Abstract
A fusion protein of antigen 85B (Ag85B) and ESAT-6 administered in cationic lipid vesicles conferred a highly significant level of protection against Mycobacterium tuberculosis in the guinea pig aerosol model of infection. The protection was manifested as delayed clinical illness and prolonged survival. Neither Ag85B nor ESAT-6 (independently or as a cocktail) induced significant protection in this model.
Today it is clear that while Mycobacterium bovis BCG protects children very efficiently against tuberculosis (TB) (7), estimates of protection against adult pulmonary TB vary from 0 to 80% in large well-controlled field trials (8). In sharp contrast to its varying efficacy in human trials, BCG generally provides very efficient protection in animal models of TB (15). So far, most attempts to develop TB subunit vaccines have resulted in vaccines of too low activity to be practical for use in humans. In particular, in the guinea pig model, subunit vaccines have not been able to compete with the high activity of BCG (2, 13). The reasons for this paradox and for the failure of BCG in some trials have been the subject of debate since the 1950s. Today the prevailing hypothesis centers on the interaction between BCG and a diverse group of nonpathogenic soil saprophytes, the so-called environmental mycobacteria (8). It was recently demonstrated with the mouse model that in contrast to BCG, TB subunit vaccines are not compromised by exposure to environmental mycobacteria (5) or prior BCG vaccination (6). Therefore, this observation suggests that although a subunit vaccine may not be more efficient than BCG under conditions where the efficacy of BCG is high, it would nonetheless have a profound influence on the global TB epidemic.
We have recently applied a strategy based on molecular engineering of vaccine candidate antigens into fusion proteins to develop a novel (anti-)TB vaccine(s) (12). The antigens we have chosen are antigen 85B (Ag85B) and ESAT-6, antigens that are generally recognized as immunodominant antigens (3). In the present study, we have tested this vaccine in the aerosol guinea pig model of TB infection by monitoring the clinical development of disease and the survival time of vaccinated guinea pigs.
Outbred Dunkin Hartley guinea pigs (Charles River, Sulzfeld, Germany) were infected and housed in cages contained within a biosafety level 3 laminar-flow enclosure. Guinea pigs were weighed weekly and euthanized if they had lost 17 to 20% of their maximum weight or if other signs of severe illness were observed, in accordance with the Statens Serum Institute and Health Protection Agency, Porton Down, ethical committees.
Mycobacterium tuberculosis Erdman and H37Rv were grown at 37°C in modified Sauton medium enriched with 0.5% sodium pyruvate and 0.5% glucose, while BCG Danish 1331 was obtained as a freeze-dried vaccine, and recombinant ESAT-6 dimer (dESAT-6), Ag85B, and Ag85B-ESAT-6 were produced as described previously (12, 14).
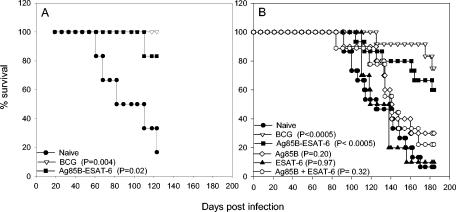
All vaccines were formulated with cationic lipid vesicles based on dioctadecylammonium bromide (DDA) (Eastman Kodak, Rochester, N.Y.) as described previously (1). Each injection contained 500 μg of DDA made as an adjuvant with either monophosphoryl lipid A (MPL; Corixa, Seattle, Wash.) (50 μg/guinea pig) or trehalose dibehenate (TDB; Sigma Chemical Co.) (200 μg/guinea pig). When combined with DDA, these two immunomodulators have recently been demonstrated to promote efficacy similar to that of potential TB vaccines (10). Guinea pigs were immunized with 20 μg of antigen in either DDA-MPL (Fig. 1A) or DDA-TDB (Fig. 1B). The cocktail of Ag85B and ESAT-6 contained 10 μg of Ag85B and 10 μg of dESAT-6. The experimental vaccines were given three times subcutaneously with a 3-week interval between each administration. BCG Danish 1331 (5 × 104 bacilli/guinea pig) was injected subcutaneously once at the same time as the first vaccination. The immune response promoted by the vaccines was evaluated by measuring skin test responses 4 weeks after the last immunization. The backs of the guinea pigs were shaven, and intradermal injections of 2.5 μg of BCG culture filtrate, dESAT-6, and Ag85B in physiological phosphate-buffered saline (pH 7.4) were administered. The delayed-time hypersensitivity (DTH) reactions were read in a blinded fashion after 24 h by two independent readers. The DTH reaction was associated with both induration and erythema, and we measured the erythema lesions to minimize variability in judgment of the readers. Guinea pigs were challenged 12 weeks after the initial vaccination (6 weeks after the third vaccination) in either a Glass-Col inhalation exposure system with M. tuberculosis Erdman (Fig. 1B) or with H37Rv by use of a contained Henderson apparatus (Fig. 1A). In both aerosol systems, the guinea pigs received approximately 10 to 20 CFU/lung.
FIG. 1.
(A) Survival curves of guinea pigs that were aerosol-infected with M. tuberculosis H37Rv throughout an observation period of 123 days (n = 6 mice) (CAMR, Salisbury, United Kingdom); (B) survival curves of guinea pigs that were aerosol-infected with M. tuberculosis Erdman throughout an observation period of 184 days (n = 9 to 15 mice) (Statens Serum Institute). The figure shows combined data from two experiments. A P of <0.05 was considered significant by the Kaplan-Meier rank sum test. Ag85B-ESAT-6, fusion protein; Ag85B + ESAT-6, antigen cocktail.
Groups of 6 (experiment 1) and groups of up to 15 (experiment 2) guinea pigs were vaccinated with either the BCG vaccine, the fusion protein (Ag85B-ESAT-6), Ag85B, dESAT-6, or a cocktail of the two antigens (Ag85B and ESAT-6 produced separately and then mixed). One group of animals was left untreated. Before their challenge, four guinea pigs from each group (experiment 2) were tested for DTH responses to the vaccine components (Table 1). All groups responded strongly to the vaccination, with both components of the vaccine being recognized after immunization with the fusion protein and the cocktail of Ag85B and ESAT-6. ESAT-6 was, however, recognized more strongly after vaccination with the fusion protein (lesion diameter, 21.0 mm) than after vaccination with the cocktail (lesion diameter, 17.7 mm). In particular, vaccination with ESAT-6 alone induced a significantly lower ESAT-6 DTH response (lesion diameter, 12.8 mm) than the fusion protein (P = 0.03; analysis of variance followed by the Tukey test). The development of clinical disease was monitored for a period of 123 days (experiment 1) (Fig. 1A) or 184 days (experiment 2) (Fig. 1B) postchallenge. In both experiments, control animals showed progressive loss of body weight and were euthanized between day 60 and day 123 (Fig. 1A) (median survival time [MST] = 82 days) or between day 92 and day 170 (Fig. 1B) (MST = 125 days). One guinea pig in each control group was still alive when the experiments were terminated. Neither the individual antigens nor the cocktail of the two antigens prolonged the life spans of the infected animals significantly, and similar survival curves were observed for these groups. The ESAT-6-vaccinated group had an MST of 128.5 days, the Ag85B-vaccinated group had an MST of 140 days, and the group vaccinated with the cocktail of the two antigens had an MST of 141 days. This was in direct contrast to the significant levels of protection induced by the fusion protein vaccine and BCG (P <0.05) at both time points. Importantly, no significant differences in the levels of protection induced by BCG and the fusion protein vaccine were found (P > 0.05; Kaplan-Meier survival curves and the log rank test).
TABLE 1.
DTH responses (skin test) in vaccinated guinea pigs
| Vaccine group | DTH response (mm) (mean ± SEM)a
|
||
|---|---|---|---|
| BCG CF | Ag85B | dESAT-6 | |
| BCG | 17.4 ± 1.2 | ND | ND |
| Ag85B-ESAT-6 fusion | ND | 14.3 ± 1.4 | 21.0 ± 2.8 |
| Ag85B | ND | 19.1 ± 1.4 | ND |
| ESAT-6 | ND | ND | 12.8 ± 1.1 |
| Ag85B+ESAT-6 cocktail | ND | 15.1 ± 1.2 | 17.7 ± 1.1 |
DTH responses were read 24 h after vaccination with 2.5 μg of BCG culture filtrate (CF), Ag85B, or dESAT-6. The values given are the mean diameters of the lesions for four guinea pigs. Responses larger than 5 mm were regarded as positive. Responses to phosphate-buffered saline were less than 5 mm. ND, not done (only antigens included in the vaccine were tested for DTH reactivity).
When the experiment was terminated (only one control left), a number of survivors were still present in the BCG- and fusion protein-vaccinated groups. To study the long-term fate of the vaccinated animals, six guinea pigs from the fusion protein group and six from the BCG-vaccinated group were monitored for more than 1 year postinfection. By week 46, the majority of the guinea pigs had been euthanized, leaving just two BCG-vaccinated guinea pigs alive but with weight loss indicating severe clinical disease. They were subsequently euthanized at weeks 56 and 66.
The guinea pig model is generally accepted as the most demanding test for TB vaccines. In this study, both the individual components and the cocktail of the two molecules failed to give significant protection. This is striking as Ag85B (11), ESAT-6 (4), and the cocktail of the two molecules (12) have been demonstrated to promote efficient protection in the mouse model of TB. Of relevance in this regard is the observation in the present paper that the immune response induced by the ESAT-6 vaccine is much lower than the response induced by this antigen fused to Ag85B. This difference, which is seen as a twofold increase in the DTH response to ESAT-6, may in fact reflect a very substantial difference in the biological activities of these vaccines. Of relevance in this regard is a logarithmic relationship between dose or sensitivity and skin reactions, which must be taken into account when interpreting differences. Taking this relationship into consideration, it can be deduced that the observed difference of 8 mm probably corresponds to an ESAT-6 reactivity that is as much as 10 times higher in the guinea pigs immunized with the fusion protein than in those immunized with ESAT-6 alone (9), which is in agreement with the relatively low inherent immunogenicity previously demonstrated for ESAT-6 (4).
It seems, therefore, that in the guinea pig model only the most efficient vaccines provide significant long-term protection against the development of disease. This observation is in agreement with earlier reports of protection against TB challenge with a culture filtrate-based subunit vaccine in mice (1), which subsequently failed to provide long-term protection, measured by survival, in the guinea pig model (2).
The present study demonstrates that TB subunit vaccines can promote efficient protection that is close to the level provided by live BCG in the highly susceptible guinea pig model. This is a highly significant finding that can be truly appreciated only when one takes into account the negative outcome from a search of the literature for earlier evidence of a nonviable TB vaccine with activity in the very demanding guinea pig model. None of the 20 or more experimental TB subunit vaccines with reported activity in the mouse model have as yet passed this test.
Acknowledgments
We thank the staff in the P3 animal facility and Lene K. Rasmussen for excellent technical assistance.
This study was supported by TB Vaccine Cluster EU contract QLK2-1999-01093 and EC contract LSHP-CT-2003503367 TBVAC (FP6 call for proposals: FR&-2002-LifeSciHealth).
Editor: J. D. Clements
REFERENCES
- 1.Andersen, P. 1994. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect. Immun. 62:2536-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin, S. L., C. D'Souza, A. D. Roberts, B. P. Kelly, A. A. Frank, M. A. Lui, J. B. Ulmer, K. Huygen, D. M. McMurray, and I. M. Orme. 1998. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66:2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boesen, H., B. N. Jensen, T. Wilcke, and P. Andersen. 1995. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect. Immun. 63:1491-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt, L., M. Elhay, I. Rosenkrands, E. B. Lindblad, and P. Andersen. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt, L., J. Feino Cunha, A. Weinreich Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks, J. V., A. A. Frank, M. A. Keen, J. T. Bellisle, and I. M. Orme. 2001. Boosting vaccine for tuberculosis. Infect. Immun. 69:2714-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colditz, G. A., C. S. Berkey, F. Mosteller, T. F. Brewer, M. E. Wilson, E. Burdick, and H. V. Fineberg. 1995. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96:29-35. [PubMed] [Google Scholar]
- 8.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 9.Hasløv, K., M. W. Bentzon, and S. Moller. 1986. Comparison in sensitized and unsensitized guinea-pigs of tuberculin PPDs RT 23 and PPD-M by skin tests and lymphocyte stimulation tests. Effect of immunization time. J. Biol. Stand. 14:143-152. [DOI] [PubMed] [Google Scholar]
- 10.Holten-Andersen, L., T. M. Doherty, K. S. Korsholm, and P. Andersen. 2004. Combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and synthetic mycobacterial cord factor as an efficient adjuvant for tuberculosis subunit vaccines. Infect. Immun. 72:1608-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz, M. A., B. W. Lee, B. J. Dillon, and G. Harth. 1995. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 92:1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen, A. W., L. A. H. van Pinxteren, L. M. Okkels, P. B. Rasmussen, and P. Andersen. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and ESAT-6. Infect. Immun. 69:2773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orme, I. M., D. N. McMurray, and J. T. Belisle. 2001. Tuberculosis vaccine development: recent progress. Trends Microbiol. 9:115-118. [DOI] [PubMed] [Google Scholar]
- 14.Pollock, J. M., J. McNair, H. Bassett, J. P. Cassidy, E. Costello, H. Aggerbeck, I. Rosenkrands, and P. Andersen. 2003. Specific delayed-type hypersensitivity responses to ESAT-6 identify tuberculosis-infected cattle. J. Clin. Microbiol. 41:1856-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith, D. W. 1985. Protective effect of BCG in experimental tuberculosis. Adv. Tuberc. Res. 22:1-97. [PubMed] [Google Scholar]