1 was reduced in the Null mice, and reduced Pecam1 expression may explain the switch from proinflammatory M1 macrophages to anti-inflammatory M2 macrophages in the Null post-MI LV (33). These findings confirm our previous data of improved angiogenesis in aging Null mice and in young Null mice post-MI (10,12).
The inflammatory profile of aged WT and Null mice revealed gene differences that were all directly associated with macrophage function. Each of the genes increased in WT mice were proinflammatory, whereas in Null mice, 7 out of 10 genes were anti-inflammatory and 3 proinflammatory. Out of the inflammatory gene changes, MMP-9 is known to proteolytically process Ccl4, Cx3cl1, Pf4, IL8, and TGF-β1, all of which can directly regulate macrophage polarization (34–37). MMP-9, therefore, may indirectly regulate macrophage polarization by proteolytically processing cytokine substrates. The number of macrophages in aged mice post-MI was not affected by MMP-9 deletion, indicating that MMP-9 deletion contributes to macrophage polarization rather than infiltration. Macrophages express different polarization phenotypes post-MI, including proinflammatory M1 and anti-inflammatory M2 phenotypes. Proinflammatory M1 macrophages dominate early post-MI and their major role is to amplify the inflammatory response, whereas M2 anti-inflammatory macrophages become more active at days 4–7 post-MI and their major roles are to activate endothelial cells and myofibroblasts and engulf apoptotic neutrophils (11,38). In aged mice post-MI, MMP-9 deletion had little to no effect on M1 polarization but significantly increased the expression of M2 phenotype markers. These findings showed that with aging, MMP-9 deletion promotes M2 macrophage polarization, which may explain the increased survival and improved LV remodeling. The anti-inflammatory macrophages that prevail in the WT infarcts from Day 4 contain lower content of inflammatory molecules and proteases and increased content of growth factors (39). Nahrendorf and colleagues (40) showed that anti-inflammatory macrophages populate infarcts at Day 4 post-MI and release vascular endothelial growth factor and TGF-β. The prevalence of anti-inflammatory M2 macrophages in Null mice at Day 7 post-MI may provide an additional explanation for the higher collagen content. The increased expression of TGF-β1 in isolated macrophages and a strong age-dependent correlation of proangiogenic markers at Day 7 post-MI in the infarcts of Null mice indicate that MMP-9 deletion improves cardiac remodeling and function post-MI by altering both scar formation and angiogenic potential. Increased expression of IL-10 and TGF-β in Null macrophages may promote the transition of cardiac fibroblasts toward a profibrotic phenotype and reveal M2 macrophage–dependent mechanisms mediated by MMP-9. However, further investigation of the mechanisms by which M2 macrophages determine dynamic plasticity of cardiac fibroblasts is needed.
We have demonstrated that MMP-9 deletion promotes M2 anti-inflammatory macrophage polarization at Day 7 post-MI in aged mice. Whether the effects of MMP-9 deletion on macrophage polarization is indirectly due to alterations in the ECM, or whether this effect is directly related to effects on the macrophage itself (by proteolytically processing cytokine and chemokine substrates or a yet unrecognized role of intracellular MMP-9 inside the macrophages) is the focus of future studies. In conclusion, our combined data reveal a negative role for MMP-9 in the aging post-MI LV. Targeting MMP-9 to regulate macrophage polarization may provide a novel therapeutic approach for the aging post-MI patient.
Funding
We acknowledge support from the American Heart Association (13POST14350034 to K.Y.D.-P.). We acknowledge support from National Institutes of Health, National Heart, Lung, and Blood Institute SC2 (HL101430 to Y.-F. J., HL095852 to H.-C.H.), support from National Institutes of Health, National Center for Complementary and Integrative Health (R00AT006704 to G.V.H.), and from National Institutes of Health HHSN 268201000036C (N01-HV-00244) for the San Antonio Cardiovascular Proteomics Center and R01HL075360 and from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award (5I01BX000505 to M.L.L.). We also acknowledge support from National Institutes of Health HL051971 and GM104357.
Acknowledgments
We thank Elizabeth R. Flynn, Dustin R. Bratton, Courtney A. Cates, Wesley Lowell, and Jianhua Zhang for technical assistance in this study.
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi:10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi:10.1016/S0140-6736(03)12113-7 [DOI] [PubMed] [Google Scholar]
- 3. Widimsky P, Wijns W, Fajadet J, et al. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur Heart J. 2010;31:943–957. doi:10.1093/eurheartj/ehp492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gharacholou SM, Alexander KP, Chen AY, et al. Implications and reasons for the lack of use of reperfusion therapy in patients with ST-segment elevation myocardial infarction: findings from the CRUSADE initiative. Am Heart J. 2010;159:757–763. doi:10.1016/j.ahj.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 5. Schwark C, Schellinger PD. Is old age really a reason to withhold thrombolytic therapy? J Neurol Neurosurg Psychiatry. 2006;77:289. doi:10.1136/jnnp.2005.080192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelly DJ, Gershlick T, Witzenbichler B, et al. Incidence and predictors of heart failure following percutaneous coronary intervention in ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. Am Heart J. 2011;162:663–670. doi:10.1016/j.ahj.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 7. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi:10.1161/CIR.0b013e31823ac046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase-9: many shades of function in cardiovascular disease. Physiology (Bethesda). 2013;28:391–403. doi:10.1152/physiol.00029.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiao YA, Ramirez TA, Zamilpa R, et al. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovasc Res. 2012;96:444–455. doi:10.1093/cvr/cvs275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yabluchanskiy A, Ma Y, Chiao YA, et al. Cardiac aging is initiated by matrix metalloproteinase-9 mediated endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2014; 306:H1398-H407. doi:10.1152/ajpheart.00090.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ducharme A, Frantz S, Aikawa M, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi:10.1172/JCI8768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lindsey ML, Escobar GP, Dobrucki LW, et al. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;290:H232–H239. doi:10.1152/ajpheart.00457.2005 [DOI] [PubMed] [Google Scholar]
- 13. Spinale FG, Coker ML, Bond BR, Zellner JL. Myocardial matrix degradation and metalloproteinase activation in the failing heart: a potential therapeutic target. Cardiovasc Res. 2000;46:225–238. doi:10.1016/S0008-6363(99)00431-9 [DOI] [PubMed] [Google Scholar]
- 14. Li PJ, Yang XH, Zhang LP, Cao W, Qin J, Yao W. [Clinical significance of soluble selectins and matrix metalloproteinases-9 in patients after successful cardiopulmonary resuscitation]. Zhongguo wei zhong bing ji jiu yi xue. 2004;16:137–141. [PubMed] [Google Scholar]
- 15. Ramirez TA, Iyer RP, Ghasemi O, et al. Aliskiren and valsartan mediate left ventricular remodeling post-myocardial infarction in mice through MMP-9 effects. J Mol Cell Cardiol. 2014;72:326–335. doi:10.1016/j.yjmcc.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–511. doi:10.1016/j.yjmcc.2009.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Phatharajaree W, Phrommintikul A, Chattipakorn N. Matrix metalloproteinases and myocardial infarction. Can J Cardiol. 2007;23:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao L, Hu X, Liu YQ, Xue Q, Feng QZ. Percutaneous coronary intervention in the elderly with ST-segment elevation myocardial infarction. Clin Interv Aging. 2014;9:1241–1246. doi:10.2147/CIA.S62642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Velders MA, James SK, Libungan B, et al. Prognosis of elderly patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention in 2001 to 2011: a report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) registry. Am Heart J. 2014;167:666–673. doi:10.1016/j.ahj.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 20. Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–1392. doi:10.1016/j.jacc.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu F, Li Y, Zhang J, et al. Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS One. 2013;8:e74535. doi:10.1371/journal.pone.0074535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ikeuchi M, Tsutsui H, Shiomi T, et al. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc Res. 2004;64:526–535. doi:10.1016/j.cardiores.2004.07.017 [DOI] [PubMed] [Google Scholar]
- 23. van Dijk A, Niessen HW, Ursem W, Twisk JW, Visser FC, van Milligen FJ. Accumulation of fibronectin in the heart after myocardial infarction: a putative stimulator of adhesion and proliferation of adipose-derived stem cells. Cell Tissue Res. 2008;332:289–298. doi:10.1007/s00441-008-0573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh M, Foster CR, Dalal S, Singh K. Osteopontin: role in extracellular matrix deposition and myocardial remodeling post-MI. J Mol Cell Cardiol. 2010;48:538–543. doi:10.1016/j.yjmcc.2009.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaplan RC, Smith NL, Zucker S, Heckbert SR, Rice K, Psaty BM. Matrix metalloproteinase-3 (MMP3) and MMP9 genes and risk of myocardial infarction, ischemic stroke, and hemorrhagic stroke. Atherosclerosis. 2008;201:130–137. doi:10.1016/j.atherosclerosis.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spinale FG, Mukherjee R, Zavadzkas JA, et al. Cardiac restricted overexpression of membrane type-1 matrix metalloproteinase causes adverse myocardial remodeling following myocardial infarction. J Biol Chem. 2010;285:30316–30327. doi:10.1074/jbc.M110.158196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romanic AM, Burns-Kurtis CL, Gout B, Berrebi-Bertrand I, Ohlstein EH. Matrix metalloproteinase expression in cardiac myocytes following myocardial infarction in the rabbit. Life Sci. 2001;68:799–814. doi:10.1016/S0024-3205(00)00982-6 [DOI] [PubMed] [Google Scholar]
- 28. Ikonomidis JS, Hendrick JW, Parkhurst AM, et al. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol. 2005;288:H149–H158. doi:10.1152/ajpheart.00370.2004 [DOI] [PubMed] [Google Scholar]
- 29. BenEzra D. Inhibition of angiogenesis by tissue inhibitor of metalloproteinase-3. Invest Ophthalmol Vis Sci. 1997;38:2433–2434. [PubMed] [Google Scholar]
- 30. Ma Y, de Castro Brás LE, Toba H, et al. Myofibroblasts and the extracellular matrix network in post-myocardial infarction cardiac remodeling. Pflugers Arch. 2014;466:1113–1127. doi:10.1007/s00424-014-1463-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Belkin AM, Stepp MA. Integrins as receptors for laminins. Microsc Res Tech. 2000;51:280–301. doi:10.1002/1097-0029(20001101)51:3<280::AID-JEMT7>3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- 32. Han Z, Ni J, Smits P, et al. Extracellular matrix protein 1 (ECM1) has angiogenic properties and is expressed by breast tumor cells. FASEB J. 2001;15:988–994. doi:10.1096/fj.99-0934com [DOI] [PubMed] [Google Scholar]
- 33. Serebruany VL, Gurbel PA. Effect of thrombolytic therapy on platelet expression and plasma concentration of PECAM-1 (CD31) in patients with acute myocardial infarction. Arterioscler Thromb Vasc Biol. 1999;19:153–158. doi:10.1161/01.ATV.19.1.153 [DOI] [PubMed] [Google Scholar]
- 34. Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi:10.1016/j.pharmthera.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Starr AE, Dufour A, Maier J, Overall CM. Biochemical analysis of matrix metalloproteinase activation of chemokines CCL15 and CCL23 and increased glycosaminoglycan binding of CCL16. J Biol Chem. 2012;287:5848–5860. doi:10.1074/jbc.M111.314609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van den Steen PE, Husson SJ, Proost P, Van Damme J, Opdenakker G. Carboxyterminal cleavage of the chemokines MIG and IP-10 by gelatinase B and neutrophil collagenase. Biochem Biophys Res Commun. 2003;310:889–896. [DOI] [PubMed] [Google Scholar]
- 37. Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]
- 38. Wan E, Yeap XY, Dehn S, et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res. 2013;113:1004–1012. doi:10.1161/CIRCRESAHA.113.301198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. 2013;112:1624–1633. doi:10.1161/CIRCRESAHA.113.300890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi:10.1084/jem.20070885 [DOI] [PMC free article] [PubMed] [Google Scholar]