Abstract
Plague caused by Yersinia pestis is an ancient disease, responsible for millions of deaths in human history. Unfortunately, there is no FDA-approved vaccine available. Recombinant subunit vaccines based on two major antigens, Caf 1 (F1) and LcrV (V), have been under investigation and showed promise. However, there are two main problems associated with these vaccines. First, the Yersinia capsular protein F1 has high propensity to aggregate, particularly when expressed in heterologous systems such as Escherichia coli, thus affecting vaccine quality and efficacy. Second, the subunit vaccines do not induce adequate cell-mediated immune responses that also appear to be essential for optimal protection against plague. We have developed two basic approaches, structure-based immunogen design and phage T4 nanoparticle delivery, to construct new plague vaccines that may overcome these problems. First, by engineering F1 protein, we generated a monomeric and soluble F1V mutant (F1mutV) which has similar immunogenicity as wild-type F1V. The NH2-terminal β-strand of F1 was transplanted to the COOH-terminus and the sequence flanking the β-strand was duplicated to retain a key CD4+ T cell epitope. Second, we generated a nanoparticle plague vaccine that can induce balanced antibody- and cell-mediated immune responses. This was done by arraying the F1mutV on phage T4 via the small outer capsid (Soc) protein which binds to T4 capsid at nanomolar affinity. Preparation of these vaccines is described in detail and we hope that these would be considered as candidates for licensing a next-generation plague vaccine.
Keywords: Yersinia pestis, F1V, Bacteriophage T4, Nanoparticle vaccine
1 Introduction
Plague caused by Yersinia pestis (Y. pestis) is a deadly disease that wiped out one-third of Europe’s population in the 14th century and it still exists in parts of the world today [1, 2]. Due to its exceptional virulence, the organism is listed by the CDC as Tier-1 biothreat agent. Although it has been a national priority to stockpile an efficacious plague vaccine, no FDA-approved plague vaccine is yet available. Previously, a killed whole-cell (KWC) vaccine was in use in the USA. But this vaccine was discontinued because it requires multiple immunizations, exhibits high reactogenicity, and does not provide complete protection [3]. Although a live-attenuated plague vaccine (strain EV76) is still in use in the states of former Soviet Union [4], it may not meet FDA approval because of the highly infectious nature of the Y. pestis and the virulence mechanisms of vaccine strains have not been fully understood [3, 5].
Efforts in the past two decades led to recombinant vaccine candidates containing two Y. pestis virulence factors, the capsular protein Caf1 (F1) and the low calcium response protein LcrV (V) [3, 6]. F1 assembles into flexible linear fibers via a chaperone/usher mechanism [7]http://www.plospathogens.org/article/info%3Adoi/10.1371/journal.ppat.1003495-ppat.1003495-Zavialov1, forming a capsular layer that is pivotal for bacteria to escape phagocytosis [8] (Fig. 1). The V forms a “pore” at the tip of the “injectisome” structure of the type 3 secretion system (T3SS), regulating delivery of bacterial virulence factors into the host cytosol [9]. Two types of F1/V recombinant vaccines have been under investigation, one containing a mixture of F1 and V antigens (F1 + V) [10], and another, a single F1 − V fusion protein (F1V) [11, 12]. The problem, however, is that F1 naturally assembles into a fiber on the surface of the bacterium, and when expressed in heterologous systems such as Escherichia coli (E. coli), it forms insoluble and heterodisperse aggregates. These properties cause variability in the structure of the vaccine components and might compromise the efficacy [11, 13–16]. Second, the subunit vaccines do not induce adequate cell-mediated immune responses that also appear to be essential for optimal protection against plague [17].
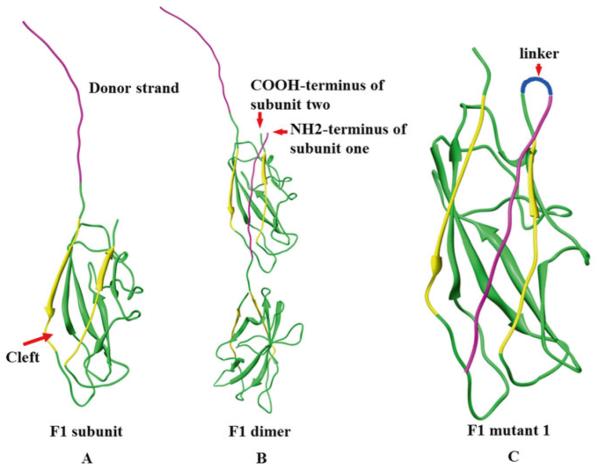
Fig. 1.
Reorientation of the NH2-terminal β-strand of F1 protein to generate monomeric F1. (a) Structure of F1 subunit, purple strand indicates donor β-strand, yellow strands indicate the strands that form groove. (b) F1 dimer showing how F1 monomers oligomerize to generate a linear fiber. (c) Schematic of F1 mutant 1 in which the NH2-terminal β-strand is reoriented
In this chapter, we first describe methods to engineer a soluble F1V mutant, which we call F1mutV [18]. We then describe the preparation of nanoparticle F1mutV vaccine in which the F1mutV antigen is arrayed on bacteriophage T4 capsid using our recently developed phage T4 vaccine delivery system [18–20].
1.1 Construction of a Soluble F1mutV Vaccine
Previous structural studies have demonstrated that F1 polymerizes into a linear fiber by head-to-tail interlocking of F1 subunits through a donor strand complementation mechanism [7] (Fig. 1a, b). Each subunit consists of a seven-stranded antiparallel β-barrel with immunoglobulin-like fold. Six of the β-strands form an incomplete sandwich with a hydrophobic cleft that exposes the hydrophobic core. The cleft is then filled with the NH2-terminal β-strand of the adjacent subunit, thereby connecting the two subunits. By repeating this process, referred to as intermolecular complementation, a linear F1 fiber is assembled (Fig. 1a, b). Prior to filling with the β-strand of adjacent subunit, the cleft is occupied by a “spare” β-strand of Caf1M, a chaperone for F1 fiber assembly, with the assistance of an outer membrane usher protein, Caf1A. Over-expression of F1 protein in E. coli exposes the unfilled hydrophobic cleft causing aggregation and formation of insoluble inclusion bodies [21, 22]. We have constructed an F1 mutant by shifting the NH2-terminal β-strand of F1 to the COOH-terminus and reorienting the β-strand such that it could fill its own cleft (intramolecular complementation) (Fig. 1c). Furthermore, it no longer requires the assistance of chaperone and usher proteins. Consequently, a soluble F1 monomer was produced.
In order to construct this F1 mutant, we first deleted the NH2-terminal donor strand [amino acid (aa) residues 1–14] and fused it to the COOH-terminus with a short two aa serine-alanine linker in between (Fig. 1c and 2). This was named pET-F1mut1 (Fig. 2) and over-expressed the F1mut1 protein in E. coli and confirmed that it is soluble and monomeric [18]. The aa residues 7–20 are reported to contain a mouse H-2-IAd-restricted CD4+ T cell epitope [23], which was disrupted in pET-F1mut1. To restore this epitope, we added these residues to the switched β-strand, which resulted in the duplication of the residues 15–21 at the COOH-terminus (Fig. 2). We then fused the mutated F1 to the NH2-terminus of V with a two aa linker in between to generate the F1mutV fusion protein (Fig. 2). F1mutV was over-expressed in E. coli and also shown to be soluble and monomeric. Importantly, F1mutV retained full immunogenicity of wild-type F1V and conferred complete protection against challenge with Y. pestis CO92 [18].
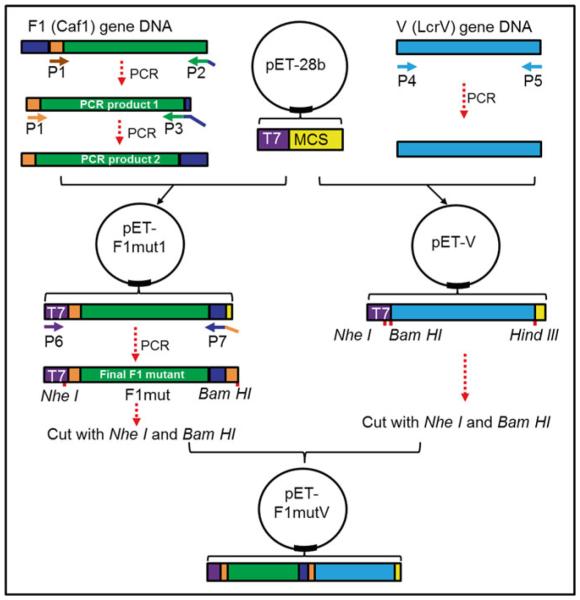
Fig. 2.
Cloning strategy to generate pET-F1mutV plasmid. T7 indicates T7 promoter sequence, MCS indicates multiple cloning site, P1–P7 indicate primers 1–7, blue indicates the first 14-aa residues of F1, and orange indicates aa residues 15–21 of F1. The remaining region of F1 is shown in green. V gene is shown in cyan. The linkers in F1mut or between F1mut and V are not shown
1.2 Generate a T4 Bacteriophage Nanoparticle-Displayed F1mutV Vaccine
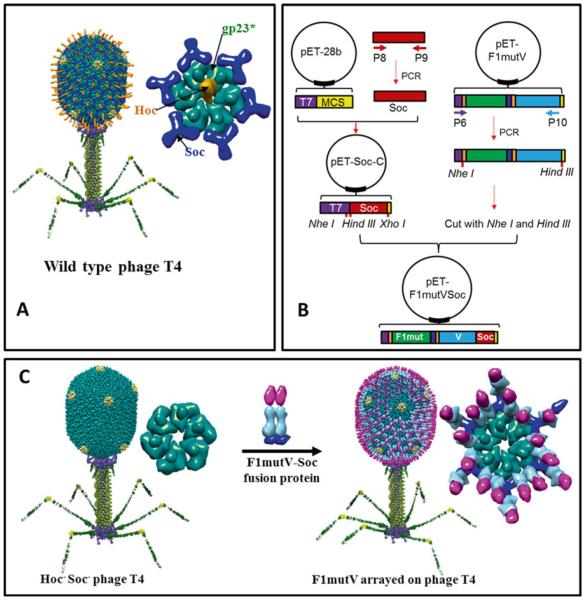
Recently, we have developed a novel bacteriophage T4 platform to deliver vaccine antigens [18, 20, 24, 25]. Pathogen antigens are displayed on the 120 nm × 86 nm phage T4 capsid at high density. The T4 capsid (head) is composed of three essential capsid proteins: 930 copies of major capsid protein, gp23* (“*” refers to cleaved and matured form); 55 copies of vertex protein, gp24*; and 12 copies of portal protein, gp20 (Fig. 3a) [26, 27]. A unique feature of T4 head is that it is decorated with two nonessential proteins, the small outer capsid protein (Soc) and the highly antigenic outer capsid protein (Hoc) (Fig. 3a) [28].
Fig. 3.
Preparation of T4 nanoparticle-arrayed plague vaccine. (a) Structural model of bacteriophage T4. The enlarged capsomer on right shows the major capsid protein gp23* (green; “*” represents the cleaved mature form) (930 copies), Soc (blue; 870 copies), and Hoc (orange; 155 copies). Yellow subunits at the fivefold vertices correspond to gp24*. The portal vertex (not visible in the picture) connects the head to the tail. (b) Cloning strategy to generate pET-F1mutVSoc. Red indicates Soc. The other components are shown as in Fig. 2. P6–P10 indicate primers 6–10. (c) Display of F1mutV-Soc fusion protein on the Hoc−Soc− phage particle. Shown on the right are models of the enlarged capsomers before and after F1mutV-Soc display
Approximately 870 molecules of Soc protein (9 kDa) assemble into trimers at the quasi threefold axes of the head. The binding sites appear after the head undergoes maturation cleavages and expansion [29]. Each Soc molecule clamps two adjacent gp23 capsomers, forming a reinforced cage around the shell (Fig. 3a) [29]. Hoc consists of a string of four domains of which three are Ig-like. One hundred and fifty-five copies of these fiber-like Hoc molecules assemble on the capsid, one each at the center of the capsomer (Fig. 3a) [30]. The COOH-terminal domain contains the capsid-binding site; hence it is attached to the capsid surface whereas the NH2-terminal domain is projected out to ~160 Å distance from the capsid wall. Soc and Hoc are completely dispensable under laboratory conditions showing no significant effect on phage productivity or infectivity when deleted from the genome [28]. Purified Soc and Hoc proteins bind to Hoc− Soc− capsid in vitro with high specificity and nanomolar affinity. Pathogen antigens as large as 116 kDa can be fused to Soc without compromising its ability to bind to the capsid (Fig. 3c) [18–20, 25]. Alternatively, multilayered oligomeric complexes of >500 kDa can be assembled through Hoc or Soc. Essentially all the Soc and Hoc molecules can be occupied by pathogen antigens [19, 31]. Importantly, these T4 nanoparticle-displayed antigens induce strong humoral and cellular immune responses [18, 20], making it a robust antigen display and delivery system.
In order to array F1mutV on T4 capsid we first constructed a “universal” Soc vector, pET-Soc-C, which contains multiple cloning sites upstream of NH2-terminus of Soc, by inserting Soc into expression vector pET28b (Fig. 3b). Any foreign protein can be inserted upstream of NH2-terminus of Soc, generating an in-frame fusion protein (Fig. 3b, c). F1mutV was amplified by PCR from pET-F1mutV and inserted into pET-Soc-C. The F1mutV-Soc was over-expressed in E. coli in soluble form and purified by nickel affinity chromatography and gel filtration chromatography. The purified F1mutV-Soc was then arrayed on T4 capsid by incubation with Hoc− Soc− T4 phage (Fig. 3c).
2 Materials
Prepare all solutions using autoclaved Milli-Q water (Millipore) and analytical grade reagents. Prepare and store all reagents at room temperature (unless indicated otherwise).
2.1 Construction of Plasmids
E. coli expression vector pET28b (Novagen, MA).
Competent E. coli DH5α cells (NEB, MA).
Luria–Bertani (LB) medium (Quality Biological, MD).
SOC medium (Quality Biologicals, MD).
1000× Kanamycin (50 mg/ml): Add 0.5 g kanamycin (Gold Biotechnology, MD) to 10 ml Milli-Q water.
Kanamycin LB plates: Add 2 g LB powder (Affymetrix, OH) and 1.5 g agar to 100 ml Milli-Q water, and sterilize by autoclaving. When cooled to about 50 °C, add 0.1 ml 1000× kanamycin. Mix and pour into sterile petri plates.
PCR kit: 2× Phusion High-Fidelity PCR Master Mix (Thermo Scientific).
Restriction enzymes: FastDigest NheI, FastDigest HindIII, FastDigest BamHI, FastDigest XhoI (all were purchased from Thermo Scientific).
FastAP Thermosensitive Alkaline Phosphatase (Thermo Scientific).
T4 DNA Ligase (Thermo Scientific).
Agarose gel running buffer: Add 100 ml 10× AccuGENE™ Tris-borate-EDTA (TBE) agarose gel running buffer (Lonza Chemicals Company, Switzerland) to 900 ml Milli-Q water to make 1× agarose gel running buffer.
GeneJET Gel Extraction kit (Thermo Scientific).
GeneJET Plasmid Miniprep Kit (Thermo Scientific).
2.2 Protein Purification
Competent E. coli BL21-CodonPlus (DE3)-RIPL cells (Agilent Technologies, CA).
SOC medium (Quality Biologicals, MD).
1000× Kanamycin (50 mg/ml): Add 0.5 g kanamycin (Gold Biotechnology, MD) to 10 ml Milli-Q water.
1000× Chloramphenicol (50 g/ml): Add 0.5 g chloramphenicol (Amresco) to 10 ml ethanol.
Kanamycin/chloramphenicol LB plates: Add 2 g LB powder (Affymetrix, OH) and 1.5 g agar to 100 ml Milli-Q water, and sterilize by autoclaving. When cooled to about 50 °C, add 0.1 ml 1000× kanamycin and 0.1 ml 1000× chloramphenicol. Mix and pour into sterile petri plates.
Moore’s medium: To 800 ml Milli-Q water, add 20 g tryptone, 15 g yeast extract, 8 g NaCl, 2 g dextrose, 2 g Na2HPO4, and 1 g KH2PO4, adjust to 1 L with Milli-Q water, and sterilize by autoclaving. Add 1 ml 1000× kanamycin and 1 ml 1000× chloramphenicol before use.
Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Gold Biotechnology, MO).
Complete proteinase inhibitor cocktail (Roche).
HisTrap binding buffer: 50 mM Tris–HCl pH 8.0, 300 mM NaCl, and 20 mM imidazole.
HisTrap washing buffer: 50 mM Tris–HCl pH 8.0, 300 mM NaCl, and 50 mM imidazole.
HisTrap elution buffer: 50 mM Tris–HCl pH 8.0, 300 mM NaCl, and 400 mM imidazole.
Gel filtration buffer: 20 mM Tris–HCl, pH 8.0 and 100 mM NaCl. All protein purification buffers have to go through 0.22 μm filter and be made the day before use and kept at 4 °C.
Nickel affinity chromatography column: 1 ml HisTrap HP column (GE Healthcare).
Amicon Ultra-4 centrifugal filter units (Millipore, MA).
Gel filtration chromatography column: Hi-load 16/60 Superdex 200 column (GE Healthcare).
4–20 % (w/v) polyacrylamide gel (Life Technologies).
Acetylated bovine serum albumin (BSA) standard (Affymetrix, OH).
SDS-loading buffer: 20 mM Tris–HCl pH 6.8, 100 mM dithiothreitol, 2.5 % β-mercaptoethanol, 1 % SDS (w/v), 0.1 % bromophenol blue, and 10 % glycerol.
Tris–glycine running buffer: Add 100 ml 10× Tris–glycine running buffer (Bio-Rad) to 900 ml Milli-Q water to make 1× Tris–glycine running buffer.
Coomassie blue R-250 staining solution (Teknova, CA).
Destaining solution: Add 100 ml methanol and 100 ml acetic acid to 800 ml Milli-Q water.
2.3 T4 Phage Purification
E. coli P301 (stored in our lab).
Hoc−Soc− phage T4 mutant.
LB medium (Quality Biological, MD).
M9CA medium broth: To 800 ml Milli-Q water, add 12.5 g M9CA medium broth powder (Amresco, OH), adjust to 1 L with Milli-Q water, and sterilize by autoclaving.
Top-agar: Add 2 g LB powder (Affymetrix, OH) and 0.75 g agar to 100 ml Milli-Q water, sterilize, and keep it at 42 °C.
LB plates: Add 2 g LB powder (Affymetrix, OH) and 1.5 g agar to 100 ml Milli-Q water, and sterilize by autoclaving. Mix and pour into sterile petri plates.
Pi-Mg buffer: 26 mM Na2HPO4, 22 mM KH2PO4, 70 mM NaCl, 1 mM MgSO4.
Deoxyribonuclease I (DNase I) (Sigma-Aldrich).
HPLC-grade chloroform (Fisher Scientific).
Cesium chloride (CsCl) stock solution: 8 M CsCl, 100 mM Tris–HCl pH7.5, 85 mM NaCl, 20 mM NH4Cl.
Slide-A-lyzer dialysis cassette (0.5–3 ml capacity; Pierce).
Dialysis buffer I: 10 mM Tris–HCl pH 7.5, 200 mM NaCl, 5 mM MgCl2.
Dialysis buffer II: 10 mM Tris–HCl pH 7.5, 50 mM NaCl, 5 mM MgCl2.
2.4 Antigen Preparation
Alhydrogel (Al3+ concentration: 10 mg/ml) (Brenntag Biosector, Denmark).
PBS pH 7.4 (Quality Biological, MD).
5 M NaCl (Quality Biological, MD).
3 Methods
Here, we first provide a procedure to construct and test the immunogenicity of our recently developed monomeric F1mutV. Then, we provide a step-by-step protocol for how to prepare our bacteriophage T4 nanoparticle vaccine to deliver monomeric F1mutV.
3.1 F1mutV Immunogens as Next-Generation Plague Vaccines
3.1.1 Construction of F1mutV
In order to generate pET-F1mutV, we first constructed two intermediate plasmids (pET-F1mut1 and pET-V) as depicted in Fig. 2. Plasmid pET-F1mut1 was constructed by two rounds of PCR. The first round of PCR was performed to amplify F1 fragment in which the NH2-terminal 14 aa residues were deleted and at the same time the NH2-terminal 7 amino acids were fused to the COOH-terminus of F1. This PCR product was used as a template for the second round of PCR using a forward primer containing NheI restriction site and a reverse primer containing the NH2-terminal 14 aa residues and XhoI restriction site. The PCR fragment was then inserted into NheI and XhoI linearized pET28b vector to generate pET-F1mut1. The final F1 mutant, in which the NH2-terminal 14 aa residues were deleted but the NH2-terminal 21 aa residues were added to the COOH-terminus, was amplified from pET-F1mut1. Finally, the PCR product was inserted into the upstream of V in pET-V plasmid to generate pET-F1mutV.
3.1.1.1 Construct pET-F1mut1
-
Amplify F1 mutant intermediate 1, in which NH2-terminal 14 aa residues (see Note 1) were removed and at the same time NH2-terminal 7 amino acids were added to the C-terminus of F1, with primers 1 and 2 using Thermo 2× Phusion High-Fidelity PCR Master Mix. Y. pestis CO92 plasmid pMT1 was used as a template.
Primer 1: Forward primer binding to nt 43–61 of 5′-end of F1, including the Nhe I restriction site (highlighted in bold) at the 5′-end (see Note 2).
5′-CCATCT GCTAGC GAACCAGCCCGCATCACTC-3′
Primer 2: Reverse primer binding to the 3′-end nucleotide sequence of F1, including the first 21 nt of the F1 (underline) at the 5′-end.
5′-GGTGCTTGCAGTTAAATCTGC TGCAGA TTGGTTAGATACGGTTACGGTTACAGC-3′ (Italics indicates the sequence for the two aa (Ser-Ala) linker).
Purify PCR product by agarose gel electrophoresis and gel extraction using Thermo GeneJET gel extraction kit, according to the manufacturer’s instructions (see Note 3).
-
Use F1 mutant intermediate 1 as template to amplify F1 mutant intermediate 2 with primers 1 and 3 using Thermo 2× Phusion High-Fidelity PCR Master Mix.
Primer 3: Reverse primer binding to the 3′-end of F1 mutant intermediate 1, including the first 42 nt of F1 (underline) and the Xho I restriction site (bold) at the 5′-end.
5′-CGT CTCGAG TTAAACAAGAGTTGCCGTTGCAGTGGTGCTTGCAGTTAAATCTGC-3′
Purify PCR product by gel electrophoresis and gel extraction using Thermo GeneJET gel extraction kit, according to the manufacturer’s instructions.
Cut the insert (PCR product) with restriction enzymes, Nhe I and Xho I, and at the same time cut the pET28b vector plasmid DNA using the same restriction enzymes.
Purify insert and vector using gel electrophoresis and Thermo GeneJET gel extraction kit, following the manufacturer’s instructions.
Ligate insert and vector using T4 DNA ligase for 1 h at 22 °C.
Transform E. coli DH5α with the ligation product according to the manufacturer’s instructions and incubate LB plate overnight at 37 °C.
Pick a single colony, and inoculate into a 125 ml flask containing 10 ml LB medium with 50 μg/ml kanamycin. Incubate the flask in shaking incubator at 220 rpm, 37 °C overnight.
Isolate plasmid DNA using Thermo GeneJET plasmid mini-prep kit, following the manufacturer’s instructions. The generated plasmid was named pET-F1mut1.
3.1.1.2 Construct pET-V
-
Amplify V with primers 4 and 5 using Thermo 2× Phusion High-Fidelity PCR Master Mix, according to the manufacturer’s instructions. Y. pestis CO92 plasmid pCD1 was used as a template.
Primer 4: Forward primer binding to the 5′-end nucleotide sequence of V, including the BamH I restriction site (highlighted in bold) at the 5′-end.
5′-CA GGATCC ATGATTAGAGCCTACGAACA AAACC-3′
Primer 5: Reverse primer binding to the 3′-end nucleotide sequence of the V, including the Hind III restriction site (highlighted in bold) at the 5′-end (see Note 4).
5′-CCT AAGCTT TTTACCAGACGTGTCATCTAG CAGAC-3′
Purify PCR product by gel electrophoresis and gel extraction using Thermo gel extraction kit, according to the manufacturer’s instructions.
Digest the insert (PCR product) with restriction enzymes, BamH I and Hind III, and at the same time cut the pET28b vector plasmid DNA using the same restriction enzymes.
Repeat steps 6–9 of Subheading “Construct pET-F1mut1.”
Isolate plasmid DNA using Thermo GeneJET plasmid miniprep kit, following the manufacturer’s instructions. The generated plasmid was named pET-V.
3.1.1.3 Construct pET-F1mutV
-
Amplify final F1 mutant from pET-F1mut1 with primers 6 and 7 using Thermo 2× Phusion High-Fidelity PCR Master Mix.
Primer 6: Forward primer binding to the T7 promoter of pET28b vector.
5′-TAATACGACTCACTATAGGGGA-3′
Primer 7: Backward primer binding to the 3′-end of the F1 mutant intermediate 2, including the first 21 nt of the F1 mutant intermediate 2 (predicted CD4+ T cell epitope, underlined) and the BamH I restriction site (highlighted in bold) at the 5′-end.
5′-CTG GGATCC AAGAGTGATGCGGGCTGGTTC AACAAGAGTTGCCGTTGCAGTG-3′
Purify PCR product by gel electrophoresis and gel extraction using Thermo gel extraction kit, according to the manufacturer’s instructions.
Cut the insert (PCR product) with restriction enzymes, Nhe I and BamH I, and at the same time cut the pET-V vector DNA using the same restriction enzymes.
Repeat steps 6–9 of Subheading “Construct pET-F1mut1.”
Isolate plasmid DNA using Thermo GeneJET plasmid miniprep kit, following the manufacturer’s instructions. The resulting pET-F1mutV plasmid contained F1mut in-frame fusion with V, a 23-aa vector sequence containing the hexa-histidine sequence at the NH2-terminus of F1, and also a 13-aa vector sequence containing the hexa-histidine sequence at the COOH-terminus of V.
3.1.2 Purification of Recombinant F1mutV from E. coli
Transform 10 ng of pET-F1mutV into 25 μl BL21-CodonPlus (DE3)-RIPL competent cells, according to the manufacturer’s instructions (see Note 5). Incubate plate overnight at 37 °C.
Pick a single colony, and inoculate into a 125 ml flask containing 30 ml Moore’s medium with 50 μg/ml kanamycin and 50 μg/ml chloramphenicol. Incubate the flask in shaking incubator at 220 rpm, 37 °C overnight.
Inoculate 20 ml of overnight cultures into a 2 L flask containing 1 L of Moore’s medium with 50 μg/ml kanamycin and 50 μg/ml chloramphenicol. Incubate the flask in a shaking incubator at 220 rpm, 37 °C, until the cell density raises to 3.0 × 108 cells/ml.
Transfer the flask to 30 °C shaking incubator and shake for 30 min at 220 rpm (see Note 6).
Add IPTG to the culture to a final concentration of 1 mM, and induce protein expression for 2 h at 30 °C.
Harvest the cells by centrifugation at 7000 rpm (8,288 g) for 10 min at 4 °C with GS3 rotor in Sorvall RC-5C plus centrifuge. Discard the supernatant, and store the pellet at −80 °C for further purification.
Resuspend the pellet with 40 ml HisTrap binding buffer supplemented with one pill of complete proteinase inhibitor cocktail (see Note 7).
Lyse the cells by French press at 12,000 psi twice.
Centrifuge the cell lysate at 17,000 rpm (34,572 g) for 20 min at 4 °C with SS34 rotor in Sorvall RC-5C plus centrifuge.
Collect the supernatant which contains soluble F1mutV protein, and filter it through 0.22 μm filters (see Note 8).
Set up 1 ml HisTrap HP column on AKTA-prime system. First, wash column with 20 ml of water, and then equilibrate the column with 20 ml of HisTrap binding buffer.
Load the supernatant collected in step 10 onto HisTrap HP column and wash the column with 20 ml HisTrap washing buffer (see Note 9).
-
Elute protein with 20–400 mM linear imidazole gradient with HisTrap binding buffer as buffer A and HisTrap elution buffer as buffer B. AKTA-prime was set as follows:
Concentration (% buffer B): 0; gradient length: 40; target (% buffer B): 100; flow rate: 1 ml/min; fraction base: ml; fraction size: 1; pressure limit: 0.3.
Collect and pool the peak fractions, and concentrate using Amicon Ultra-4 centrifugal filtration (10 kDa cutoff) according to the manufacturer’s instructions.
Wash Hi-load 16/60 Superdex 200 column with 150 ml gel filtration buffer, and then load the concentrated peak fractions onto Hi-load 16/60 Superdex 200 column with flow rate of 1.0 mg/ml.
Collect and pool the peak fractions from gel filtration elution and concentrate using Amicon Ultra-4 centrifugal filtration (10 kDa cutoff).
Aliquot the concentrated F1mutV protein and store at −80 °C for future use. Following steps are intended to determine the concentration of F1mutV protein by SDS-PAGE with BSA (1 mg/ml) as standard.
Mix equal volume of F1mutV or BSA with 2× SDS loading buffer, boiled for 5 min.
Load 1 μl, 2 μl, 3 μl, and 4 μl of F1mutV protein, and 1 μg, 2 μg, 4 μg, and 8 μg of BSA to each well, and run SDS-PAGE using 4–20 % Tris–glycine gel, according to the manufacturer’s instructions.
Disassemble the gels, transfer gel into a clean tray, and rinse with water.
Add Coomassie blue R-250 staining solution to the tray, microwave for 1 min, and keep shaking gently at room temperature for 15 min.
Discard Coomassie blue R-250 staining solution, add destain solution to the tray, and keep shaking gently at room temperature overnight (see Note 10).
Scan gel with laser densitometry (PDSI, GE Healthcare), and quantify protein bands with ImageQuant 5.2 software (GE Healthcare), according to the manufacturer’s instructions.
Generate BSA standard curve using Microsoft Excel with the number calculated in step 23, and calculate the concentration of F1mutV protein based on BSA standard curve.
3.1.3 Preparation of Vaccine Formulations for Animal Immunization
The exact amount of protein depends on the number of animals per group. Here, we describe the dose for one animal (10 μg antigen/animal).
Prepare 2× aluminum hydrogel solution. The final dose of aluminum for each mouse is 100 μg in 50 μl buffer (2.0 mg/ml); hence concentration of aluminum in 2× aluminum hydrogel solution should be 4.0 mg/ml. Add 1.0 ml aluminum hydrogel solution (10 mg/ml), 72.6 μl 5 M NaCl, and 1427.4 μl H2O to make 2.5 ml of 2× aluminum hydrogel solution (Al3+ concentration: 4 mg/ml, NaCl: 0.145 mol/L = 0.85 %) (see Note 11).
Mix antigen with 2× aluminum hydrogel solution. For naïve group: take 25 μl 2× aluminum hydrogel solution, add 25 μl PBS (pH 7.4) to make the total volume to 50 μl, and vortex. This is for one mouse (50 μl/mouse). For F1mutV group: take 25 μl 2× aluminum hydrogel solution, and mix with 23.5 μl PBS (pH 7.4) and 1.5 μl F1mutV (6.5 mg/ml). The total volume is 50 μl. Vortex the mixture. This is for one mouse (50 μl/mouse).
3.1.4 Immunization
Immunizations of mice and immunological analyses and challenges with Y. pestis CO92 were performed using methods as described elsewhere [18, 31].
3.2 Preparation of Bacteriophage T4 Nanoparticles Arrayed with Mutated F1mutV Plague Antigen
Two nonessential proteins of T4, Hoc and Soc, can bind to Hoc−Soc− T4 capsid with nanomolar affinity in vitro. In order to display plague antigens, we first have to fuse F1mutV to Soc protein. The reason we chose Soc is because it has 5.6 times higher binding sites (870 per capsid) when compared to Hoc (155 per capsid). Thus, Soc fusion can display more antigen molecules per capsid.
3.2.1 Construction of pET-F1mutVSoc
We first constructed a “universal” vector, pET-Soc-C, which contains multiple cloning sites upstream of NH2-terminus of Soc, by inserting Soc into expression vector pET28b. Any foreign protein can be inserted into upstream of NH2-terminus of Soc and generate an in-frame fusion with the NH2-terminal end of SOC. F1mutV was amplified by PCR from pET-F1mutV and inserted into pET-Soc-C to generate pET-F1mutVSoc.
-
Use Thermo 2× Phusion High-Fidelity PCR Master Mix to amplify RB69 Soc gene DNA from RB69 phage genome DNA, using the end primers 8 and 9 containing Hind III and Xho I restriction sites (highlighted in bold), respectively (see Note 12).
Primer 8: 5′-TCGAC AAGCTT CT GCT GGTGGTT ATGTAAACATCAAA-3′
Primer 9: 5′-TGGTG CTCGAG ACCACTTACTGGTGTAGGGGT-3′
Purify PCR product by gel electrophoresis and gel extraction using Thermo gel extraction kit.
Cut the insert (PCR product) with restriction enzymes, Hind III and Xho I, and at the same time cut the pET28b vector plasmid DNA using the same restriction enzymes.
Repeat steps 6–9 of Subheading “Construct pET-F1mut1.”
Isolate plasmid DNA using Thermo GeneJET plasmid mini-prep kit, following the manufacturer’s instructions. The generated plasmid was named pET-Soc-C.
-
Amplify F1mutV DNA from template, pET-F1mutV, using the primer 6 and primer 10 which contains HindIII restriction sites using Thermo 2× Phusion High-Fidelity PCR Master Mix.
Primer 6: 5′-TAATACGACTCACTATAGGGGA-3′
Primer 10: 5′-CCT AAGCTT CTTTACCAGACGTGTCATCTAGCAGAC-3′
Cut the insert (PCR product) with restriction enzymes, NheI and Hind III, and at the same time cut the pET-Soc-C vector DNA (described in step 5) using the same restriction enzymes.
Repeat steps 6–9 of Subheading “Construct pET-F1mut1.”
Isolate plasmid DNA using Thermo GeneJET plasmid mini-prep kit, following the manufacturer’s instructions. The resulting clone, pET-F1mutVSoc, contained F1mutV fused in-frame to the NH2-terminus of RB69 Soc and also the flanking vector sequences containing two hexa-histidine tags at both NH2- and COOH-termini.
3.2.2 Purification of Recombinant F1mutV-Soc from E. coli
Transform 10 ng of pET-F1mutVSoc into 25 μl BL21-CodonPlus (DE3)-RIPL competent cells, according to the manufacturer’s instructions. Incubate plate overnight at 37 °C.
Repeat all procedures from steps 2 to 24 of Subheading 3.1.2.
3.2.3 Purification of Hoc−Soc− Phage T4
Use pipette tip to streak the glycerol stock of E. coli P301 cells on an LB plate. Incubate at 37 °C overnight.
Pick a single colony, inoculate into a 125 ml flask containing 20 ml LB medium, and incubate the flask in shaking incubator at 220 rpm, 37 °C, for 8 h.
Inoculate 10 ml of cultures in step 2 into a 2 L flask containing 500 ml of LB and M9CA medium (250 ml LB + 250 ml M9CA). Incubate the flask in a shaking incubator at 220 rpm and 37 °C until the cell density raises to 2.0 × 108 cells/ml.
Infect the culture with Hoc−Soc− phage T4 at multiplicity of infection (MOI) of 0.2 by adding 2 × 1010 plaque-forming units (PFU) Hoc-Socphage T4 (see Note 13).
Incubate the flask in shaking incubator at 200 rpm, 37 °C, for 30 min, allow the culture to grow for 2–3 h, and observe for phage growth (see Note 14).
After confirmation of phage growth, add 10 ml chloroform into the flask and keep it shaking at 200 rpm for 10 min at 37 °C.
Distribute the culture into 250 ml centrifugation tubes, and centrifuge for 45 min at 12,000 rpm (23,440 g) with GSA rotor in Sorvall RC-5C plus centrifuge.
Discard the supernatant and resuspend the pellet by adding 15 ml Pi-Mg buffer.
Add DNase I to a final concentration of 10 μg/ml and 500 μl chloroform, and keep shaking at 220 rpm in 37 °C for 30 min.
Centrifuge at 6000 rpm (4,300 g) for 10 min with SS34 rotor in Sorvall RC-5C plus centrifuge, discard the pellet, and collect the supernatant which contains phage.
Centrifuge at 16,000 rpm (30,624 g) for 45 min with SS34 rotor in Sorvall RC-5C plus centrifuge, discard the supernatant, and resuspend the pellet in 2 ml Pi-Mg buffer.
- Make CsCl gradient solution. First, prepare layer buffer as in the table below; then from the bottom to the top, sequentially add 750 μl of layer buffer No. 6, No. 5, No. 4, No. 3, No. 2, and No. 1 to 5 ml Beckman centrifuge tube.
Layer no. Stock CsCl (ml) H2O (ml) Total volume (ml) 1 1 4 5 2 1.5 3.5 5 3 2 3 5 4 2.5 2.5 5 5 3 2 5 6 3.5 1.5 5 Add 0.5 ml resuspended phage sample from step 11 to the top of the CsCl gradient solution, and centrifuge at 35,000 rpm (148,596 g) for 1 h at 4 °C using SW55 Ti rotor in Beckman L-60 Ultracentrifuge.
Following centrifugation, observe for the visible turbid phage bands. Fasten the tube to a vertical holder and using syringe needle, pierce the wall of centrifugation tube at the bottom of the phage band. Aspirate the phage band into 5 ml syringe.
Injection of phage sample into Slide-A-lyzer dialysis cassette and dialyze first against dialysis buffer I for 5 h at 4 °C and then against dialysis buffer II overnight at 4 °C.
Collect the phage sample and store at 4 °C for future use. The following steps will determine the titer of the phage using standard method.
Take seven clean glass tubes labeled 1–7, and add 990 μl Pi-Mg buffer into each tube.
Add 10 μl phage sample into the tube 1 and vortex to mix. Transfer 10 μl sample from tube 1 into tube 2 and vortex to mix. Continue to dilute the phage sample till tube 6. Add 10 μl Pi-Mg buffer into tube 7 which services as a control (see Note 15).
Transfer 100 μl of diluted phage samples or control from tubes 1 to 7 separately into 7 new clean glass tubes.
Add 400 μl of freshly grown E. coli P301 into each tube and keep tubes at 37 °C for 7–8 min.
Add 2.5 ml of top-agar to each tube and mix; immediately pour onto LB plates, shake the plates for uniform distribution, and keep the plates at room temperature for 10 min to solidify the top-agar (see Note 16).
Incubate the plates at 37 °C overnight.
Count the number of plaques in each plate and calculate the titer of phage sample.
3.2.4 Preparation of Antigen and Animal Immunizations
The exact amount of Hoc−Soc− T4 phages and protein depends on how many animals will be used. Here the dose we will describe is for one animal (10 μg antigen/animal).
Take about 1.5 × 1011 phage particles and centrifuge at 15,000 rpm (21,130 g) in 1.5 ml LoBind Eppendorf tubes for 45 min at 4 °C using AM 2.18 rotor in Jouan MR-23i centrifuge (see Note 17).
Wash the pellet by adding 1.0 ml PBS and another round of centrifugation as in step 1.
Discard the supernatant, add 400 μl PBS to the tube, and resuspend the phage pellet overnight.
Add 290 μg F1mutV-Soc to the tube that contains phage, adjust the volume to 800 μl with PBS, gently vortex to mix, and incubate at 4 °C for 45 min.
Sediment the phage particles at 15,000 rpm (21,130 g) for 45 min at 4 °C using AM 2.18 rotor in Jouan MR-23i centrifuge, and the supernatant containing the unbound protein was discarded.
Wash the phage pellet containing the bound plague antigen (F1mutV) twice by adding 1.0 ml PBS and centrifugation as in step 1.
Add 50 μl PBS to the pellet, resuspend at 4 °C overnight, and analyze by SDS-PAGE as described in procedures from steps 18 to 24 of Subheading 3.1.2. Determine the copy numbers of F1mutV per capsid.
Immunize the animals by i.m. injection.
4 Notes
Acknowledgements
This work was supported by grants from the National Institutes of Health (NIAID U01-AI082086 and R01-AI111538). The authors thank Dr. Ashok Chopra, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX, for his collaboration in testing the immunogenicity and protective efficacy of the vaccine formulations described in this chapter.
Footnotes
F1 protein has a 21-aa signaling peptide, which was not included in the numbering of nucleotides (nt) or amino acids (aa). Thus F1 starts with GCAGATTTAACTGCAA…(nt) or ADLTASTTATATL…(aa).
To ensure restriction enzyme digestion after PCR amplification, an additional 2–6 bp (depends on the enzyme) should be included at the 5′-end of the primers.
Purification is necessary in order to remove resident primer 2 as well as F1 gene template. Both of these should be avoided in order to get a pure F1 mutant intermediate 2.
There is no stop codon before restriction site in reverse primer. So, the resultant plasmid contains V gene fused in-frame with a 13-aa vector sequence that includes the hexa-histidine sequence at the C-terminus.
RIPL cells produce higher yields when compared to any other BL21 host cells available in the Novagen.
The flask should be kept shaking at 30 °C for at least 30 min to cool down the E coli. The higher temperature may increase the chance of partitioning the over-expressed protein into the inclusion bodies, thus reducing the yield of soluble protein.
Proteinase inhibitors are necessary during purification. Steps 7–17 have to be carried out at 4 °C.
All the solutions and samples that go to column have to go through 0.22 μm filter; otherwise the viscous sample will clog the column.
Keep the flow rate at 1 ml/min for 1 ml HisTrap column or max 5 ml/min for 5 ml HisTrap column. Increased flow rate will decrease protein binding to column.
BIORAD Bio-Safe™ Coomassie stain is used, which does not need special handing. If regular Coomassie stain is used, follow procedures on use and disposal of the stain.
Alhydrogel is thick and settles down. Shake vigorously to get a uniform suspension before using.
Since recombinant phage T4 Soc protein is not soluble, it is strongly recommended to use the T4-related phage RB69 Soc to construct fusion protein. Previous studies showed that the recombinant RB69 Soc is soluble and binds to T4 capsid at nearly the same affinity as T4 Soc.
Mix it immediately after adding the phage so that phage will be distributed uniformly.
The growth of phage can be assessed either by looking for turbidity and floating cell debris in the culture flask or after chloroform treatment, and by observation under light microscope.
Chloroform treatment: Take 1 ml of culture in a test tube and add four drops of chloroform. If the cells are infected well, they lyse instantly clearing the cell suspension and cellular debris can be seen floating in the sample.
Observation under light microscope: Put a drop of culture on the chamber of cell counter and cover it with a cover slip. Focus at individual E. coli cells by fine adjustment. The appearance of clear center and black/dark spots at the poles (ends) of the cells indicates good phage infection.
To get accurate titer, keep changing the pipette tip during the dilution of the phage sample.
The plates have to be kept at room temperature for at least 10 min to make sure that the top-agar solidifies.
Protein and DNA may nonspecifically bind to Eppendorf tube. Thus it is recommended to use low-binding tubes such as LoBind Eppendorf tube.
References
- 1.Wagner DM, et al. Yersinia pestis and the plague of Justinian 541–543 AD: a genomic analysis. Lancet Infect Dis. 2014;14:319–326. doi: 10.1016/S1473-3099(13)70323-2. [DOI] [PubMed] [Google Scholar]
- 2.Guernier V, et al. Fleas of small mammals on Reunion Island: diversity, distribution and epidemiological consequences. PLoS Negl Trop Dis. 2014;8(9):e3129. doi: 10.1371/journal.pntd.0003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smiley ST. Current challenges in the development of vaccines for pneumonic plague. Expert Rev Vaccines. 2008;7:209–221. doi: 10.1586/14760584.7.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zilinskas RA. The anti-plague system and the Soviet biological warfare program. Crit Rev Microbiol. 2006;32:47–64. doi: 10.1080/10408410500496896. [DOI] [PubMed] [Google Scholar]
- 5.Sha J, et al. Deletion of Braun lipoprotein encoding gene and altering the function of lipopolysaccharide attenuate plague bacterium. Infect Immun. 2013;81:815–828. doi: 10.1128/IAI.01067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenzweig JA, et al. Progress on plague vaccine development. Appl Microbiol Biotechnol. 2011;91:265–286. doi: 10.1007/s00253-011-3380-6. [DOI] [PubMed] [Google Scholar]
- 7.Zavialov AV, et al. Structure and biogenesis of the capsular F1 antigen from Yersinia pestis: preserved folding energy drives fiber formation. Cell. 2003;113:587–596. doi: 10.1016/s0092-8674(03)00351-9. [DOI] [PubMed] [Google Scholar]
- 8.Stenseth NC, et al. Plague: past, present, and future. PLoS Med. 2008;5(1):e3. doi: 10.1371/journal.pmed.0050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derewenda U, et al. The structure of Yersinia pestis V-antigen, an essential virulence factor and mediator of immunity against plague. Structure. 2004;12:301–306. doi: 10.1016/j.str.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Williamson ED, et al. A new improved sub-unit vaccine for plague: the basis of protection. FEMS Immunol Med Microbiol. 1995;12:223–230. doi: 10.1111/j.1574-695X.1995.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson GW, Jr, Heath DG, Bolt CR, Welkos SL, Friedlander AM. Short- and long-term efficacy of single-dose subunit vaccines against Yersinia pestis in mice. Am J Trop Med Hyg. 1998;58:793–799. doi: 10.4269/ajtmh.1998.58.793. [DOI] [PubMed] [Google Scholar]
- 12.Heath DG, et al. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine. 1998;16:1131–1137. doi: 10.1016/s0264-410x(98)80110-2. [DOI] [PubMed] [Google Scholar]
- 13.Goodin JL, et al. Purification and protective efficacy of monomeric and modified Yersinia pestis capsular F1-V antigen fusion proteins for vaccination against plague. Protein Expr Purif. 2007;53:63–79. doi: 10.1016/j.pep.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodin JL, et al. Purification and characterization of a recombinant Yersinia pestis V-F1 “Reversed” fusion protein for use as a new subunit vaccine against plague. Protein Expr Purif. 2011;76:136–144. doi: 10.1016/j.pep.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Mizel SB, et al. Flagellin-F1-V fusion protein is an effective plague vaccine in mice and two species of nonhuman primates. Clin Vaccine Immunol. 2009;16:21–28. doi: 10.1128/CVI.00333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell BS, et al. Design and testing for a nontagged F1-V fusion protein as vaccine antigen against bubonic and pneumonic plague. Biotechnol Prog. 2005;21:1490–1510. doi: 10.1021/bp050098r. [DOI] [PubMed] [Google Scholar]
- 17.Parent MA, et al. Cell-mediated protection against pulmonary Yersinia pestis infection. Infect Immun. 2005;73:7304–7310. doi: 10.1128/IAI.73.11.7304-7310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao P, et al. Mutated and bacteriophage T4 nanoparticle arrayed F1-V immunogens from Yersinia pestis as next generation plague vaccines. PLoS Pathog. 2013;9(7):e1003495. doi: 10.1371/journal.ppat.1003495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Shivachandra SB, Leppla SH, Rao VB. Bacteriophage T4 capsid: a unique platform for efficient surface assembly of macromolecular complexes. J Mol Biol. 2006;363:577–588. doi: 10.1016/j.jmb.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 20.Sathaliyawala T, et al. Assembly of human immunodeficiency virus (HIV) antigens on bacteriophage T4: a novel in vitro approach to construct multicomponent HIV vaccines. J Virol. 2006;80:7688–7698. doi: 10.1128/JVI.00235-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews GP, Heath DG, Anderson GW, Jr, Welkos SL, Friedlander AM. Fraction 1 capsular antigen (F1) purification from Yersinia pestis CO92 and from an Escherichia coli recombinant strain and efficacy against lethal plague challenge. Infect Immun. 1996;64:2180–2187. doi: 10.1128/iai.64.6.2180-2187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J, et al. Macromolecular organisation of recombinant Yersinia pestis F1 antigen and the effect of structure on immunogenicity. FEMS Immunol Med Microbiol. 1998;21:213–221. doi: 10.1111/j.1574-695X.1998.tb01168.x. [DOI] [PubMed] [Google Scholar]
- 23.Musson JA, et al. Sequential proteolytic processing of the capsular Caf1 antigen of Yersinia pestis for major histocompatibility complex class II-restricted presentation to T lymphocytes. J Biol Chem. 2006;281:26129–26135. doi: 10.1074/jbc.M605482200. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Shivachandra SB, Zhang Z, Rao VB. Assembly of the small outer capsid protein, Soc, on bacteriophage T4: a novel system for high density display of multiple large anthrax toxins and foreign proteins on phage capsid. J Mol Biol. 2007;370:1006–1019. doi: 10.1016/j.jmb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shivachandra SB, et al. Multicomponent anthrax toxin display and delivery using bacteriophage T4. Vaccine. 2007;25:1225–1235. doi: 10.1016/j.vaccine.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black LW, Rao VB. Structure, assembly, and DNA packaging of the bacteriophage T4 head. Adv Virus Res. 2012;82:119–153. doi: 10.1016/B978-0-12-394621-8.00018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fokine A, et al. Molecular architecture of the prolate head of bacteriophage T4. Proc Natl Acad Sci U S A. 2004;101:6003–6008. doi: 10.1073/pnas.0400444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishii T, Yanagida M. The two dispensable structural proteins (soc and hoc) of the T4 phage capsid; their purification and properties, isolation and characterization of the defective mutants, and their binding with the defective heads in vitro. J Mol Biol. 1977;109:487–514. doi: 10.1016/s0022-2836(77)80088-0. [DOI] [PubMed] [Google Scholar]
- 29.Qin L, Fokine A, O’Donnell E, Rao VB, Rossmann MG. Structure of the small outer capsid protein, Soc: a clamp for stabilizing capsids of T4-like phages. J Mol Biol. 2009;395:728–741. doi: 10.1016/j.jmb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sathaliyawala T, et al. Functional analysis of the highly antigenic outer capsid protein, Hoc, a virus decoration protein from T4-like bacteriophages. Mol Microbiol. 2010;77:444–455. doi: 10.1111/j.1365-2958.2010.07219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao P, et al. In vitro and in vivo delivery of genes and proteins using the bacteriophage T4 DNA packaging machine. Proc Natl Acad Sci U S A. 2013;110:5846–5851. doi: 10.1073/pnas.1300867110. [DOI] [PMC free article] [PubMed] [Google Scholar]