Abstract
After chikungunya virus (CHIKV) transmission was detected in Puerto Rico in May 2014, multiple surveillance systems were used to describe epidemiologic trends and CHIKV-associated disease. Of 28 327 cases reported via passive surveillance, 6472 were tested for evidence of CHIKV infection, and results for 4399 (68%) were positive. Of 250 participants in household cluster investigations, 70 (28%) had evidence of recent CHIKV infection. Enhanced surveillance for chikungunya at 2 hospitals identified 1566 patients who tested positive for CHIKV, of whom 10.9% were hospitalized. Enhanced surveillance for fatal cases enabled identification of 31 cases in which CHIKV was detected in blood or tissue specimens. All surveillance systems detected a peak incidence of chikungunya in September 2014 and continued circulation in 2015. Concomitant surveillance for dengue demonstrated low incidence, which had decreased before CHIKV was introduced. Multifaceted chikungunya surveillance in Puerto Rico resolved gaps in traditional passive surveillance and enabled a holistic description of the spectrum of disease associated with CHIKV infection.
Keywords: chikungunya, dengue, Puerto Rico, surveillance
Following the introduction of chikungunya virus (CHIKV) into the Caribbean in late 2013 [1, 2], multiple challenges to case surveillance were expected throughout the region, including in the United States territory of Puerto Rico. First, although recommendations for surveillance had been provided by the Centers for Disease Control and Prevention (CDC) and the Panamerican Health Organization in anticipation of CHIKV introduction into the Americas [3], surveillance for chikungunya in Puerto Rico was not yet in place, where clinical and public awareness of chikungunya was low. Second, laboratory capacity to routinely test suspected cases did not exist. Third, although severe and potentially fatal manifestations of CHIKV infection [4–7] had been reported to occur most frequently in infants and the elderly [8–10], the expected incidence of such manifestations was unclear owing to differences in surveillance methods and potential population-specific differences (eg, underlying comorbidities, demographic age structure, and genetic predisposition). Fourth, multiple acute febrile illnesses (AFIs) are endemic in Puerto Rico, including dengue [11], influenza [12], leptospirosis [13], and melioidosis [14], all of which may be misdiagnosed as chikungunya and vice versa. Thus, accurate diagnosis of chikungunya was needed to enable appropriate and early initiation of clinical management of AFIs. Last, all of these challenges were expected to be exacerbated by the anticipated rapid spread and high rate of infection (eg, 38%–63% [3, 15]) associated with chikungunya outbreaks. For these reasons, a variety of approaches was implemented to monitor the epidemiologic and clinical trends of the chikungunya epidemic in Puerto Rico.
PASSIVE CASE SURVEILLANCE
Passive surveillance for infectious diseases occurs when ill individuals seek medical care, a clinician diagnoses the case as being due to a suspected etiologic agent, and the case is reported to public health authorities either before or after laboratory diagnostic testing is performed. The primary goal of such passive surveillance is to monitor epidemiologic trends in diseases. In the case of chikungunya, such monitoring was expected to be delayed due to overwhelming case counts, as had been reported during prior outbreaks in immunologically naive populations [8, 10]. In Puerto Rico, the infrastructure for a passive chikungunya surveillance system (PCSS) was already in place, since the Puerto Rico Department of Health (PRDH) and the CDC had collaboratively operated the passive dengue surveillance system (PDSS) since the 1960s [11]. The epidemiologic trends of dengue have been monitored for decades via the PDSS, including detection of recent dengue epidemics in 2010 [16] and 2012–2013 (PRDH and CDC, unpublished data).
To initiate passive chikungunya surveillance, the existing dengue case investigation form (available at: http://www.cdc.gov/dengue/resources/dengueCaseReports/DCIF_English.pdf) was modified to specify clinical suspicion of chikungunya. Clinicians reported suspected chikungunya cases to the PRDH using the modified surveillance form, and the existing PDSS infrastructure was used to transport surveillance forms and serum specimens to the PRDH. Laboratory capacity was established at the PRDH for chikungunya diagnostic testing, first by reverse transcription–polymerase chain reaction (RT-PCR) [17] with updated primers and later by anti-CHIKV immunoglobulin M (IgM) capture (MAC) enzyme-linked immunosorbent assay(ELISA) [18]. After substantial increases in the number of reported cases of suspected chikungunya, diagnostic testing was prioritized for hospitalized patients and residents of municipalities that had not yet identified cases that had positive results of laboratory tests. Only patients reported to both the PDSS and the PCSS were tested for evidence of infection with both dengue virus (DENV) and CHIKV.
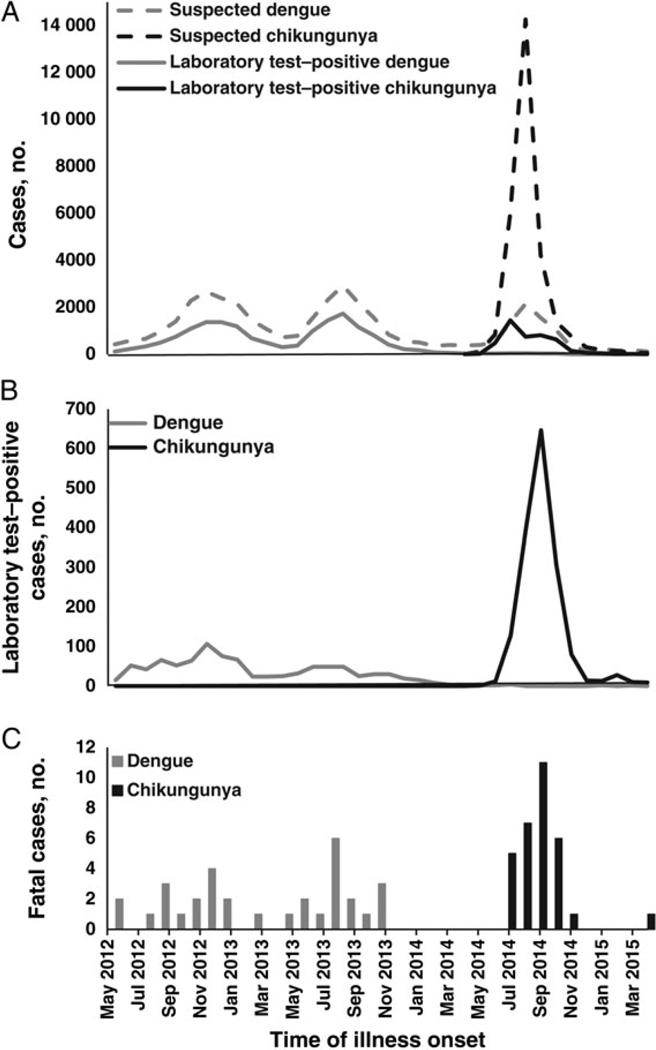
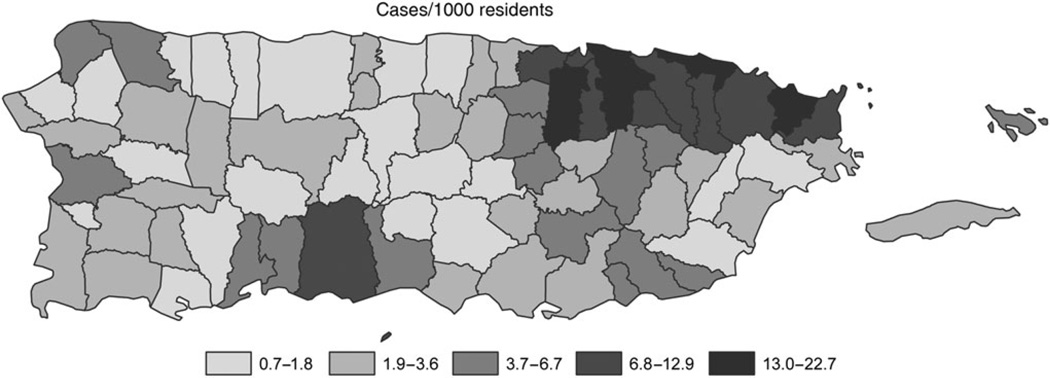
Suspected travel-associated and locally acquired chikungunya cases were reported via the PCSS beginning in January 2014. The first identified chikungunya case with positive results of laboratory tests had illness onset on 5 May 2014, did not report recent travel outside of Puerto Rico, and was a resident of the San Juan metropolitan area [19]. Soon thereafter, additional chikungunya cases were reported from throughout the San Juan metropolitan area and subsequently from throughout the island [19]. As case counts increased from hundreds to thousands per week (Figure 1A), the laboratory capacity of the PRDH was exceeded, and not all reported cases could be tested. An administrative order was issued in mid-September stating that only hospitalized cases of suspected chikungunya should be reported via the PCSS. Both hospitalized and nonhospitalized cases continued to be reported through 2015, and by April several dozen cases, including cases with positive results of laboratory tests, were being reported per week. During the first year of the CHIKV transmission, 28 327 suspected chikungunya cases (8.0 cases per 1000 population) had been reported via the PCSS from all 78 municipalities of Puerto Rico (Figure 2). The incidence of reported cases of suspected chikungunya was highest in the San Juan and Ponce metropolitan areas. Diagnostic testing was performed on 6472 suspected chikungunya cases, of which 4399 (68%) had positive results of laboratory tests; all tested positive by RT-PCR except 2 cases that were positive by MAC ELISA. Because MAC ELISA was not implemented into routine diagnostic testing algorithms until relatively late in the epidemic, it is likely that some chikungunya cases were misdiagnosed; however, the relative benefit of having this testing routinely performed versus the likelihood of detecting false-positive cases (ie, detection of anti-CHIKV IgM antibody in individuals who had been recently infected with CHIKV but were presenting for care due to AFI of another etiology) is unclear.
Figure 1.
Dengue and chikungunya cases detected through various surveillance systems, by month of illness onset, Puerto Rico, May 2012–April 2015. A, Cases of suspected dengue and chikungunya and cases with positive results of laboratory testing reported by passive surveillance to the Puerto Rico Department of Health. B, Cases of laboratory test–positive dengue and chikungunya detected through the Sentinel Enhanced Dengue Surveillance System. C, Fatal cases of dengue and chikungunya with positive results of laboratory testing detected through the Enhanced Fatal Acute Febrile Illness Surveillance System.
Figure 2.
Cases of suspected chikungunya reported to the Passive Chikungunya Surveillance System, by municipality, Puerto Rico, May 2014–April 2015.
Cases of suspected dengue and cases with positive results of laboratory testing that were detected by the PDSS were at low levels in mid-2014 following the dengue epidemic of 2012–2013 (Figure 1A). A peak in suspected dengue cases reported to the PDSS occurred concomitantly with the peak of the chikungunya epidemic in 2014; however, the proportion of cases with positive results of laboratory testing that were reported to PDSS did not increase, suggesting that the peak in reported cases of suspected dengue was attributable to chikungunya cases being reported to both the PCSS and the PDSS. During the first year of CHIKV circulation, 8142 suspected dengue cases were reported via the PDSS, of which 225 (2.8%) had positive results of laboratory tests, representing the lowest annual number of dengue cases reported to the PDSS in several decades. A total of 2552 cases were reported to both the PDSS and the PCSS, of which 916 (35.9%) were tested for evidence of infection with both CHIKV and DENV. Of these, 577 (63%) had evidence of infection with CHIKV, 20 (2.2%) had evidence of infection with DENV, and 1 (0.1%) had evidence of coinfection with both CHIKV and DENV, based on results of RT-PCR.
HOUSEHOLD-BASED CLUSTER INVESTIGATIONS
Because underreporting occurs in all passive surveillance systems owing to some ill individuals not seeking medical care, lack of clinical suspicion of chikungunya in clinically apparent cases, and lack of case reporting of clinically suspected cases [20, 21], the PCSS was not expected to detect all chikungunya cases in Puerto Rico. To provide a cross-sectional view of the reasons for underreporting of cases and subsequently attempt to correct such deficiencies, household-based cluster investigations were conducted during the first weeks of the epidemic [19]. A convenience sample of chikungunya cases with positive results of laboratory testing that were reported via the PCSS was contacted, and household visits were scheduled within 30 days of illness onset among index case patients. All households within a 50-m radius of the index case patients’ households were offered participation, which included completing a questionnaire and providing a serum specimen for chikungunya diagnostic testing.
A total of 21 cluster investigations were conducted, with 250 individuals from 137 households participating [19]. Of these, 70 individuals had evidence of current or recent CHIKV infection by RT-PCR or MAC ELISA [18], respectively, of whom 58 (83%) reported a current or recent AFI. The majority (63%) of recently ill individuals who had positive results of laboratory tests had sought care for their illness, and 13% reported having been hospitalized. However, few (20%) reported having received a diagnosis of chikungunya, and only 2 (8%) had been reported via the PCSS, demonstrating prominent underreporting of clinically apparent chikungunya cases. After conducting cross-testing for DENV infection, anti-DENV IgM antibody was detected in serum specimens from 12 household-investigation participants (5%); DENV nucleic acid was not detected in any specimens by RT-PCR. Thus, although there was no evidence of concomitant DENV and CHIKV circulation, serologic diagnostic test results demonstrated recent circulation of DENV.
Although useful in elucidating the reasons for the underreporting of chikungunya cases and estimating the ratio of symptomatic to asymptomatic CHIKV infections, limitations of the interpretation of the data gathered through household investigations included (1) a lack of representativeness, as participants in the cluster investigations tended to be older than individuals who were not available when surveys were conducted; and (2) potential changes after the initial months of the epidemic in the trends of ill individuals seeking care, clinical suspicion of chikungunya, and reporting of suspected cases.
ENHANCED, FACILITY-BASED SURVEILLANCE
The facility-based Sentinel Enhanced Dengue Surveillance System (SEDSS) was established in mid-2012 to better elucidate the incidence, etiology, and clinical course of febrile patients presenting for care at 2 hospitals in southern Puerto Rico. Rather than relying on clinicians to elect to report suspected cases, as occurs during passive surveillance, the SEDSS identifies cases by querying all patients for the presence of fever when they register at the emergency department. Febrile patients are then offered participation in the SEDSS, and those who agree to participate are tested for DENV, influenza virus, and other common causes of febrile illness. In April 2014, laboratory diagnostic testing for chikungunya by RT-PCR and MAC ELISA was added to the SEDSS diagnostic algorithm, and the first chikungunya case with positive results of laboratory testing was identified in late May (Figure 1B). Case counts began to steadily increase thereafter until nearly 650 chikungunya cases with positive results of laboratory tests were identified in September. Case detection steadily decreased thereafter and throughout early 2015. One year after it had been first detected, CHIKV was still being detected in patients enrolled in the SEDSS, although at comparatively low levels (n = 9 in April 2015). In total, 1566 chikungunya cases with positive results of laboratory tests were detected via the SEDSS during the first year following introduction of CHIKV, of which 94% tested positive by RT-PCR and 6% tested positive by MAC ELISA.
Similar to the patterns of detection of dengue cases observed through passive surveillance, the proportion of dengue cases with positive results of laboratory testing detected by the SEDSS was declining following the dengue epidemic in 2012–2013. This pattern continued during the first year of CHIKV circulation, wherein just 11 dengue cases with positive results of laboratory tests were identified. No CHIKV/DENV coinfections were identified by the SEDSS.
Whereas most previous studies of hospitalization rates and severe manifestations associated with chikungunya were based on passive surveillance, enhanced, facility-based surveillance enabled calculation of a more precise denominator by which to measure the frequency of hospitalization and potentially life-threatening complications directly or indirectly associated with chikungunya. Previously documented complications of CHIKV infection, namely bullous skin lesions and encephalitis, were observed in 8 patients (0.5%) and 7 patients (0.4%) with clinically apparent chikungunya, respectively. Of 171 patients (10.9%) with clinically apparent chikungunya who were hospitalized, many (39%) were infants or elderly. Common admitting diagnoses were generally associated with dehydration, pain management, and/or complications of preexisting conditions (eg, asthma, diabetes, and chronic heart disease); 8 patients (0.5%) with chikungunya were admitted to the intensive care unit. Two patients (0.1%) died, both of whom were elderly and had multiple comorbidities. Because tissue specimens were not available for either case, it is unclear whether these fatal cases were associated with exacerbation of preexisting conditions and/or whether CHIKV played a role in the fatal outcome.
ENHANCED DETECTION OF FATAL CASES
The Enhanced Fatal AFI Surveillance System (EFASS) has been collaboratively conducted by the PRDH, the Institute of Forensic Sciences of Puerto Rico, and the CDC since 2010. Owing to documented underreporting of fatal dengue cases [22], the EFASS was established to better enumerate the incidence, etiology, and clinical course of fatal cases with dengue-like illness. Under the EFASS, fatal cases were compiled for which premortem serum specimens were collected via passive and enhanced hospital-based surveillance systems and postmortem serum and tissue specimens were collected by forensic pathologists during autopsy of patients who died following dengue-like illness. During the first 3 years of operation of the EFASS, fatal cases of dengue, leptospirosis, and melioidosis were identified that would likely not otherwise have been detected [13, 14, 16, 23]. Diagnostic testing for CHIKV infection was added to the EFASS diagnostic algorithm in March 2014.
The first case of suspected fatal chikungunya was identified by forensic pathologists in late May 2014 and had positive results of RT-PCR [19]. Over the following 12 months, 31 CHIKV-associated fatal cases were detected through the EFASS that were positive by RT-PCR in serum and/or tissue specimens (0.9 cases per 100 000 population per year; Figure 1C). As 7 fatal cases were reported to the PRDH via passive surveillance, enhanced surveillance enabled detection of >3 times more CHIKV-associated fatal cases than passive surveillance alone. Of the 31 CHIKV-associated fatal cases identified, 10 (32%) died at home, and 6 (19%) had never sought medical care, both circumstances of which would have made identification through passive surveillance impossible. No fatal dengue cases were detected during the chikungunya epidemic.
Three additional pertinent findings were gleaned through enhanced surveillance for fatal cases. First, all but 2 (94%) of the identified CHIKV-associated fatal cases had one or multiple underlying comorbidities, most frequently diabetes, obesity, and hypertension. Such findings have been previously associated with fatal chikungunya cases [4]. Second, postmortem collection of tissue specimens from 25 cases enabled detection of CHIKV antigen by immunohistochemistry analysis in 11 cases (44%), of which 8 (32%) showed viral antigen in multiple organs. CHIKV antigen was identified most often in mesenchymal tissues and cells of the mononuclear-phagocytic system. Third, although most cases had died from complications of preexisting conditions and/or coinfection, at least 3 of the fatal cases with CHIKV antigen detected in tissue specimens had a clinical picture consistent with septic shock in the absence of evidence of bacterial coinfection, which has been reported as a rare outcome of CHIKV infection [4, 24]. Taken together, these findings suggested a rare but important role for CHIKV in fatal outcome. Histologic examination of tissue specimens from fatal cases therefore assisted in elucidating the pathophysiology of CHIKV infection in patients with severe disease.
COMPARATIVE DATA
Epidemiologic patterns detected through the PCSS, SEDSS, and EFASS were all in agreement regarding temporal trends of the chikungunya epidemic in Puerto Rico; however, each system provided a unique perspective of its magnitude and patients’ clinical course. Comparison of the incidence of cases detected through the SEDSS, which operated in just 2 of 62 hospitals in Puerto Rico and does not include cases seen at outpatient clinics or private physicians’ offices, suggested a burden of clinically apparent chikungunya larger than was captured through passive surveillance. Such enhanced surveillance systems are therefore necessary to more accurately quantitate the burden of chikungunya, dengue, and other AFIs [25, 26]. Also, all surveillance systems demonstrated circulation of CHIKV for several months after the peak of the epidemic. How long CHIKV circulation will continue in Puerto Rico is unclear.
Collecting similar clinical data via all 4 surveillance systems enabled indirect comparison of the clinical signs and symptoms associated with chikungunya (Table 1). In agreement with the expected manifestations of CHIKV infection, fever and arthralgia were reported in the large majority (≥90% and ≥70%, respectively) of patients. Although these characteristic manifestations of chikungunya were not identified in all cases of chikungunya with positive results of laboratory tests, their absence may be attributable to lack of collection or reporting of such manifestations, preverbal status (as among infants) and hence an inability to report subjective symptoms, and/or a variable spectrum of disease. Also, all surveillance systems that captured hospitalization status identified a similar rate of hospitalization among symptomatically infected individuals (approximately 10%). Severe manifestations were rarely documented (<1% of cases) in all surveillance systems, and even more rare was death in association with CHIKV infection (≤0.1% of cases). Of those patients who died while infected with CHIKV, death may more likely have been a result of exacerbation of preexisting medical conditions.
Table 1.
Demographic Characteristics, Clinical Signs and Symptoms, and Outcomes of People or Patients With Evidence of Chikungunya Virus Infection, as Gathered by Multiple Surveillance Systems, Puerto Rico, May 2014 – April 2015
| Variable | Household Investigations (n = 59) |
Passive Chikungunya Surveillance System (n = 4399) |
Sentinel Enhanced Dengue Surveillance System (n = 1566) |
Enhanced Fatal AFI Surveillance System (n = 31) |
|---|---|---|---|---|
| Diagnostic methods | ||||
| RT-PCR | 10 (17) | 4397 (100) | 1498 (96) | 31 (100) |
| MAC ELISA | 52 (88) | 2 (<0.1) | 148 (9) | 14 (45) |
| Immunohistochemistry | NT | NT | NT | 11 (35) |
| Demographic characteristics | ||||
| Age | 46 y (9 y–94 y) | 38 y (0 d–101 y) | 25 y (0 d–97 y) | 61 y (6 d–85 y) |
| Male sex | 32 (54) | 1876 (42.6)a | 739 (47.2) | 19 (61) |
| Pregnantb | 1 (2) | 8 (0.2) | 55 (4) | 1 (3) |
| Comorbidities | ||||
| Diabetes | 11 (19) | NC | 170 (11) | 15 (48) |
| Hypertension | 22 (37) | NC | 286 (18) | 16 (52) |
| Obesity | NC | NC | NC | 14 (45) |
| Asthma | 11 (19) | NC | 234 (15) | 4 (13) |
| Joint disease/arthritis | 15 (25) | NC | NC | 4 (13) |
| Lupus | NC | NC | NC | 2 (6) |
| Heart disease | 6 (10) | NC | 91 (6) | 4 (13) |
| Kidney disease | NC | NC | 24 (2) | 5 (16) |
| Signs and symptomsb | ||||
| Fever | 55 (93) | 712 (90) | 1459 (99.6) | 25 (81) |
| Arthralgia | 56 (95) | 627 (79) | 1294 (83) | 19 (61) |
| Myalgia | 48 (81) | 635 (80) | 1263 (81) | 15 (48) |
| Headache | 41 (69) | 544 (68) | 1120 (72) | 8 (26) |
| Eye pain | 18 (31) | 344 (43) | 718 (46) | 3 (10) |
| Anorexia | NC | 157 (20) | 888 (57) | 11 (35) |
| Lethargy | NC | 30 (4) | 1270 (81) | 19 (61) |
| Nausea/vomiting | 19 (32) | 226 (28) | 893 (57) | 7 (26) |
| Rash | 32 (54) | 327 (41) | 970 (62) | 12 (39) |
| Arthritis | 29 (42) | 114 (14) | 694 (44) | 6 (19) |
| Bleeding manifestations | 7 (12) | 220 (28) | 632 (40) | 11 (35) |
| Minorc | 7 (12) | 214 (27) | 102 (6.5) | 11 (35) |
| Majord | 0 (0) | 10 (1) | 1 (<0.1) | 2 (6) |
| Severe manifestationse | 0 (0) | 29 (4) | 46 (2.9) | 14 (27) |
| Clinical outcomesb | ||||
| Duration of illness, d | 6 (2–21) | NC | NC | 6 (1–28) |
| Sought medical care | 25 (63)f | NA | NA | 24 (77) |
| Hospitalized | 3 (12)f | 263 (12) | 171 (10.9) | 12 (39) |
| Duration of hospitalization, d |
7 (3–15)f | NC | … | 2 (0–23) |
| Admitted to ICU | 0 (0) | NC | 8 (0.5) | 8 (26) |
| Death | 0 (0) | 7 (0.1) | 2 (0.1) | 27 (100) |
Data are no. (%) of individuals or median value (range).
Abbreviations: AFI, acute febrile illness; ICU, intensive care unit; MAC ELISA, immunoglobulin M antibody capture enzyme-linked immunosorbent assay; NA, not applicable; NC, not captured; NT, none tested; RT-PCR, reverse transcription–polymerase chain reaction.
Sex was not provided for 472 cases (10.7%).
Cases detected by the Passive Chikungunya Surveillance System only include 795 for which clinical and demographic data were available or 2171 cases for which status of hospitalization was reported. Cases detected by the Enhanced Fatal AFI Surveillance System only include 27 for which data from medical records or family interviews were available.
Petechiae, bleeding gums, epistaxis, unspecified mucosal bleeding, and hematuria.
Purpura/ecchymoses, melena, hematemesis, and vaginal bleeding.
Jaundice, convulsions, effusion, edema, encephalitis, and hepatomegaly.
Data exclude index case patients who were reported to the Puerto Rico Department of Health and associated with subsequently initiated household investigations.
Use of various surveillance systems permitted 2 additional observations on dengue incidence during the first year of CHIKV circulation. First, dengue incidence had been steadily decreasing for several months before the introduction of CHIKV, suggesting that the low incidence of dengue during the chikungunya epidemic may have been attributable to natural decline due to high herd immunity following dengue epidemics in 2010 and 2012–2013. Second, both passive and enhanced surveillance demonstrated that, although dengue incidence was at a historic low, DENV was still circulating during the chikungunya epidemic.
CONCLUSIONS
Any single approach to chikungunya surveillance is unable to capture all clinical outcomes and epidemiologic characteristics of interest; however, all of the approaches described herein are associated with both strengths and weaknesses, which public health organizations seeking to characterize the burden of chikungunya and other emerging infectious diseases should consider with respect to their surveillance objectives. Passive surveillance is likely the most feasible approach to define temporal and geographic trends but is subject to inherent underreporting and unclear biases in case detection, both of which may be difficult to accurately estimate. Community-based case detection can be used to identify deficiencies in healthcare-seeking behaviors, estimate infection rates, and, if conducted in an appropriately selected sample of the population, estimate the actual number of CHIKV infections that occurred during an outbreak. The clinical spectrum of disease and burden of clinically apparent cases can both be accurately estimated by conducting facility-based surveillance; however, such systems are resource intensive and necessitate both a well-designed study protocol and the participation of multiple partners. Caution should be taken when interpreting the findings of facility-based surveillance, as results are biased toward cases that are clinically more severe (ie, those with illness severe enough to merit seeking medical care). Surveillance for fatal cases is similarly resource intensive and only detects an extreme outcome associated with CHIKV infection. Although autopsy is not always logistically possible or culturally acceptable, findings from surveillance for fatal cases can be used to better understand the pathophysiology of CHIKV infection in individuals with severe manifestations. In areas where conducting all or some combination of these surveillance systems is possible, determination of the rates of symptomatic infection, clinically apparent disease, hospitalization, severe manifestations, and death in association with CHIKV infection may be possible.
Several questions remain regarding both the endemicity of CHIKV and the effect of CHIKV transmission on DENV circulation. Because CHIKV and DENV are from distinct viral families, antibodies directed against CHIKV neither neutralize DENV nor cross-react in serologic diagnostic tests and vice versa, suggesting that there should not be direct immunologic suppression of DENV circulation following CHIKV infection in humans. Therefore, whether infection with CHIKV may result in a decreased likelihood of infection with DENV, either in humans or mosquitoes, is unknown. Thus, continued surveillance for dengue and chikungunya will be conducted to detect CHIKV circulation in Puerto Rico and its potential effects on the concomitant incidence of dengue.
As with chikungunya, there will be also be a need for multifaceted surveillance during the emergence of pathogens for which questions remain regarding public health burden, medical complications, and/or severe manifestations of disease. Puerto Rico benefited from several preestablished surveillance systems that were able to be expeditiously modified to detect chikungunya cases, and similar approaches may be feasible in other settings. In many jurisdictions, passive surveillance will be the only approach available to monitor chikungunya and other emerging infectious diseases. However, region-specific differences in healthcare-seeking behaviors, clinical reporting practices, availability of laboratory diagnostic testing, and cocirculation of other pathogens that cause AFI are likely to affect trends in cases reported via passive surveillance. Nonetheless, passive surveillance remains the most practical and feasible approach to compare epidemiologic trends across regions affected by chikungunya and other emerging infectious diseases.
Acknowledgments
We thank Jomil Torres, Olga Lorenzi, and Aidsa Rivera for providing surveillance data.
Financial support. This work was supported by the Centers for Disease Control and Prevention (CDC), the Puerto Rico Department of Health, and the CDC Dengue Branch (support to Sentinel Enhanced Dengue Surveillance System via cooperative agreement 5U01CK000246-03).
Footnotes
Presented in part: 2016 Council for State and Territorial Epidemiologists Annual Meeting, Anchorage, Alaska, 20 June 2016. Abstract number 6441; American Society for Tropical Medicine and Hygiene 65th Annual Meeting, Philadelphia, Pennsylvania, 26 October 2015. Abstract number 2824; International Conference on Emerging Infectious Diseases 2015, Atlanta, Georgia, 25 August 2015; and La Sociedad de Enfermedades Infecciosas de Puerto Rico Reunionn Anual 2015, Rincon, Puerto Rico, 25 April 2015.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fischer M, Staples JE. Notes from the field: chikungunya virus spreads in the Americas—Caribbean and South America, 2013–2014. MMWR Morb Mortal Wkly Rep. 2014;63:500–501. [PMC free article] [PubMed] [Google Scholar]
- 2.Staples JE, Fischer M. Chikungunya virus in the Americas–what a vectorborne pathogen can do. N Engl J Med. 2014;371:887–889. doi: 10.1056/NEJMp1407698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention and Panamerican Health Organization (PAHO) Washington, DC: PAHO; 2011. Preparedness and response for chikungunya virus introduction in the Americas. [Google Scholar]
- 4.Economopoulou A, Dominguez M, Helynck B, et al. Atypical chikungunya virus infections: clinical manifestations, mortality, and risk factors for severe disease during the 2005–2006 outbreak on Reunion. Epidemiol Infect. 2009;137:534–541. doi: 10.1017/S0950268808001167. [DOI] [PubMed] [Google Scholar]
- 5.Ernould S, Walters H, Alessandri JL, et al. Chikungunya in paediatrics: epidemic of 2005–2006 in Saint-Denis, Reunion Island [in French] Arch Pediatr. 2008;15:253–262. doi: 10.1016/j.arcped.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Kumar NP, Suresh A, Vanamail P, et al. Chikungunya virus outbreak in Kerala, India, 2007: a seroprevalence study. Mem Inst Oswaldo Cruz. 2011;106:912–916. doi: 10.1590/s0074-02762011000800003. [DOI] [PubMed] [Google Scholar]
- 7.Mavalankar D, Shastri P, Bandyopadhyay T, Parmar J, Ramani KV. Increased mortality rate associated with chikungunya epidemic, Ahmedabad, India. Emerg Infect Dis. 2008;14:412–415. doi: 10.3201/eid1403.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manimunda SP, Sugunan AP, Rai SK, et al. Outbreak of chikungunya fever, Dakshina Kannada District, South India, 2008. Am J Trop Med Hyg. 2010;83:751–754. doi: 10.4269/ajtmh.2010.09-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2010;169:67–72. doi: 10.1007/s00431-009-0986-0. [DOI] [PubMed] [Google Scholar]
- 10.Renault P, Solet JL, Sissoko D, et al. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005–2006. Am J Trop Med Hyg. 2007;77:727–731. [PubMed] [Google Scholar]
- 11.Noyd DH, Sharp TM. Recent advances in dengue: relevance to Puerto Rico. P R Health Sci J. 2015;34:65–70. [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez Rodriguez E, Tomashek KM, Gregory CJ, et al. Co-infection with dengue virus and pandemic (H1N1) 2009 virus. Emerg Infect Dis. 2010;16:882–884. doi: 10.3201/eid1605.091920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez Rodriguez NM, Galloway R, Blau DM, et al. Case series of fatal leptospira spp./dengue virus co-infections-Puerto Rico, 2010–2012. Am J Trop Med Hyg. 2014;91:760–765. doi: 10.4269/ajtmh.14-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doker TJ, Sharp TM, Rivera-Garcia B, et al. Contact investigation of melioidosis cases reveals regional endemicity in Puerto Rico. Clin Infect Dis. 2015;60:243–250. doi: 10.1093/cid/ciu764. [DOI] [PubMed] [Google Scholar]
- 15.Chopra A, Anuradha V, Ghorpade R, Saluja M. Acute chikungunya and persistent musculoskeletal pain following the 2006 Indian epidemic: a 2-year prospective rural community study. Epidemiol Infect. 2012;140:842–850. doi: 10.1017/S0950268811001300. [DOI] [PubMed] [Google Scholar]
- 16.Sharp TM, Hunsperger E, Santiago GA, et al. Virus-specific differences in rates of disease during the 2010 dengue epidemic in Puerto Rico. PLoS Negl Trop Dis. 2013;7:e2159. doi: 10.1371/journal.pntd.0002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanciotti RS, Kosoy OL, Laven JJ, et al. Chikungunya virus in US travelers returning from India, 2006. Emerg Infect Dis. 2007;13:764–767. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Micro. 2000;38:1823–1826. doi: 10.1128/jcm.38.5.1823-1826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharp TM, Roth NM, Torres J, et al. Chikungunya cases identified through passive surveillance and household investigations–Puerto Rico, May 5-August 12, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:1121–1128. [PMC free article] [PubMed] [Google Scholar]
- 20.German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, Waller MN. Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep. 2001;50:1–35. quiz CE1–7. [PubMed] [Google Scholar]
- 21.Thacker SB, Birkhead GS. Surveillance. In: Gregg M, editor. Field epidemiology. 3rd. New York: Oxford University Press, Inc.; 2008. pp. 38–64. [Google Scholar]
- 22.Tomashek KM, Gregory CJ, Rivera Sanchez A, et al. Dengue deaths in Puerto Rico: lessons learned from the 2007 epidemic. PLoS Negl Trop Dis. 2012;6:e1614. doi: 10.1371/journal.pntd.0001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Investigation of leptospirosis underreporting- Puerto Rico, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:421. [PubMed] [Google Scholar]
- 24.Torres JR, Leopoldo Códova G, Castro JS, et al. Chikungunya fever: Atypical and lethal cases in the Western hemisphere. ID Cases. 2015;2:6–10. doi: 10.1016/j.idcr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beatty ME, Stone A, Fitzsimons DW, et al. Best practices in dengue surveillance: a report from the Asia-Pacific and Americas Dengue Prevention Boards. PLoS Negl Trop Dis. 2010;4:e890. doi: 10.1371/journal.pntd.0000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization (WHO) Geneva, Switzerland: WHO; 2009. Dengue: guidelines for diagnosis, treatment, prevention and control. [PubMed] [Google Scholar]