Abstract
Purpose
Tissue fibrosis caused by pathological activation of fibroblasts with increased synthesis of extracellular matrix components is a major hallmark of systemic sclerosis (SSc). Notch signalling regulates tissue differentiation and pathologic activation of Notch signaling has been implicated in the pathogenesis of various malignancies. The aim of the present study was to investigate the role of Notch signaling in SSc and to evaluate the therapeutic potential of Notch inhibition for the treatment of fibrosis.
Methods
Activation of the Notch pathways was analyzed by staining for the Notch intracellular domain (NICD) and quantification of the mRNA levels hes-1. Notch signaling was inhibited in the mouse model of bleomycin induced dermal fibrosis and in tight-skin-1 mice by the γ-secretase inhibitor DAPT and by overexpression of a Notch-1 antisense construct.
Results
Notch signaling is activated in SSc in vivo with accumulation of the NICD and increased transcription of the target gene hes-1. Overexpression of a Notch antisense construct prevented bleomycin-induced fibrosis and hypodermal thickening in tight-skin 1 mice. Potent anti-fibrotic effects were also obtained by treatment with DAPT. In addition to prevention of fibrosis, targeting Notch signaling resulted in almost complete regression of pre-established experimental fibrosis.
Conclusion
We demonstrate that pharmacologic as well as genetic inhibition of Notch signaling exerts potent anti-fibrotic effects in different murine models of SSc. These findings might have direct translational implications because different inhibitors of the γ-secretase complex are available and yielded promising results in cancer trials.
Introduction
Systemic sclerosis (SSc) is a chronic fibrotic disease of unknown etiology that affects the skin and several internal organs such as the lungs, heart, gastrointestinal tract and kidneys. Early stages of SSc are characterized by an activation of the immune system and infiltration of affected tissues with leukocytes (1). The infiltrating leukocytes, primarily T cells, monocytes and B cells, release pro-fibrotic mediators that may initially drive the activation of resident fibroblasts. However, the activation of SSc fibroblasts persists even after the inflammatory infiltrates have resolved suggesting an endogenous activation (2). The excessive release of collagen by SSc fibroblasts leads to progressive fibrosis and is a major cause of death in SSc patients (3). Treatment of tissue fibrosis represents a particular challenge for physicians. Current approaches to treat fibrosis are of limited efficacy and therapies targeting specifically the activation of fibroblasts and the release of extracellular matrix are not available for clinical use to date (4–6).
The Notch gene was first discovered in Drosophila mutants, which developed notches at the tips of their wing blades (7). The Notch gene transcribes into receptors consisting of one large transmembrane domain and is evolutionarily conserved (8). Binding of ligands such as Jagged-1 (Jag-1) to the Notch receptor induces cleavage of Notch at two different sites. The cleavage step catalyzed by the γ-secretase complex results in the release of the Notch intracellular domain (NICD) (8). The NICD then translocates into the nucleus, where it stimulates together with other transcription factors such as CSL (mammalian CBF-1, Drosophila Suppressor of Hairless, C. elegans LAG-1) and Mastermind (MAM) the transcription of target genes such as the Hairy/Enhancer of Split (Hes) (9). Notch-1 signaling plays a central role in the regulation of cell differentiation (10). However, Notch signaling is not restricted to development, and pathologic activation of Notch signaling has been implicated in the pathogenesis of human diseases such as T cell acute lymphoblastic leukemias (T-ALL) and melanoma (11, 12). In this context, first clinical trials with γ-secretase inhibitors have demonstrated promising results (13). Further clinical trials currently analyze an inhibition of Notch signaling for the treatment of solid tumors such as gastrointestinal neuroendocrine tumors, metastatic thyroid cancer and advanced breast cancer (www.clinicaltrials.gov).
In the present study, we demonstrate that the Notch pathway is activated in skin of SSc patients. Inhibition of the Notch pathway by antisense constructs or by inhibitors of the γ-secretase complex prevents fibrosis in different animal models and induces the regression of established fibrosis. Thus, inhibition of the Notch-1 pathway might be a novel strategy for the treatment of SSc and other fibrotic diseases.
Material and Methods
Patient and control skin biopsy samples
Biopsies from SSc patients (n = 11) were taken from involved skin of the forearm (Supplementary Table 1). All patients fulfilled the criteria for SSc as suggested by LeRoy et al (14). Control biopsies (n = 8) were obtained from skin biopsies of healthy age- and sex-matched volunteers. All patients and controls signed a consent form approved by the local institutional review boards.
Immunohistochemistry for NICD and α-smooth muscle actin
To analyze whether the Notch pathway is activated in SSc patients, immunohistochemistry for the NICD was performed. Fresh frozen sections from SSc patients, healthy individuals and from patients with hypertrophic scars and keloids were incubated with 3% H2O2 followed by serum blocking with 10 % goat serum in 5 % bovine serum albumin. The NICD was detected by staining with polyclonal rabbit-anti-human NICD (Abcam, Cambridge, UK) antibodies overnight at 4 °C.
The expression of α-smooth muscle actin (αSMA) was analyzed in paraffin embedded sections. After deparaffinization, samples were incubated with 3% bovine serum albumin followed by incubation with 3 % H202. αSMA positive cells in mouse sections were detected by incubation with monoclonal anti-αSMA antibodies (clone 1A4, Sigma-Aldrich, Steinheim, Germany). Irrelevant isotype antibodies in the same concentration were used for control (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Antibodies labeled with horseradish peroxidase (Dako, Hamburg, Germany) were used as secondary antibodies. The expression of the NICD and αSMA was visualized with DAB peroxidase substrate solution (Sigma-Aldrich). The number of myofibroblasts was counted from 6 different sections of lesional skin for each mouse by an examiner blinded to the treatment of the mice.
Quantitative real time PCR
Total RNA was isolated with the NucleoSpin RNA II extraction system (Machery-Nagel, Düren, Germany) and converted into cDNA as described (15). The following primer pairs were used for SYBR Green real-time PCR: human hes-1 (forward primer 5’ – TACCCAGCCAGTGTCAAC – 3’, reverese primer 5’ – CAGATGCTGTCTTTGGTTTATCC – 3’), murine hes-1 (forward primer 5’ – CCAAGCTAGAGAAGGCAGACA – 3’, reverse primer 5’ – CCGGAGGTGCTTCACAGT – 3’). Quantification of β-actin was used to normalize for the amounts of loaded cDNA.
Microtiter tetrazolium (MTT) assay
The metabolic activity of dermal fibroblasts incubated with DAPT was measured using the MTT [3, (4, 5-dimethylthiazol-2-yl) 2, 5-diphenyl-tetrazolium bromide] method as described (16). Untreated fibroblasts were used as negative controls and all other results were normalized to untreated cells. Fibroblasts incubated with 50 % DMSO served as positive controls.
Prevention of bleomycin-induced experimental fibrosis
Skin fibrosis was induced in 6-week-old, pathogen-free, female DBA/2 mice (Charles River, Sulzfeld, Germany) by local injections of bleomycin for 21 days (15). One hundred μl of bleomycin dissolved in 0.9 % sodium chloride (NaCl) at a concentration of 0.5 mg/ml was administered every other day by subcutaneous injections in defined areas of the upper back (n = 10). Subcutaneous injections of 100 μl 0.9 % NaCl, the solvent for bleomycin, were used as controls (n = 10). To investigate whether the γ-secretase inhibitor DAPT exerts anti-fibrotic effects in vivo, two groups of mice were treated with DAPT dissolved in 10 % dimethylsulfoxide (DMSO) in 0.9 % NaCl at concentrations of 1.5 and 6.0 mg/kg/d by oral gavage (n = 7 for both groups). These doses are pharmacologically relevant and were used in previous studies (17, 18). After 21 d, animals were sacrificed by cervical dislocation.
The mouse model of bleomycin-induced dermal fibrosis was also used to evaluate the outcome of Notch-1 antisense transgenic mice (Notch-1 as tg) upon challenge with bleomycin. Notch-1 as tg mice were previously described (17, 19). These mice appear phenotypically grossly normal. The protein levels of Notch-1 are reduced by 50 % in several organs including the skin (17, 19). Genotyping of the mice was performed by PCR with the following primers: Notch-1 forward primer: 5’ – GCTCCCATTCATCAGTTC – 3’, reverse primer: 5’ – TCTGTGAGAGTGAGCAGG – 3’. For our experiments, Notch-1 antisense transgenic mice were backcrossed onto a C57BL/6 background for at least twelve generations. Four groups of mice were analyzed: Two groups consisted of transgenic mice (n = 7 for each group), whereas the other two groups consisted of wildtype littermates (n = 8 for each group). Dermal fibrosis was induced in one group each by repeated injections of bleomycin, whereas the other two groups received sham treatment with subcutaneous injections of NaCl and served as controls.
All mouse experiments were approved by the local ethical committee.
Inhibition of Notch signaling in tight-skin 1 mice
In addition to the mouse model of bleomycin-induced dermal fibrosis, the tight-skin (Tsk-1) mouse model of SSc was used to evaluate the anti-fibrotic potential of an inhibition of Notch signaling. Due to a dominant mutation in fibrillin-1, the phenotype of Tsk-1 is characterized by an increased hypodermal thickness (20). Four groups of mice were analyzed: The first group of Tsk-1 mice (n =7) was sacrificed at an age of five weeks to evaluate the degree of fibrosis before anti-fibrotic treatment. One group of Tsk-1 mice (n = 5) was treated with DAPT at a concentration of 6 mg/kg/d by oral gavage, another Tsk-1 group (n = 12) received the solvent DMSO. The last group consisted of pa/pa (control) (n = 21) mice, which also received sham treatment with the solvent DMSO. The treatment was started at an age of five weeks. After five weeks of treatment, mice were sacrificed by cervical dislocation.
In addition to the treatment with the γ-secretase inhibitor DAPT, Tsk-1 mice were crossed with Notch-1 antisense transgenic mice to generate Tsk-1 mice expressing the Notch as construct. The offspring consisting of Notch-1 as tg / Tsk-1 mice, wt / Tsk-1 mice, Notch-1 as tg− mice not carrying the mutated Tsk-1 allele (Notch-1 as tg / pa mice) and wt / pa mice were sacrificed at an age of ten weeks to analyze the hypodermal thickness, the collagen content as well as the number of myofibroblasts. The two wildtype groups consisted of eight, the two Notch-1 as tg groups of five mice each.
Genotyping of Tsk-1 mice was performed by PCR with the following primers: mutated fibrillin-1/ Tsk-1 forward primer: 5’ – GTTGGCAACTATACCTGCAT – 3’, reverse primer: 5’ – CCTTTCCTGGTAACATAGGA – 3’.
Treatment of established fibrosis
Potential regression of established fibrosis by treatment with DAPT was evaluated in a modified bleomycin model (21). 6-week-old DBA/2 mice were divided into six groups (n = 8 for all groups). The first group of mice was sacrificed after 3 weeks of bleomycin challenge to analyze fibrotic changes before anti-fibrotic treatment. Another group of mice was sacrificed after 6 weeks of bleomycin injections. The third group was injected for 3 weeks with bleomycin and afterwards for another 3 weeks with 0.9 % NaCl to control for spontaneous regression of fibrosis. To analyze the potential of DAPT for treatment of established fibrosis, one group of mice challenged for six weeks with bleomycin received anti-fibrotic treatment with DAPT at a dose of 6 mg/kg/d only for the last three weeks. The last two groups received intracutaneous injections of 0.9 % NaCl for three and six weeks, respectively.
Histological analysis
The injected skin areas were fixed in 4 % formalin and embedded in paraffin. Histological sections were stained with hematoxylin and eosin for the determination of dermal thickness. Dermal thickness at the injection sites was analyzed with a Nikon Eclipse 80i microscope (Nikon, Badhoevedorp, Netherlands) at 200 fold magnification. The dermal thickness was determined by measuring the largest distance between the epidermal-dermal junction and the dermal-subcutaneous fat junction as described (22). The measurements were done by an examiner blinded to the treatment of the mice.
Hydroxyproline assay
To analyze the collagen content in skin samples, hydroxyproline assay was performed as previously described (23). After digestion of punch biopsies (Ø 3mm) in 6M HCl for three hours at 120°C, chloramine T (0.06 M) was added and samples were mixed and incubated for 20 min at room temperature. 3.15 M perchloric acid and 20 % p-dimethylaminobenzaldehyde were added and samples were incubated for additional 20 min at 60 °C. The absorbance was determined at 557 nm with a Spectra MAX 190 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
Statistics
Data are expressed as mean ± standard error of the mean. The Wilcoxon signed rank tests for related samples and the Mann-Whitney-U-test for non-related samples were used for statistical analyses. A p-value of less than 0.05 was considered statistically significant.
Results
Notch signaling is activated in SSc patients
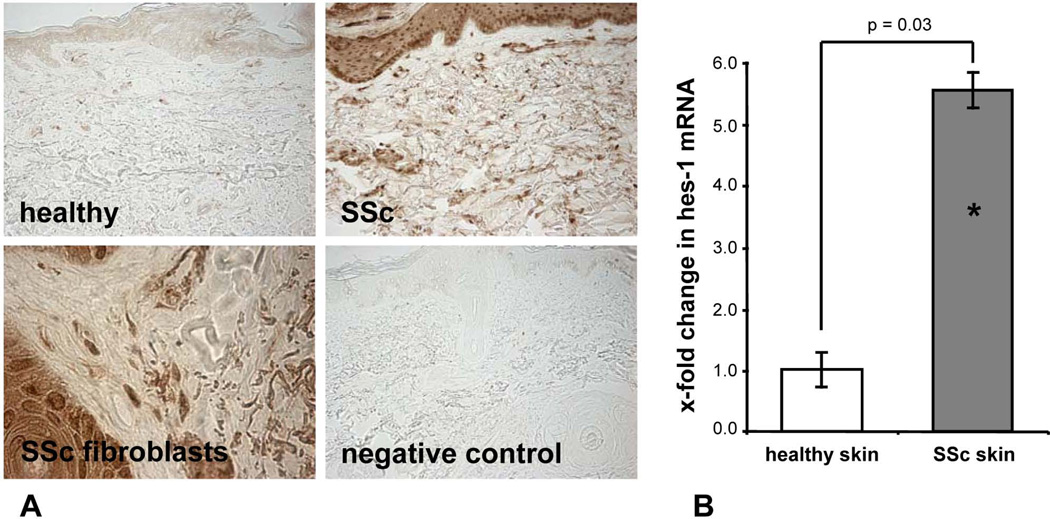
First, we analyzed whether Notch signaling is activated in SSc. Using antibodies that detect selectively the NICD, but not uncleaved, inactive Notch-1, we could demonstrate an activation of the Notch pathway in SSc patients. The NICD was barely detectable by immunohistochemistry in the skin of healthy individuals (Figure 1a). In contrast, all SSc patients stained positive for the NICD. Intensive staining of the NICD was particularly observed in fibroblasts (Figure 1a). In addition, mRNA levels of the Notch target gene hes-1 were elevated by 453 ± 30 % in skin biopsies of SSc patients as compared to skin biopsies from healthy individuals (p < 0.05) (Figure 1b). Thus, Notch signaling is prominently activated in SSc patients, but not in controls.
Figure 1.
Notch signaling is activated in SSc. A, The NICD, as a marker of active Notch signaling, was barely detectable in skin sections of healthy controls by immunohistochemistry. However, an intense staining for the NICD was detected in skin sections of SSc patients, in particular in fibroblasts. Representative skin sections of SSc patients, healthy volunteers and isotype controls are shown at 200 fold magnification, fibroblasts are shown at 1000 fold magnification. B, The mRNA levels of the Notch target gene hes-1 are elevated in skin of SSc patients compared to healthy controls. * indicates statistical significant differences compared to healthy controls.
We additionally analyzed the activation of Notch signaling in other fibrotic diseases and performed immunohistochemistry for NICD in hypertrophic scars and keloids. We also observed an accumulation of the NICD in patients with hypertrophic scars and keloids. Similar to SSc, we observed a prominent staining in fibroblasts, but also in keratinocytes and endothelial cells (Supplementary Figure 1).
Inhibition of Notch signaling prevents bleomycin-induced dermal fibrosis
To investigate the role of the Notch pathway in fibrosis, we used the mouse model of bleomycin-induced dermal fibrosis as a model for early inflammatory stages of SSc (24). An increased activation of the Notch pathway with prominent staining for the NICD was observed in bleomycin challenged mice compared to vehicle (NaCl) injected controls (Supplementary Figure 2a). In particular, fibroblasts from bleomycin challenged mice stained positively for NICD (Supplementary Figure 2a). Consistent with the increased NICD levels, the transcription of the Notch target gene hes-1 expression was activated in the skin of bleomycin injected mice. The mRNA levels of hes-1 increased by 323 ± 35 % in bleomycin challenged mice compared to controls (p = 0.02) (Supplementary Figure 2b). This induction was completely prevented upon treatment with DAPT even with the lower dose of 1.5 mg/kg/d (Supplementary Figure 2b).
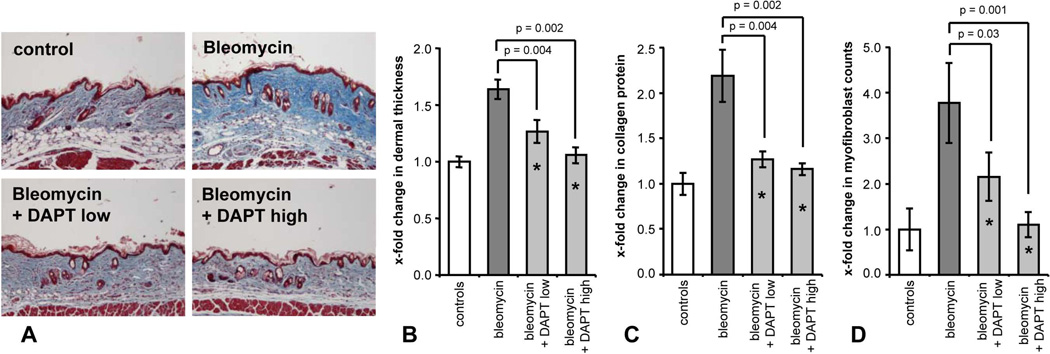
Next, we investigated whether inhibition of the Notch pathway by DAPT can prevent bleomycin-induced fibrosis. We first performed MTT assays to assess whether the γ-secretase inhibitor DAPT affects cell proliferation. No changes were observed in metabolic activity at both concentrations of 1 and 10 µM (data not shown). Further, we incubated dermal fibroblasts to evaluate the effect of DAPT treatment on collagen synthesis. Incubation of fibroblasts with DAPT effectively reduced the collagen release from SSc fibroblasts but not from healthy controls (p < 0.05 for SSc fibroblasts) (Supplementary Figure 3). Treatment of mice with DAPT was well tolerated and no differences in body weight or other obvious signs of toxicity were observed between DAPT and vehicle treated groups. In bleomycin-injected mice without anti-fibrotic treatment, a massive accumulation of collagen bundles with prominent dermal thickening was observed (Figure 2a). Treatment with DAPT reduced dermal thickening in a dose-dependent manner (Figure 2b). At a dose of 1.5 mg/kg/d, DAPT inhibited dermal thickening by 58 ± 10 % (p = 0.004 compared to bleomycin). The increase in dermal thickness was completely prevented at doses of 6 mg/kg/d DAPT (p = 0.002) (Figure 2b).
Figure 2.
Inhibition of γ-secretase complex by DAPT prevents bleomycin-induced dermal fibrosis. A, Treatment with DAPT prevented fibrosis in bleomycin challenged mice. Representative tissue sections at 100 fold magnification are shown: control mice injected intracutaneously with NaCl; bleomycin challenged mice without anti-fibrotic treatment; mice receiving bleomycin and DAPT at a dose of 1.5 mg/kg/d; mice treated with bleomycin and DAPT at a dose of 6 mg/kg/d. B, Treatment with DAPT at doses of 1.5 mg/kg/d and 6 mg/kg/d prevented dermal thickening. C, Dose-dependent reduction of the collagen content in lesional skin as analyzed with hydroxyproline assay. D, Treatment with DAPT prevented differentiation of resting fibroblasts into myofibroblasts. * indicates statistical significant differences compared to mock treated mice challenged with bleomycin.
Consistent with reduced dermal thickening, accumulation of lesional collagen and myofibroblast differentiation were also prevented by treatment with DAPT (Figure 2c and 2d). DAPT reduced the hydroxyproline content by 87 ± 6 % compared to bleomycin (p = 0.002) (Figure 2c). Treatment with DAPT also decreased the myofibroblast counts in a dose-dependent manner with almost complete inhibition at doses of 6 mg/kg/d (p = 0.001) (Figure 2d and Supplementary Figure 4).
To further evaluate the mechanism by which inhibition of Notch signaling reduces fibrosis, we measured the expression of different cytokines in the affected skin. As expected, the mRNA levels of the cytokines IL-4, IL-6, TGFβ, CTGF and IFNγ were upregulated in bleomycin-challenged mice which was completely prevented upon inhibition of Notch signaling by DAPT (p < 0.05 for all cytokines except for IFNγ) (Supplementary Figure 5).
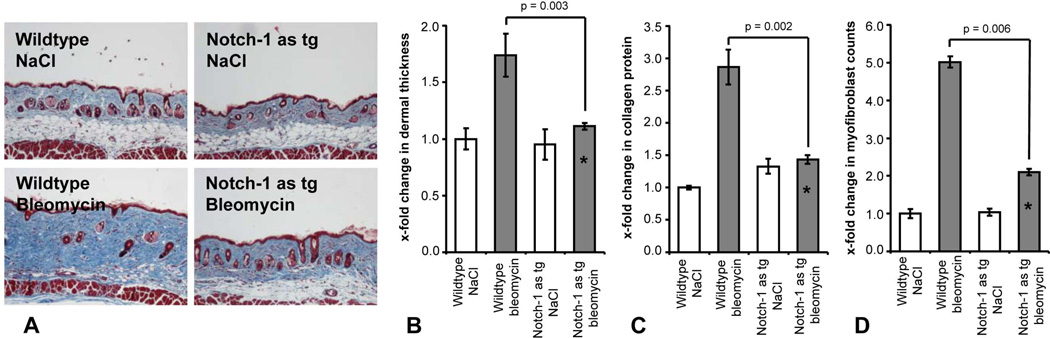
To confirm our results obtained with the chemical inhibitor DAPT with a genetic approach, we evaluated the outcome of Notch-1 antisense transgenic (Notch-1 as tg) mice challenged with bleomycin. Notch-1 as tg mice have significantly reduced Notch-1 signaling in several organs including the skin (17, 19). No histological differences of the skin were observed between wildtype and Notch-1 as tg mice injected with NaCl (Figure 3a). However, Notch-1 as tg mice were protected from bleomycin-induced dermal fibrosis (Figure 3a). Dermal thickening upon bleomycin challenge was reduced by 85 ± 19 % in Notch-1 as tg mice (p = 0.003 vs bleomycin injected wildtype mice) (Figure 3b). The collagen content in lesional skin was also significantly decreased by 77 ± 7 % (p = 0.002) (Figure 3c) and myofibroblast differentiation was almost completely prevented (p = 0.006) (Figure 3d).
Figure 3.
Notch-1 as tg mice are protected from bleomycin-induced fibrosis. A, Dermal fibrosis with accumulation of thickened collagen bundles was strongly reduced by knockdown of Notch-1. Representative tissue sections at 100 fold magnification are shown: Wildtype mice injected with NaCl; Notch-1 as tg mice with NaCl; Wildtype mice challenged with bleomycin; Notch-1 as tg mice receiving injections with bleomycin. B, Overexpression of a Notch-1 antisense construct prevented dermal thickening upon bleomycin challenge. C, Decreased accumulation of collagen protein in lesional skin of Notch-1 as tg mice. D, Knockdown of Notch-1 prevented differentiation of resting fibroblasts into myofibroblasts. * indicates statistical significant differences compared to bleomycin challenged wildtype mice.
Inhibition of the Notch pathway corrects the tight skin phenotype
We further analyzed the anti-fibrotic potential of DAPT in the Tsk-1 model, which resembles later stages of SSc characterized by endogenous activation of fibroblasts without inflammatory infiltrates. Notch signaling was activated in Tsk-1 mice with increased staining for the NICD, particularly in fibroblasts (Supplementary Figure 6a). In addition to elevated NICD levels, the expression of hes-1 was upregulated in Tsk-1 mice to 543 ± 31 % compared to control mice not carrying the Tsk-1 allele (p < 0.05) (Supplementary Figure 6b). Treatment with DAPT completely reduced the expression of hes-1 to control levels (p < 0.05) (Supplementary Figure 6b).
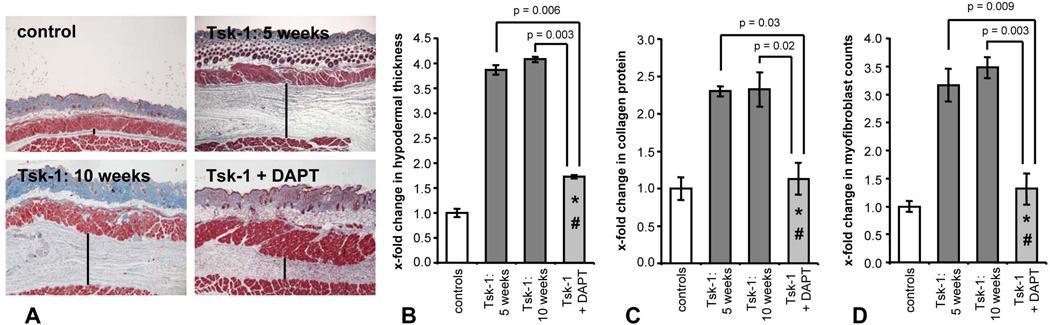
Skin fibrosis was already manifest at an age of 5 weeks and the degree of fibrosis at this age was only slightly lower compared to 10-week-old Tsk-1 mice (Figure 4). Treatment with the γ-secretase inhibitor DAPT ameliorated the histological changes in Tsk-1 mice (Figure 4a). The prominent hypodermal thickening in Tsk-1 mice was significantly decreased by 75 ± 4 % upon treatment with 6 mg/kg/d DAPT (p = 0.003) (Figure 4b). Moreover, the hydroxyproline content and the number of myofibroblasts were strongly reduced by 90 ± 21 % (p = 0.02) and by 87 ± 28 % (p = 0.003), respectively (Figure 4c and d). Of note, hypodermal thickness and myofibroblast counts in mice treated with DAPT from an age of 5 weeks to an age of 10 weeks were not only reduced compared to 10-week-old mice, but were also significantly lower than in 5-week-old Tsk-1 mice and thus below pre-treatment levels (Figure 4). Treatment with DAPT does therefore not only prevent progression of fibrosis, but also induces regression of fibrosis in Tsk-1 mice.
Figure 4.
Inhibition of the γ-secretase complex ameliorates the Tsk-1 phenotype. A, Representative tissue sections at 40 fold magnification are shown: control mice, untreated 5-week-old Tsk-1 mice, untreated Tsk-1 mice at an age of 10 weeks, Tsk-1 mice treated with DAPT for five weeks. Bars indicate hypodermal thickness. Treatment of Tsk-1 mice with DAPT reduced hypodermal thickening (B), reduced hydroxyproline content of the skin (C) and reduced the myofibroblast counts (D) below pre-treatment levels in Tsk-1 mice. * indicates statistical significant differences compared to untreated 10-week-old Tsk-1 mice. # indicates statistical significant differences compared to untreated 5-week-old Tsk-1 mice.
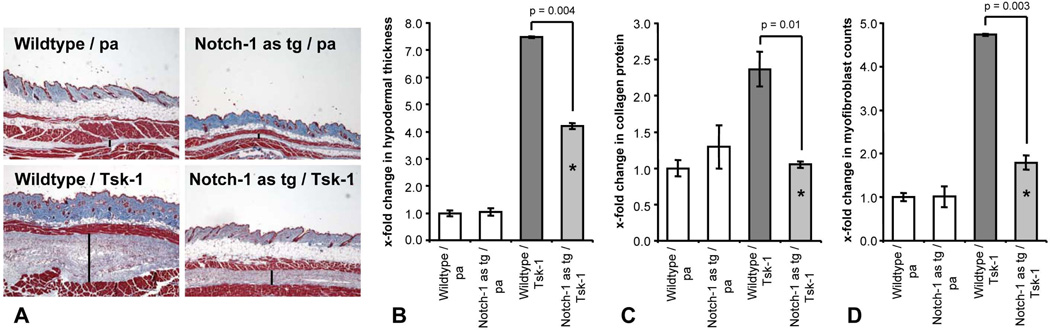
Finally, Notch-1 as tg mice were interbred with Tsk-1 mice to yield animals bearing the Notch-1 antisense construct and the Tsk-1 allele. The accumulation of collagen and tissue fibrosis were significantly reduced in Notch-1 as tg/ Tsk-1 mice compared to wt / Tsk-1 littermates (Figure 5a). The hypodermal thickening was significantly decreased by 51 ± 11 % in Notch-1 as tg/ Tsk-1 mice (p = 0.004) (Figure 5b). Consistently, the collagen content and the number of αSMA positive myofibroblasts were reduced by 96 ± 5 % and by 79 ± 16 %, respectively (p = 0.01 and 0.003, respectively) (Figure 5c and d).
Figure 5.
Overexpression of a Notch-1 antisense construct corrects histological changes in Tsk-1 mice. A, Representative tissue sections of wildtype mice without the Tsk-1 allele (wildtype / pa), Notch-1 as tg / pa mice, Notch-1 wildtype / Tsk-1 mice and Notch-1 as tg / Tsk-1 mice are shown at 40 fold magnification. Bars indicate hypodermal thickness. B, Hypodermal thickening is strongly reduced in Tsk-1 mice bearing the Notch-1 antisense construct. C, The hydroxyproline content is significantly decreased in Notch-1 as tg / Tsk-1 mice. D, Myofibroblast counts in Tsk-1 mice are decreased upon downregulation of Notch-1. * indicates statistical significant differences compared to wildtype / Tsk-1 mice.
Inhibition of Notch signaling induces regression of pre-established fibrosis
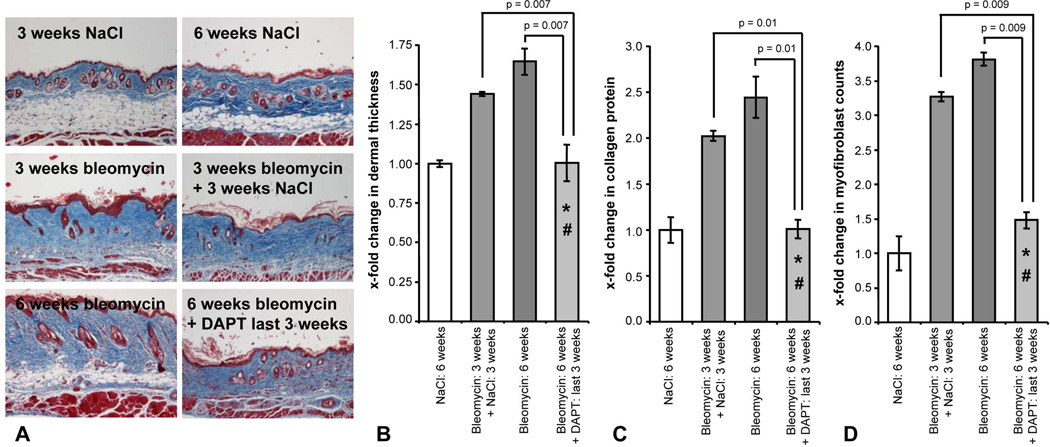
In addition to the preventive effects, we investigated whether inhibition of Notch signaling can induce regression of fibrosis. Therefore, we evaluated the effects of DAPT in a modified bleomycin model for pre-established fibrosis. The dermal thickening observed after three weeks of bleomycin challenge further progressed in mice challenged with bleomycin for six weeks (Figure 6a). The dermal thickening of mice challenged with bleomycin for six weeks and treated with DAPT for the last three weeks was significantly less pronounced than in mice treated with bleomycin for six weeks (p = 0.007). Moreover, the dermal thickness in the DAPT treated group was also reduced compared to mice treated with bleomycin for three weeks followed by injections with vehicle for another three weeks (p = 0.007) (Figure 6b). Thus, treatment with DAPT did not only prevent progression of fibrosis, but induced significant regression of fibrosis. Consistently, a significant reduction of the hydroxyproline content was observed in mice treated with DAPT for the last three weeks of bleomycin treatment (Figure 6c). Treatment with DAPT reduced the collagen content in lesional skin by 97 ± 9 % compared to three weeks of bleomycin injections (p = 0.01) (Figure 6c). Similarly, differentiation of resting fibroblasts into myofibroblasts was effectively reduced by 79 ± 12 % compared to mice challenged with bleomycin for three weeks (p = 0.009) (Figure 6d).
Figure 6.
Inhibition of Notch signaling induces regression of established fibrosis. A, A progressive dermal thickening was observed with bleomycin challenge over time. Treatment with DAPT induced potent regression of pre-established fibrosis. Representative tissue sections at 100 fold magnification are shown. B, Treatment with DAPT at a dose of 6 mg/kg/d for the last three weeks reduced dermal thickness almost back to baseline levels. C, The collagen content in lesional skin was significantly reduced upon treatment with DAPT. D, Myofibroblast counts were almost normalized by treatment with DAPT. * indicates statistical significant differences compared to mice injected with bleomycin for three weeks followed by injection with NaCl for another three weeks. # indicates statistical significant differences compared to six weeks of bleomycin challenge.
Discussion
We demonstrate that Notch signaling is activated in SSc patients and in different models of experimental fibrosis with accumulation of the NICD and increased transcription of the target gene hes-1. A particularly pronounced activation was observed in SSc fibroblasts. Inhibition of the Notch pathway either pharmacologically by blockade of the γ-secretase complex or by overexpression of a Notch antisense construct exerted potent anti-fibrotic effects. Inhibition of Notch signaling prevented the development of dermal fibrosis in inflammation-dependent models of dermal fibrosis such as bleomycin induced fibrosis and in inflammation-independent fibrosis in Tsk-1 mice. These data suggest that targeting Notch directly reduces the collagen synthesis of fibroblasts and might be effective in early inflammatory stages of SSc as well as in later, non inflammatory stages, when the inflammatory infiltrates have already resolved. These results are consistent with another very recent report in which DAPT prevented hypochlorite-induced fibrosis (25). We further showed that inhibition of Notch signaling by DAPT reduces the expression of pro-fibrotic cytokines such as IL-4, IL-6, TGFβ and CTGF. The profound decrease in IL-4 is consistent with the recent finding that IL-4 is a direct Notch target gene (26). In addition to prevention of fibrosis, targeting Notch signaling also potently induced regression of experimental fibrosis. Treatment with DAPT reduced dermal thickness, myofibroblast counts and the hydroxyproline content of the skin almost back to levels observed in control mice. We believe that these results might have direct translational implications. The Notch pathway has been implicated in the pathogenesis of a variety of different malignancies and different strategies for the inhibition of the Notch pathway are currently under investigation for novel therapeutic approaches (13, 27). Studies analyzing the therapeutic potential of inhibitors of the γ-secretase complex that is required for the release of the active NICD, are currently the most common approach for targeting Notch (13, 28). Two different inhibitors of the γ-secretase complex, MK0752 and PF-03084014, are currently evaluated in clinical trials for the treatment of resistant T-ALLs and advanced breast cancer (www.clinicaltrials.gov). In addition, clinical trials with LY450139 are currently ongoing in Alzheimer disease (www.clinicaltrials.gov). However, targeting γ-secretase is problematic as all Notch receptors from Notch-1 to Notch-4 are affected. Thus, clinical trials with γ-secretase inhibitors revealed several adverse events such as gastrointestinal toxicity (29, 30). These side effects might be strongly ameliorated by short interruptions in the administration of γ-secretase inhibitors. A short time period seems to be sufficient to allow at least some intestinal stem cells to correctly differentiate into enterocytes (28). To avoid these adverse events, other approaches to target the Notch pathway such as antibodies specifically targeting individual Notch receptors or direct inhibition of the assembly of the transcription factor complex by synthetic, cell permeable peptides were recently evaluated and showed promising effects in preclinical models without intestinal toxicity (31, 32). Thus, different pharmacologic inhibitors of the Notch pathway are available for clinical trials. Due to the prominent activation of the Notch pathway in experimental fibrosis and in human fibrotic skin and the potent anti-fibrotic effects observed upon inhibition of Notch signaling on multiple experimental levels, targeting Notch might be a promising novel therapeutic approach for the treatment of SSc. This is particularly important because the uncontrolled accumulation of extracellular matrix often leads to severe organ dysfunction with high morbidity and mortality and because efficient anti-fibrotic therapies are not yet available (4, 6).
In summary, we demonstrate that the Notch pathway is activated in SSc and that inhibition of Notch signaling exerts potent anti-fibrotic effects in preclinical models. Our data suggest that inhibition of Notch signaling might be a promising molecular target for anti-fibrotic therapeutic approaches. Since inhibitors of the Notch pathway are currently evaluated in clinical trials for other indications, these findings might initiate clinical trials in patients with SSc and other fibrotic diseases.
Supplementary Material
Acknowledgments
The study was supported by the Deutsche Forschungsgesellschaft (DI 1537/2-1), grant A20 of the Interdisciplinary Center of Clinical Research (IZKF) in Erlangen, by the Career Support Award of Medicine of the Ernst Jung Foundation, and by the Intramural Research Program of the National Institute on Aging, NIH.
We also would like to thank Maria Halter for excellent technical support.
Footnotes
The authors declare to have no financial support or other benefits from commercial sources, or any other financial interests, which could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
References
- 1.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360(19):1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 2.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117(3):557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung L, Krishnan E, Chakravarty EF. Hospitalizations and mortality in systemic sclerosis: results from the Nationwide Inpatient Sample. Rheumatology (Oxford) 2007;46(12):1808–1813. doi: 10.1093/rheumatology/kem273. [DOI] [PubMed] [Google Scholar]
- 4.Charles C, Clements P, Furst DE. Systemic sclerosis: hypothesis-driven treatment strategies. Lancet. 2006;367(9523):1683–1691. doi: 10.1016/S0140-6736(06)68737-0. [DOI] [PubMed] [Google Scholar]
- 5.Kim R, Meyer KC. Therapies for interstitial lung disease: past, present and future. Ther Adv Respir Dis. 2008;2(5):319–338. doi: 10.1177/1753465808096948. [DOI] [PubMed] [Google Scholar]
- 6.Swigris JJ, Brown KK, Make BJ, Wamboldt FS. Pulmonary rehabilitation in idiopathic pulmonary fibrosis: a call for continued investigation. Respir Med. 2008;102(12):1675–1680. doi: 10.1016/j.rmed.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan T, Bridges C. Sex-linked inheritance in Drosophila. Carnegie Institution of Washington. 1916;237:1–88. [Google Scholar]
- 8.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134(7):1243–1251. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- 10.Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16(5):633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I, et al. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest. 2005;115(11):3166–3176. doi: 10.1172/JCI25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy M, Pear WS, Aster JC. The multifaceted role of Notch in cancer. Curr Opin Genet Dev. 2007;17(1):52–59. doi: 10.1016/j.gde.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Purow B. Notch inhibitors as a new tool in the war on cancer: a pathway to watch. Curr Pharm Biotechnol. 2009;10(2):154–160. doi: 10.2174/138920109787315060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28(7):1573–1576. [PubMed] [Google Scholar]
- 15.Akhmetshina A, Dees C, Pileckyte M, Maurer B, Axmann R, Jungel A, et al. Dual inhibition of c-abl and PDGF receptor signaling by dasatinib and nilotinib for the treatment of dermal fibrosis. FASEB J. 2008;22(7):2214–2222. doi: 10.1096/fj.07-105627. [DOI] [PubMed] [Google Scholar]
- 16.Distler JH, Jungel A, Huber LC, Seemayer CA, Reich CF, 3rd, Gay RE, et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci U S A. 2005;102(8):2892–2897. doi: 10.1073/pnas.0409781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chigurupati S, Arumugam TV, Son TG, Lathia JD, Jameel S, Mughal MR, et al. Involvement of notch signaling in wound healing. PLoS One. 2007;2(11):e1167. doi: 10.1371/journal.pone.0001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teachey DT, Seif AE, Brown VI, Bruno M, Bunte RM, Chang YJ, et al. Targeting Notch signaling in autoimmune and lymphoproliferative disease. Blood. 2008;111(2):705–714. doi: 10.1182/blood-2007-05-087353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng P, Zlobin A, Volgina V, Gottipati S, Osborne B, Simel EJ, et al. Notch-1 regulates NF-kappaB activity in hemopoietic progenitor cells. J Immunol. 2001;167(8):4458–4467. doi: 10.4049/jimmunol.167.8.4458. [DOI] [PubMed] [Google Scholar]
- 20.Green MC, Sweet HO, Bunker LE. Tight-skin, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am J Pathol. 1976;82(3):493–512. [PMC free article] [PubMed] [Google Scholar]
- 21.Akhmetshina A, Venalis P, Dees C, Busch N, Zwerina J, Schett G, et al. Treatment with imatinib prevents fibrosis in different preclinical models of systemic sclerosis and induces regression of established fibrosis. Arthritis Rheum. 2009;60(1):219–224. doi: 10.1002/art.24186. [DOI] [PubMed] [Google Scholar]
- 22.Distler JH, Jungel A, Huber LC, Schulze-Horsel U, Zwerina J, Gay RE, et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007;56(1):311–322. doi: 10.1002/art.22314. [DOI] [PubMed] [Google Scholar]
- 23.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T, Takagawa S, Katayama I, Yamazaki K, Hamazaki Y, Shinkai H, et al. Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J Invest Dermatol. 1999;112(4):456–462. doi: 10.1046/j.1523-1747.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- 25.Kavian N, Servettaz A, Mongaret C, Wang A, Nicco C, Chereau C, et al. Targeting ADAM-17/notch signaling abrogates the development of systemic sclerosis in a murine model. Arthritis Rheum. 2010;62(11):3477–3487. doi: 10.1002/art.27626. [DOI] [PubMed] [Google Scholar]
- 26.Amsen D, Antov A, Flavell RA. The different faces of Notch in T-helper-cell differentiation. Nat Rev Immunol. 2009;9(2):116–124. doi: 10.1038/nri2488. [DOI] [PubMed] [Google Scholar]
- 27.Real PJ, Ferrando AA. NOTCH inhibition and glucocorticoid therapy in T-cell acute lymphoblastic leukemia. Leukemia. 2009 doi: 10.1038/leu.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27(38):5124–5131. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 29.Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA, et al. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch Neurol. 2008;65(8):1031–1038. doi: 10.1001/archneur.65.8.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei P, Walls M, Qiu M, Ding R, Denlinger RH, Wong A, et al. Evaluation of selective gamma-secretase inhibitor PF-03084014 for its antitumor efficacy and gastrointestinal safety to guide optimal clinical trial design. Mol Cancer Ther. 2010;9(6):1618–1628. doi: 10.1158/1535-7163.MCT-10-0034. [DOI] [PubMed] [Google Scholar]
- 31.Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, et al. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462(7270):182–188. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464(7291):1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.