Abstract
An experimental model of infection with Nocardia brasiliensis, used as an example of a facultative intracellular pathogen, was tested. N. brasiliensis was injected into the rear foot pads of BALB/c mice to establish an infection. Within 30 days, infected animals developed a chronic actinomycetoma infection. Batch cultures of N. brasiliensis were used to purify P61, P38, and P24 antigens; P61 is a catalase, and P38 is a protease with strong caseinolytic activity. Active and passive immunizations of BALB/c mice with these three purified soluble antigens were studied. Protection was demonstrated for actively immunized mice. However, immunity lasted only 30 days. Other groups of immunized mice were bled at different times, and their sera were passively transferred to naive recipients that were then infected with N. brasiliensis. Sera collected 5, 6, and 7 days after donor immunization conferred complete, long-lasting protection. The protective effect of passive immunity decreased when sera were collected 2 weeks after donor immunization. However, neither the early sera (1-, 2-, and 3-day sera) nor the later sera (30- or 45-day sera) prevented the infection. Hyperimmune sera with the highest levels of immunoglobulin G (IgG) to N. brasiliensis antigens did not protect at all. The antigens tested induced two IgM peaks. The first peak was present 3 days after immunization but was not antigen specific and did not transfer protection. The second peak was evident 7 days after immunization, was an IgM response, was antigen specific, and conferred protection. This results clearly demonstrate that IgM antibodies protect the host against a facultative intracellular bacterium.
Protective immunity against infectious microorganisms plays a major role in the eradication or control of several infectious diseases. To date, vaccines have been successful in inducing long-lasting protection, mainly because humoral immunity is induced. Immunoglobulin G (IgG) antibodies against bacterial products such as toxins are effective in neutralizing their toxic effects. IgG antibodies can also neutralize viruses and opsonize bacteria to promote phagocytosis. In experimental infections with lymphocytic choriomeningitis virus, IgM antibodies have been proven to control early infection (15). It has also been shown that natural antibodies play a critical role in preventing viral and bacterial dissemination and enhance antigen trapping in secondary lymphoid organs (7).
The role of antibodies in host protection in facultative intracellular infections was studied with Nocardia asteroides, and the conclusion was that humoral immunity did not participate in protecting the infected host (1). It was claimed that antibodies and B lymphocytes may even have worsened the experimental infections (8). In chronic infections with intracellular microbes, the induction of effective cell-mediated immunity is required to control disease (2, 4). Mycobacterium tuberculosis, Mycobacterium leprae, Nocardia asteroides, N. brasiliensis, and Listeria monocytogenes are examples of intracellular microbes. Chronic infections with these intracellular microbes induce strong antibacterial antibody responses that probably play little or no role in host protection.
Mycetoma is a chronic infectious disease that may be produced by fungi (eumycetoma) or by bacteria (actinomycetoma). In both cases, clinical signs of infection include swelling, abscess, and ulcers that discharge microcolonies of the etiologic agent. N. brasiliensis, Actinomadura madurae, and Streptomyces somaliensis are common agents producing actinomycetoma in humans. Salinas-Carmona et al. previously developed an experimental model of chronic intracellular infection in BALB/c mice by injecting N. brasiliensis ATCC 700358 in order to induce an actinomycetoma lesion (11). Immunodominant antigens from N. brasiliensis were identified in sera from infected mice as well as human patients with actinomycetoma (13). These immunodominant antigens included P61, P26, and P24, which were isolated and purified from N. brasiliensis cells (17). P24 antigen was later used to determine anti-N. brasiliensis antibody titers in an enzyme-linked immunosorbent assay (ELISA). With this technique, a clinical correlation between anti-P24 antibody concentrations and active infection was found (14). P38 purified from an N. brasiliensis crude cell extract is a protease with strong caseinolytic activity; when injected into BALB/c mice, this antigen induced protection (6). Passive humoral immunity was also transferred with sera from animals immunized with heat-killed N. brasiliensis. However, sera containing the highest levels of anti-N. brasiliensis IgG antibodies did not protect animals from infection (10).
In the present work, the facultative intracellular bacterium N. brasiliensis was injected into immunized and control mice to investigate the role of humoral immunity in host protection. We used three purified protein antigens for active and passive immunizations and found that complete protection was mediated by humoral immunity. For both actively and passively induced protection, we demonstrated that IgM antibodies were responsible for the protective effect. The presence of low-affinity polyreactive IgM antibodies against facultative intracellular bacteria is of the utmost importance in controlling infection. This is the first clear demonstration that humoral immunity is sufficient to prevent an experimental infection with an intracellular microbe, such as N. brasiliensis.
MATERIALS AND METHODS
Bacterial strain.
N. brasiliensis ATCC 700358 was isolated from a human patient with actinomycetoma. The bacterium was grown in brain heart infusion (BHI) medium (Difco Laboratories, Detroit, Mich.). Batch cultures to isolate and purify soluble antigens were prepared as previously described (17). Briefly, N. brasiliensis unicellular suspensions were incubated in 1-liter Erlenmeyer flasks with 170 ml of BHI medium for 7 days at 37°C under stationary conditions. Bacterial cells were washed with distilled water and defatted with ethanol-ethyl ether three times.
Animals.
We used 12-week-old male and female BALB/c mice. Animals were maintained under normal conditions with Purina rodent food and water available at libitum. Requirements for care and handling of experimental animals according to international and Mexican regulations (NOM-062-Z00-1999) were met.
Isolation and purification of soluble antigens.
Immunodominant antigens P61 and P24 were isolated as described elsewhere (9, 11). Briefly, N. brasiliensis ATCC 700358 bacterial mass was ground after being defatted as described above and suspended in 0.01 M Tris-HCl containing 0.01 M magnesium acetate. The suspension was magnetically stirred overnight at 4°C. The supernatant was collected after centrifugation and dialyzed for 2 days against distilled water at 4°C. The crude cellular extract (CCE) contained more than 30 bands, including the immunodominant antigens P61 and P24. The P24 protein was purified as previously described (17). After 50% ammonium sulfate precipitation, the supernatant was extensively dialyzed against water and lyophilized. The contents of each vial were resuspended in 1 ml of phosphate-buffered saline (pH 7.2) and digested for 2 h at 37°C with 150 μg of DNase I (Sigma Chemical Co., St. Louis, Mo.). After digestion, the samples were applied to a Sephadex G-100 column as previously described (17). Fractions containing purified P24 antigen were collected and used for an ELISA, for Western blotting, and for immuniziation of BALB/c mice. The ammonium sulfate precipitate was applied to a nondenaturating preparative polyacrylamide gel electrophoresis (PAGE) system as described elsewhere (17) and run at 150 V for 4 h. The P61 antigen showed a greenish color and was electroeluted from the gel at 120 V for 2 h and recovered from the cathodic chamber. This purified P61 antigen was used for an ELISA, for Western blotting, and for immunization of mice. P38 is a protease with high caseinolytic activity in a CCE. We resolved 1 mg of CCE/ml by preparative 12% PAGE under native conditions. The protease was eluted by gentle stirring at 4°C for 72 h and used for further testing.
Active immunization.
Ten 12-week-old mice per group were immunized once with 20 μg of purified antigen (P61, P38, or P24). Antigens were emulsified with incomplete Freund's adjuvant (IFA), and 0.1 ml was injected into the rear foot pad. Two weeks later, all animals in this experiment were reimmunized with 15 μg of the same antigen emulsified with IFA. Two weeks after the second injection, a third immunization was given with the homologous antigen emulsified with the same adjuvant. Mice were bled at various times, infected with 106 CFU of N. brasiliensis ATCC 700358, and examined daily for signs of infection. The control group was not immunized but was infected in the same manner on the same day under the same conditions as the experimental groups. In other experiments, animals were immunized only once with 20 μg of purified antigen emulsified with IFA and then infected at various times after this single immunization. Additional experiments were done with infection at 15 days, at 90 days, and at 120 days after immunization.
Passive immunization.
Twenty 12- to 20-week-old BALB/c mice per group were immunized only once with purified P61, P24, or P38 antigen emulsified with IFA as described above. These mice were used as donors of sera for passive humoral immunity experiments. Blood was drawn on days 1, 2, and 3 after the single immunization or on days 5, 6, and 7. In other experiments, donors were bled on days 14, 15, 16, or 45 after immunization. In all cases, sera were sterilized by Millipore membrane filtration (0.22-μm-pore size) and frozen at −20°C until used. BALB/c mice of the same ages as the donors were used as passive humoral immunity naive recipients. Ten mice per group were injected intraperitoneally every day with 0.1 ml of sterilized sera for three consecutive days. On the last day of injection, mice were infected with N. brasiliensis and examined daily for 90 days or more for signs of infection.
Experimental actinomycetoma infection.
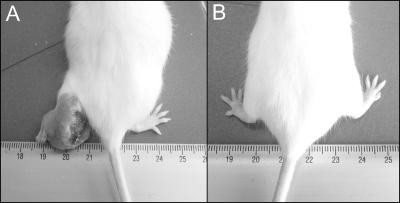
N. brasiliensis ATCC 700358 was cultured in BHI medium as described above to prepare a unicellular suspension that contained 107 CFU/ml. A 4-day culture, in the logarithmic growth phase, was injected as a 0.1-ml sample into a rear foot pad to induce a typical actinomycetoma lesion (11). Signs of infection, including abscesses, ulcers, inflammation, and deformities, were noted (see Fig. 4). Results are presented as inflammation in millimeters, as measured with a vernier caliper, plotted against days after infection.
FIG. 4.
Positive and negative controls for infection. (A) Actinomycetoma lesion in rear foot pad with severe inflammation and ulcers 1 month after infection with N. brasiliensis (positive control). (B) Normal foot pad (negative control).
Detection of anti-N. brasiliensis IgM antibodies by an ELISA.
Purified P61, P38, and P24 antigens obtained as described above were used for an ELISA as previously described (14). Briefly, 0.5 μg of purified antigen was suspended in 200 μl of pH 5.0 acetate buffer and dispensed in 96-well polystyrene plates. After washing and blocking were done, 200 μl of 1:10-diluted sera from control and experimental mice was added. Rabbit anti-mouse IgM (μ-chain specific) was used as a secondary antibody. Chromogen substrate solution contained hydrogen peroxide and o-phenylendiamine. Results were recorded with a semiautomatic ELISA plate reader at 492 nm and are presented as absorbance values.
Determination of anti-N. brasiliensis IgM antibody specificity by Western blotting.
A CCE from N. brasiliensis was analyzed by 8 to 18% gradient PAGE. Sodium dodecyl sulfate-PAGE was resolved with the Laemmli discontinuous buffering system (5). After electrophoresis was completed, the proteins were electrotransferred to nitrocellulose membranes (Trans-Blot cell; Bio-Rad) as described by Towbin et al. (16). Serum samples from immunized mice were diluted 1:5 and incubated with nitrocellulose strips. After five washes with phosphate-buffered saline-Tween, 0.1 ml of rabbit anti-mouse immunoglobulins was added; 0.2% hydrogen peroxide-3,3′-diaminobenzidine (Sigma) was used as the chromogen substrate solution.
Total IgM quantification with a nephelometer.
Control and immunized mice were bled at various times after antigen injection. Serum samples were diluted 1:5 with saline solution. A 100-μl quantity of diluted serum was mixed with 200 μl of goat anti-mouse IgM (μ-chain specific). After incubation for 30 min at room temperature, samples were read with a laser nephelometer (Behring).
Serum samples from normal nonimmunized mice of the same age and sex were used as controls. These serum samples were diluted 1:2, 1:4, 1:8, and 1:16 and incubated with a constant amount of anti-mouse IgM to obtain normal reference values. The results are presented as total micrograms of IgM.
Isolation of IgM.
Serum samples from immunized and control mice were treated to remove IgM and other euglobulins. Serum samples were dialyzed against double-distilled water for 48 h at 4°C. After centrifugation at 400 × g for 15 min, precipitates and supernatants were obtained and frozen at −20°C until used.
Statistical analysis.
Protection induced by active or passive immunization was determined as millimeters of inflammation. These experimental values were used to calculate the mean and standard deviation (SD). Differences between positive actinomycetoma control and immunized animals were assessed by Student's t test. ELISA results are presented as the mean and SD. A P value of <0.05 was considered statistically significant.
RESULTS
Antigen purity and reactivity.
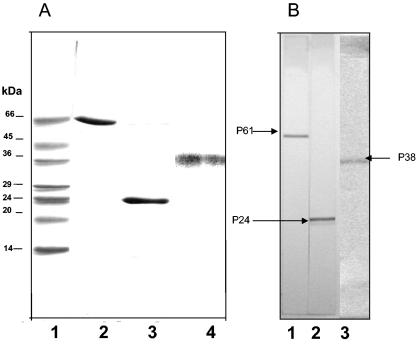
Isolated and purified antigens were analyzed to assess purity and immunogenicity by PAGE under denaturating conditions with sodium dodecyl sulfate. The Coomassie blue R-250-stained gel shown in Fig. 1 demonstrates that the antigens used in this study were pure. These antigens were recognized by an anti-P61 monoclonal antibody and by hyperimmune mouse sera against P24 and P38, as indicated on the Western blot shown in Fig. 1.
FIG. 1.
Isolated antigens from N. brasiliensis are pure. (A) Polyacrylamide gel electrophoresis under denaturating conditions and staining with Coomassie blue R-250. Lane 1, molecular mass markers; lane 2, purified P61; lane 3, purified P24; lane 4, purified P38. (B) Purified proteins (antigens) recognized by anti-P61 monoclonal antibody (lane 1), anti-P24 mouse hyperimmune serum (lane 2), and anti-P38 mouse hyperimmune serum (lane 3).
Active immunization prevents experimental infection.
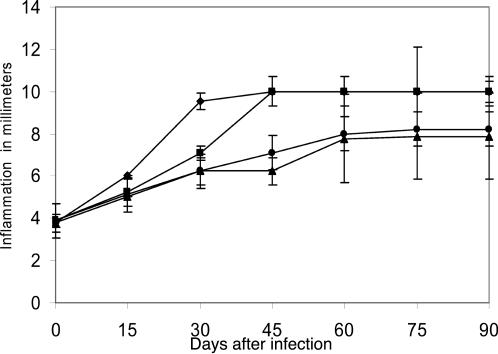
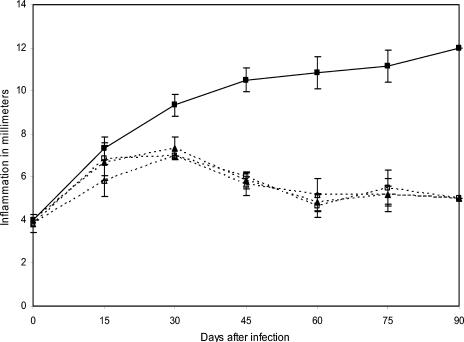
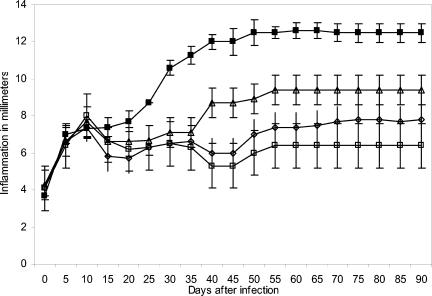
All immunized mice that received three antigen doses emulsified with incomplete Freund's adjuvant showed signs of infection less severe than those of control mice. This partial protection was maintained after 30 days of immunization with P61 and P24 antigens but not with P38 antigen, as shown in Fig. 2. The results presented here and in other experiments suggest a possible immune mechanism of protection induced by antigen injection. In other experiments, we immunized another group of BALB/c mice with the same purified antigens but with only one injection. All groups were challenged with an infective inoculum of N. brasiliensis 15 days after immunization. The results presented in Fig. 3 demonstrate that P61, P24, and P38 antigens induced complete protection. This protective effect was extraordinarily efficient because it prevented the establishment of an N. brasiliensis infection. Figure 4 shows a BALB/c mouse with actinomycetoma.
FIG. 2.
Partial protection induced by active immunization. BALB/c mice were immunized three times with purified soluble antigen emulsified with IFA. At 45 days after the last immunization, animals were infected with N. brasiliensis. Results represent means and SDs for 10 animals per group (P = 0.0001). Symbols: ▴, P24 antigen (SD, ±0.66); ♦, P61 antigen (SD, ±0.66); •, P38 antigen (SD, ±0.33); ▪, positive control (SD, ±0.40).
FIG. 3.
Complete protection after 15 days of active immunization. BALB/c mice were immunized once with purified soluble antigen emulsified with IFA. At 15 days after immunization, animals were infected with N. brasiliensis. Results represent data for 10 mice per group in one of three experiments and are shown as means and SDs (P = 0.0001). Symbols: ▴, P24 antigen (SD, ±0.53); ▵, P61 antigen (SD, ±0.37); □, P38 antigen (SD, ±0.69); ▪, positive control (SD, ±0.53).
Antigen-induced protection does not generate memory.
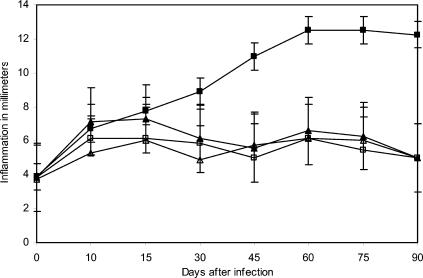
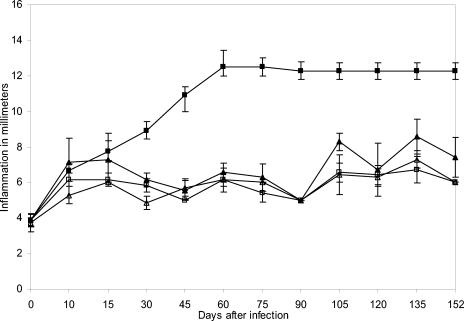
To investigate the induction of the secondary immune response that characterizes acquired immunity, we immunized mice with purified antigens P61, P24, and P38 emulsified with incomplete Freund's adjuvant. These animals were immunized only once and then were infected 15 days later with N. brasiliensis. Infection was prevented for over 90 days, as shown in Fig. 5. Then, this group of mice was challenged again with an infective dose of N. brasiliensis, and even though inflammation was present, the classical actinomycetoma lesion was absent. The same group was reinfected 120 days after immunization. The results are summarized in Fig. 5 and indicate that no protective secondary response was present.
FIG. 5.
Protection induced by active immunization is effective after 2 weeks but does not generate a secondary response. BALB/c mice were immunized once; they were infected with N. brasiliensis after 15 days and reinfected 90 and 120 days later. Results represent means and SDs for 40 animals per experiment (P = 0.00001). Symbols: ▴, P24 antigen (SD, ±0.37); ▵, P61 antigen (SD, ±0.37); □, P38 antigen (SD, ± 0.37); ▪, positive control (SD, ±0.46).
Sera transfer protective immunity from immunized mice.
BALB/c mice were immunized once with purified antigens P61, P24, and P38 emulsified with incomplete Freund's adjuvant as in the experiments described above. Blood was drawn from the animals several days after immunization, and sera were injected into naive recipients. In these experiments, 10 mice per antigen group received 0.1 ml of sera per day for three consecutive days. The donor sera were collected from the immunized mice as early as 1, 2, 3, 5, 6, 7, 14, 15, or 16 days or as late as 30 or 45 days. It is clear that sera obtained 45 days after donor immunization could not transfer protective immunity induced by active immunization. In other experiments not shown here, we found that sera from animals immunized 30 days before blood was drawn conferred only partial protection. However, sera collected as early as 5, 6, or 7 days after donor immunization transferred complete protection and prevented the establishment of an infection (Fig. 6). In other experiments not shown here, we found that sera collected as early as 1, 2, and 3 days after donor immunization did not transfer complete protection.
FIG. 6.
Protective immunity is transferred by sera collected early after donor immunization. Recipient BALB/c mice were injected intraperitoneally on a daily basis with 0.1 ml of sera from a donor that had been immunized once. Transferred sera were obtained 5, 6, or 7 days after donor immunization with purified antigens. Results represent means and SDs for one of five experiments. Symbols: ▴, anti-P24 antibody (SD, ±0.4); ▵, anti-P61 antibody (SD, ±0.75); □, anti-P38 antibody (SD, ±0.51); ▪, positive control (SD, ±0.75).
The protective effect of passive humoral immunity is partially heat sensitive.
Sera from immunized mice that had the ability to transfer complete protection to naive recipients showed diminished efficiency when heated at 56°C for 30 min. This decrease in protection was less than 50% and was seen only after 35 days of infection. The effect of heating was not evident during the first 30 days of infection, as shown in Fig. 7.
FIG. 7.
Passive humoral immunity conferred by protective sera is partially heat sensitive and is conferred by euglobulins. Recipient mice were injected intraperitoneally with 0.1 ml of supernatant not containing IgM; other mice received a precipitate containing euglobulins, including IgM antibodies. In a different experiment, protective sera were heated for 30 min at 56°C and injected intraperitoneally into naive recipient mice. Results represent data for 10 BALB/c mice per group in one of three experiments. Symbols: □, nonmodified protective sera (SD, ±1.26); ⋄, precipitate containing euglobulins (SD, ±2.9); ▵, heat-inactivated protective sera (SD, ±5.7); ▪, positive control (SD, ±0.42).
Euglobulins from protective sera confer immunity.
Sera capable of conferring protection, as demonstrated in previous experiments, were dialyzed to separate euglobulin fractions. These fractions were used in passive immunity experiments. As shown in Fig. 7, the precipitate fraction that contained the euglobulins maintained its protective capacity, as did the intact sera.
Antigen-specific IgM antibodies are responsible for protection.
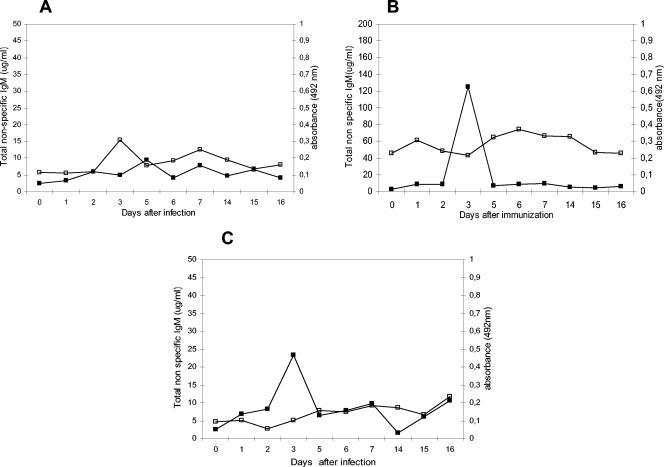
The total IgM concentrations determined with a laser nephelometer showed a dramatic increase after 3 days of immunization, but this increase was diminished shortly thereafter. By days 5, 6, and 7 after immunization, total IgM levels had decreased significantly. Using the ELISA described in Materials and Methods, we found that antigen-induced IgM antibodies were present on days 5, 6, and 7 after immunization, as shown in Fig. 8. Sera obtained on those days were the most effective in transferring protection. Interestingly, these antigen-specific IgM antibodies were polyreactive because they cross-reacted with heterologous antigens and also agglutinated red blood cells, as shown in Table 1.
FIG. 8.
Antigen-induced IgM response. Total non-antigen-specific natural IgM antibodies were determined with a laser nephelometer, and the antigen-specific IgM response was determined by an ELISA. (A) Symbols: □, antigen-specific anti-P61 antibody (SD, ±0.02); ▪, total nonspecific IgM response (SD, ±1.5). (B) Symbols: □ anti-P38 antibody (SD, ±0.05); ▪, total non-antigen-specific IgM response (SD, ±31). (C) Symbols: □, P24-specific IgM antibodies (SD, ±0.52); ▪, total non-antigen-specific IgM response (SD, ±2.04). Results represent means and SDs for one of five experiments.
TABLE 1.
Kinetics of natural polyreactive IgM antibodies and total and antigen-specific IgM responses in BALB/c mice immunized with N. brasiliensis antigens
| Antigen used to immunize mice | Day(s) after immunization | IgM
|
||
|---|---|---|---|---|
| Mean ± SD (n = 10)
| ||||
| Totala | Antigen specificb | Polyreactivec | ||
| P61 | 0 | 2.5 ± 0.6 | 0.114 ± 0.02 | Negative |
| 1 | 3.27 ± 0.8 | 0.111 ± 0.02 | Positive | |
| 3 | 5.04 ± 1.2 | 0.308 ± 0.07 | Positive | |
| 7 | 7.97 ± 1.9 | 0.251 ± 0.06 | Positive | |
| 15 | 6.68 ± 1.6 | 0.136 ± 0.03 | Positive | |
| P38 | 0 | 2.5 ± 0.6 | 0.230 ± 0.05 | Negative |
| 1 | 9.04 ± 2.2 | 0.304 ± 0.07 | Positive | |
| 3 | 125 ± 31 | 0.215 ± 0.05 | Positive | |
| 7 | 9.45 ± 2.3 | 0.330 ± 0.08 | Positive | |
| 15 | 4.54 ± 1.1 | 0.231 ± 0.05 | Positive | |
| P24 | 0 | 2.5 ± 0.6 | 0.093 ± 0.023 | Negative |
| 1 | 6.81 ± 1.7 | 0.101 ± 0.025 | Positive | |
| 3 | 23.3 ± 5.8 | 0.103 ± 0.02 | Positive | |
| 7 | 9.8 ± 2.4 | 0.183 ± 0.04 | Positive | |
| 15 | 6.13 ± 1.5 | 0.135 ± 0.03 | Positive | |
Determined by nephelometry. Results are reported in micrograms per milliliter.
Determined by an ELISA. Results are reported as absorbance at 492 nm.
Determined by hemagglutination of red blood cells.
DISCUSSION
This study demonstrates that humoral immunity prevents an experimental infection with N. brasiliensis. Protection is not IgG mediated because hyperimmune mouse serum, with high levels of anti-N. brasiliensis IgG antibodies, does not transfer the protective effect. Active immunizations with purified antigens P61, P38, and P24, with or without adjuvant, were all similar in preventing experimental infection when immunized mice were challenged at between 7 and 14 days. However, protection was short-lived and was lost 45 days after one or three immunizations. This finding also argues against IgG-mediated protection. Passive immunizations with sera collected 15 days after donor immunization conferred complete protection. These results are different from those reported by Beaman et al. (1). Using N. asteroides GUH2 to infect immunized and nonimmunized mice, they found that F1 (CBA/N × DBA/2) males and females cleared infection even though male hybrids could not produce anti-N. asteroides IgG antibodies. These results suggest that there was little or no participation from B lymphocytes. Similar results with N. brasiliensis were reported by Rico and coworkers (8); no protection was found when hyperimmune sera with high levels of anti-N. brasiliensis IgG antibodies were transferred. Moreover, when these sera were injected along with antibody-coated N. brasiliensis, the severity of the lesions increased (8). Based on these and other results, there has been a general acceptance that humoral immunity plays no role in host protection in infections produced by Nocardia strains. However, the results presented here clearly demonstrate that active immunity and passive immunity are effective in preventing experimental infection with N. brasiliensis.
Previous results also demonstrated that hyperimmune sera with anti-N. brasiliensis IgG antibodies partially prevented actinomycetoma lesions. This study suggested that humoral factors other than IgG might be responsible for the protective effect. Cytokines were proposed as candidates, but no supporting data were provided (10). In other experiments, F1 (CBA/N × DBA/2) males developed actinomycetoma 5 months after infection with N. brasiliensis, compared with 1 month for BALB/c mice. It was suggested that innate immunity might be responsible for the delay in the appearance of clinical signs of infection (12). The male hybrid mentioned here carries a B-lymphocyte defect that allows an IgM response but no IgG isotype antibody production. These findings support the notion that humoral immunity mediated by IgM might be involved in host protection.
For vesicular stomatitis virus, lymphocytic choriomeningitis virus, and Listeria monocytogenes, a typical facultative intracellular bacterium, it has been shown that natural IgM antibodies control infection and bacterial distribution (7). IgM antibodies specific for West Nile virus were also recently demonstrated to play a critical role against infection (3).
In the present work, protection induced by active immunization lasted 1 to 3 weeks and did not generate the secondary response that characterizes acquired immunity. Classic protective immune memory was not generated, even though reinfection was prevented on two occasions. In addition, passive immunity was effective only for a short time. The kinetics of the appearance and duration of induced protection suggest that IgM is responsible for these effects. The early IgM response appears 24 h after antigen stimulation, peaks at 72 h, and rapidly falls by day 5 with purified antigens P61, P38, and P24. These IgM antibodies are antigen induced but are not antigen specific. Passive transfer of sera that showed a very early IgM response and that were obtained 1, 2, or 3 days after immunization did not induce protection. In contrast, antigen-specific IgM antibodies appeared 3 days after antigen injection, and the response increased on days 4 and 5 and peaked on day 7. These findings support the notion that antigen-specific IgM antibodies are responsible for protection. Moreover, removing euglobulins from the protective sera abolished their ability to protect mice from infection. The protective effect was retained by the precipitate fraction that contained euglobulins, including IgM antibodies.
The mechanism(s) by which antigen-specific IgM antibodies prevent N. brasiliensis infection may be directly related to their capacity to bind bacteria and activate the complement cascade. This possibility is supported by our findings that protective ability decreases but that effectiveness is not abolished after heat inactivation at 56°C for 30 min. IgM antibodies can also limit N. brasiliensis dissemination and provide time for leukocyte arrival to facilitate bacterial destruction. More studies are needed to clarify the exact mechanism(s) that mediates the antigen-induced IgM-mediated protection. This information will be helpful for new vaccine development to protect against other facultative intracellular microbes.
Acknowledgments
This work was supported in part by CONACYT Mexico (grant 40236-M) and PAICYT/UANL Monterrey Mexico (grant SA 668-02).
We thank R. M. Chandler-Burns for critical reading of the manuscript and Patricia Alejandra Gallegos and Reynaldo Rodriguez for technical assistance.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Beaman, B. L., M. E. Gershwin, A. Ahmed, S. M. Scates, and R. L. Deem. 1982. Response of CBA/N × DBA2 F1 mice to Nocardia asteroides. Infect. Immun. 35:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blander, S. J., and M. G. Horwitz. 1989. Vaccination with major secretory protein of Legionella pneumophila induces cell-mediated and protective immunity in a guinea pig model of Legionnaires'disease. J. Exp. Med. 169:691-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond, M. S., B. Shresta, A. Marri, D. Mahan, and M. Engle. 2003. Cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 77:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufmann, S. H. E. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129-163. [DOI] [PubMed] [Google Scholar]
- 5.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 6.Licón-Trillo, A., M. A. Castro-Corona, and M. C. Salinas-Carmona. 2003. Immunogenicity and biophysical properties of a Nocardia brasiliensis protease involved in pathogenesis of mycetoma. FEMS Immunol. Med. Microbiol. 37:37-44. [DOI] [PubMed] [Google Scholar]
- 7.Ochsenbein, A. F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R. M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156-2159. [DOI] [PubMed] [Google Scholar]
- 8.Rico, G., R. Ochoa, A. Oliva, A. González-Mendoza, S. M. Walter, and L. Ortiz-Ortiz. 1982. Enhanced resistence to Nocardia brasiliensis infection in mice depleted of antigen-specific B-cells. J. Immunol. 129:1688-1693. [PubMed] [Google Scholar]
- 9.Salinas-Carmona, M. C., L. I. Pérez-Rivera, and E. Torres-López. 2003. Isolation and purification of immunodominant antigen P61 from Nocardia brasiliensis culture filtrate. J. Mycol. Med. 13:117-121. [Google Scholar]
- 10.Salinas-Carmona, M. C., and E. Torres-López. 1996. Role of passive humoral immunity in experimental mycetoma by Nocardia brasiliensis. Ann. N. Y. Acad. Sci. 797:263-265. [DOI] [PubMed] [Google Scholar]
- 11.Salinas-Carmona, M. C., E. Torres-López, A. I. Ramos, A. Licón-Trillo, and D. González-Spencer. 1999. Immune response to Nocardia brasiliensis antigen in an experimental model of actinomycetoma in BALB/c mice. Infect. Immun. 67:2428-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salinas-Carmona, M. C., E. Torres, and A. Revol. 2003. Experimental actinomycetoma by Nocardia brasiliensis in different mouse strains. J. Mycol. Med. 13:163-167. [Google Scholar]
- 13.Salinas-Carmona, M. C., L. Vera, O. Welsh, and M. Rodríguez. 1992. Antibody response to Nocardia brasiliensis antigens in man. Zentbl. Bakteriol. 276:390-397. [DOI] [PubMed] [Google Scholar]
- 14.Salinas Carmona, M. C., O. Welsh, and S. M. Casillas. 1993. Enzyme-linked immunosorbent assay for serological diagnosis of Nocardia brasiliensis and clinical correlation with mycetoma infections. J. Clin. Microbiol. 31:2901-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seiler, P., U. Kalinke, T. Rùlicke, E. M. Bucher, C. Bose, R. M. Zinkernagel, and H. Hengartner. 1998. Enhanced virus clearance by early inducible lymphocytic choriomeningitis virus-neutralizing antibodies in immunoglobulin-transgenic mice. J. Virol. 72:2253-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vera-Cabrera, L., M. C. Salinas-Carmona, O. Welsh, and M. A. Rodríguez. 1992. Isolation and purification of two immunodominant antigens from Nocardia brasiliensis. J. Clin. Microbiol. 30:1183-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]