Abstract
Background and Aims:
Although several noninvasive and easily accessible biomarkers for inflammatory bowel disease [IBD] are available, their sensitivity and specificity are not adequate to be used as single markers and do not overrule the need for endoscopic evaluation. We previously reported that serum leucine-rich alpha-2 glycoprotein [LRG] was a novel biomarker for rheumatoid arthritis and IBD. We herein investigated whether LRG could indicate endoscopic activity in patients with ulcerative colitis [UC].
Methods:
Serum LRG concentrations were determined by enzyme-linked immunosorbent assay [ELISA] in consecutive 129 patients with UC in two tertiary care hospitals, and associations of LRG with clinical and endoscopic activities were evaluated. Clinical activity index [CAI] < 6 was defined as clinical remission, and mucosal healing [MH] and complete mucosal healing were defined as Matts’ endoscopic grades of 1 or 2 and grade of 1, respectively.
Results:
Serum LRG levels were significantly increased and correlated with clinical and endoscopic activities in patients with UC. LRG levels were associated with both clinical and endoscopic activities even in patients with normal serum C-reactive protein [CRP] levels. Furthermore, LRG levels were significantly lower in patients with complete MH and deep remission. Serial measurements of LRG levels in a subset of patients demonstrated that LRG was significantly elevated during the endoscopically active stage compared with that during the MH stage.
Conclusions:
Serum LRG is a novel biomarker for detecting MH during disease course in patients with UC and a surrogate marker of endoscopic inflammation in patients with normal CRP levels.
Keywords: Biomarker, C-reactive protein, leucine-rich alpha-2 glycoprotein, mucosal healing
1. Introduction
Inflammatory bowel diseases [IBDs], comprising Crohn’s disease [CD] and ulcerative colitis [UC], are characterised by chronic and relapsing gastrointestinal inflammation with unknown precise aetiology, and the number of patients with IBD is increasing on a global scale. Recent therapeutic advances such as immunomodulators, calcineurin inhibitors, and biologic agents have altered the therapeutic goal of IBD from clinical remission to endoscopic remission. Critically, recent reports emphasised the importance of mucosal healing, as failure of mucosal healing was a major predictor of clinical recurrence.1,2 One of the clinical obstacles in IBD treatment is the lack of ideal biomarkers that successfully indicate the clinical course, therapeutic outcome, and mucosal healing. Endoscopy is the best modality to diagnose and monitor mucosal status of IBD patients, but cannot be frequently performed because of the cost, invasiveness, and potential risk of disease exacerbation. Therefore, clinical activity index [CAI] combined with several serological markers, such as C-reactive protein [CRP], white blood cell count, and erythrocyte sedimentation rate, are widely used in the clinical treatment of IBD. Of these, CRP is an acute phase protein whose synthesis in the liver is stimulated by interleukin [IL]-63, and it is the most widely used surrogate marker to monitor the clinical activity of IBD.4 However, CRP does not always correlate with disease activity in patients with CD.5 In addition, CRP may not always indicate disease activity in inflammatory diseases such as systemic lupus erythematosus and UC, in which inflammation is regulated mainly by cytokine[s] other than IL-6.6 Therefore, serological markers such as CRP generally lack diagnostic accuracy needed for clinical decision making in patients with UC, and colonoscopy is still the mainstay of evaluation of disease activity.
We recently identified leucine-rich alpha-2 glycoprotein [LRG] as a novel biomarker for rheumatoid arthritis and IBD by using a proteomics approach.7 LRG is a 50-kDa glycoprotein that contains repetitive sequences with a leucine-rich motif8,9 and is expressed not only by hepatocytes but also by neutrophils, macrophages, and intestinal epithelial cells.10 Although the association of LRG with angiogenesis has been reported,11 the precise pathophysiology of LRG for IBD has not been clearly elucidated. We previously showed that LRG was induced by IL-22, tumour necrosis factor [TNF]-α, and IL-1β, independently from IL-6.7 We also reported that LRG is an effective serological marker for CD and UC.10 An increase in serum LRG was also observed in other diseases such as rheumatoid arthritis, infection, heart failure,12 and malignant diseases.13 Moreover, our recent study clearly demonstrated an association between LRG upregulation and clinical activity of IBD, which was superior to conventional serological biomarkers.10 However, comprehensive analysis of the correlation between serum LRG and endoscopic disease activity of UC has not been performed. Thus, we conducted a multi-centre study to analyse serum LRG levels and endoscopic activity of UC in patients to determine whether LRG was a surrogate marker for mucosal healing.
2. Materials and Methods
2.1. Subjects
In total, a consecutive cohort of 129 patients with UC was enrolled in this study at the Department of Gastroenterology and Hepatology, Osaka University Hospital and at Keio University Hospital between 2007 and 2014. In addition, 22 healthy controls, comprising 8 females with a median age of 34 [range, 25–56] years, were recruited at the National Institute of Biomedical Innovation, Health and Nutrition. Patients were diagnosed with UC according to endoscopic, radiological, histological, and clinical criteria.14,15 Serum samples were collected from patients, and scores for clinical and endoscopic activity of disease were obtained by reviewing the clinical records of patients. Serum sampling and colonoscopy were performed within 30 days in 109 patients [84.5%] with a median interval of 7 days, and some patients with longer intervals between serum sampling and colonoscopy were also included in this study as they were clinically stable during this period [Table 1]. The ethics committee at each hospital approved the study protocol, and written informed consent was obtained from all study patients.
Table 1.
Patient characteristics.
| Gender, female/male, N | 45/84 |
| Age, median [range] | 44 [20–75] |
| Institution of patients, OUH/KUH | 77 / 52 |
| Disease location, N | |
| Extensive/left-sided/proctitis | 72 / 36 / 21 |
| Treatment | |
| 5-aminosalicycic acid, N [%] | 121 [93.4] |
| Corticosteroids, N [%] | 37 [28.7] |
| Immunomodulators, N [%] | 35 [27.1] |
| Biologic agents, N [%] | 22 [17.0] |
| Calcineurin inhibitors, N [%] | 6 [4.7] |
| Probiotics, N [%] | 70 [54.2] |
| Antibiotics, N [%] | 3 [2.3] |
| CRP, mg/dl, median [range] | 0.11 [0.01–21.3] |
| CAI, median [range] | 4 [0–19] |
| Matts grade, median [range] | 3 [1–4] |
| Intervals of blood sampling and colonoscopy, median [range] |
7 [0–272] |
OUH, Osaka University Hospital; KUH, Keio University Hospital; CAI, clinical activity index; CRP, C-reactive protein.
2.2. Assessment of clinical, endoscopic, and histological disease activity of UC
Patients with UC were classified according to the extent of disease involvement as E1 [proctitis], E2 [left-sided colitis], or E3 [pancolitis] as described by the Montreal classification.16 The clinical and endoscopic activities were determined using the Clinical Activity Index [CAI]17 and Matts’ endoscopic grading,18 respectively. CAI of 6 or more was defined as clinically active, whereas CAI of less than 6 was defined as clinical remission. Mucosal healing was defined as Matts’ grade of 1 or 2,19 and complete mucosal healing [CMH] was defined as Matts’ grade of 1,20 which is equivalent to the Mayo endoscopic score [MES] of 0.21 The proportions of clinically and endoscopically active patients were 38.3 % [46/120] and 54.2 % [70/129], respectively. Histological analysis was performed for biopsy samples where Matts’ grading was determined, and the pathologist, EM, performed central analysis using Geboes grade22 ; the grade of < 3.1 was defined as histological healing.23
2.3. Measurement of serum LRG levels
Serum LRG levels were analysed by enzyme-linked immunosorbent assay [ELISA] at the Laboratory for Immune Signal, National Institute of Biomedical Innovation, Osaka, Japan, as described previously.24 In brief, genes of the variable regions of LRG-specific antibodies were cloned from rabbits immunised with purified recombinant human LRG protein and one of the cloned gene was inserted into an expression vector containing the constant region of human IgG. Two clones [huLRB0091 and rbLRB0048] were selected to construct a sandwich ELISA for the detection of LRG.
2.4. Statistical analysis
Differences between measurements and groups were tested with the Mann–Whitney U, Kruskal–Wallis, or Steel–Dwass test. Paired t testing was used to determine the level of significance of change in LRG levels over time during the clinical course of disease. A receiver operated characteristic [ROC] curve was generated by plotting the false-positive fraction versus the true-positive fraction for every possible cutoff score,25 and area under the ROC curve [AUC] was calculated. Multivariable logistic regression analysis was performed to investigate factors associated with the increase in LRG; p- values < 0.05 were considered as statistically significant. All analyses were performed using JMP® Pro version 11 software [SAS, Cary, NC].
3. Results
3.1. Increased serum LRG levels in clinically active patients with UC
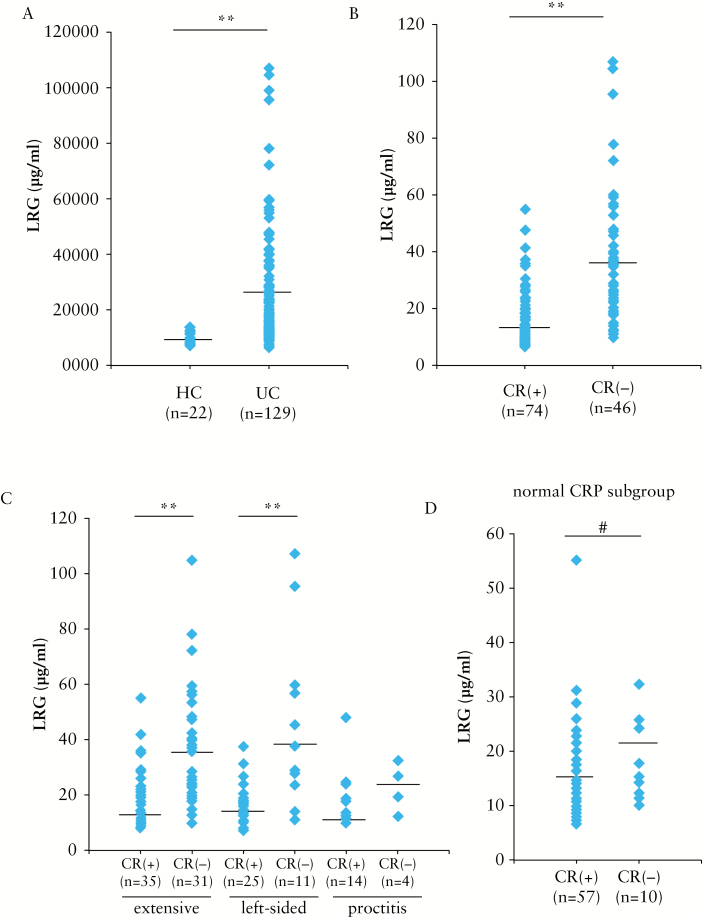
We first compared serum LRG levels between patients with UC in clinical remission and those with active disease. Patient characteristics are presented in Table 1. The median age of patients was 44 [range, 20–75] years, and the majority of patients had extensive or left-sided colitis [83.7%]; proctitis was observed only in 16.3% patients. Consistent with our previous report,10 serum LRG levels were significantly higher in patients with UC than in healthy controls [Figure 1A], and in clinically active patients with UC than in those in remission [Figure 1B]. When patients with UC were categorised into three groups according to disease location by the Montreal classification,16 LRG levels were significantly higher in clinically active patients with UC having extensive and left-sided colitis than in those in remission [Figure 1C]. The LRG levels also tended to be higher in clinically active patients with proctitis than those in remission [p = 0.09].
Figure 1.
Serum LRG levels are increased in clinically active patients with ulcerative colitis [UC]. [A] Serum LRG levels were measured in 129 patients with UC and in 22 healthy controls [HC]. [B] Serum LRG levels were measured in 120 patients with UC: 74 patients in clinical remission (CR[+]: CAI < 6), 46 patients in active (CR[−]: CAI ≥ 6) stage. [C] Serum LRG levels in patients with UC categorised according to disease activity and disease location are shown. Patients in remission: extensive colitis [n = 35], left-sided colitis [n = 25], and proctitis [n = 14]. Active patients: extensive colitis [n = 31], left-sided colitis [n = 11], and proctitis [n = 4]. [D] Serum levels of LRG were determined in UC patients with normal CRP levels [≤ 0.2mg/dl] are shown. Patients with UC were divided into clinical remission [n = 57] and active [n = 10] groups; #p < 0.05, **p < 0.001 by the Mann–Whitney U test.
We then determined LRG levels in 67 patients with UC whose CRP levels were within normal limits [≤ 0.2mg/dl] and found that LRG levels were significantly elevated in clinically active patients with UC compared with those in remission [Figure 1D]. These data demonstrated that LRG levels indicated clinical disease activity even in patients with UC showing normal CRP levels.
3.2. Correlation of serum LRG levels with endoscopic disease activity in UC
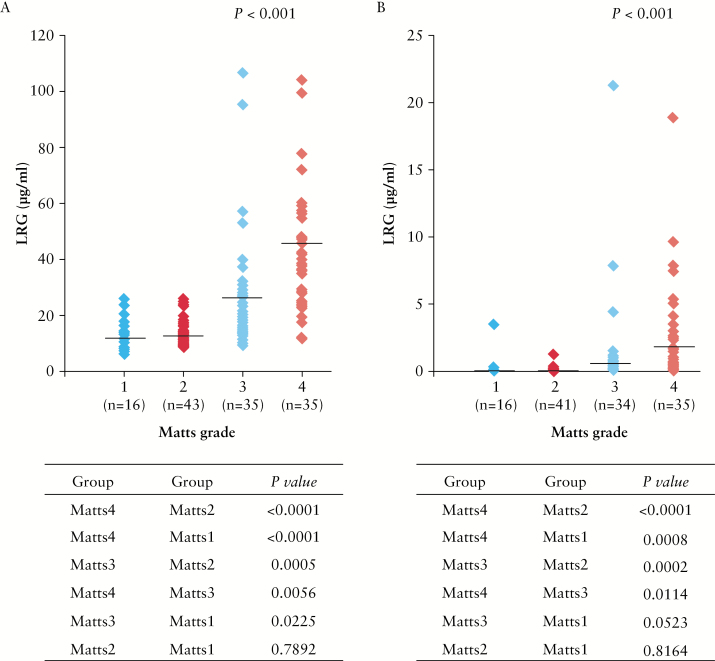
We next assessed whether serum LRG levels were associated with endoscopic disease activity in patients with UC. As shown in Figure 2A, colonic mucosal inflammation significantly correlated with increased LRG levels. Increased serum CRP levels were also significantly associated with endoscopic inflammation; however, most patients with Matts’ grade 1–2 did not have elevated serum CRP levels, and the degree of change in CRP levels across patients with different Matts’ grades was small compared with that detected for LRG [Figure 2B]. Pair-wise comparison of changes in LRG and CRP levels across endoscopic disease grades by the Steel–Dwass post hoc analysis revealed that the p-values for between-grade changes were generally lower for LRG than those for CRP [Figure 2].
Figure 2.
Serum LRG levels are positively associated with endoscopic activity in patients with ulcerative colitis [UC]. [A] Serum LRG levels of patients with UC were plotted according to the Matts’ grade: 1 [n = 16], 2 [n = 43], 3 [n = 35], and 4 [n = 35]. [B] Serum CRP levels of patients with UC were plotted according to the Matts’ grade: 1 [n = 16], 2 [n = 41], 3 [n = 34], and 4 [n = 35], p < 0.001, by the Kruskal–Wallis test. In [A] and [B], differences in each Matts’ grade between LRG and CRP levels were also analysed by the Steel–Dwass test; **p < 0.001, #p < 0.05.
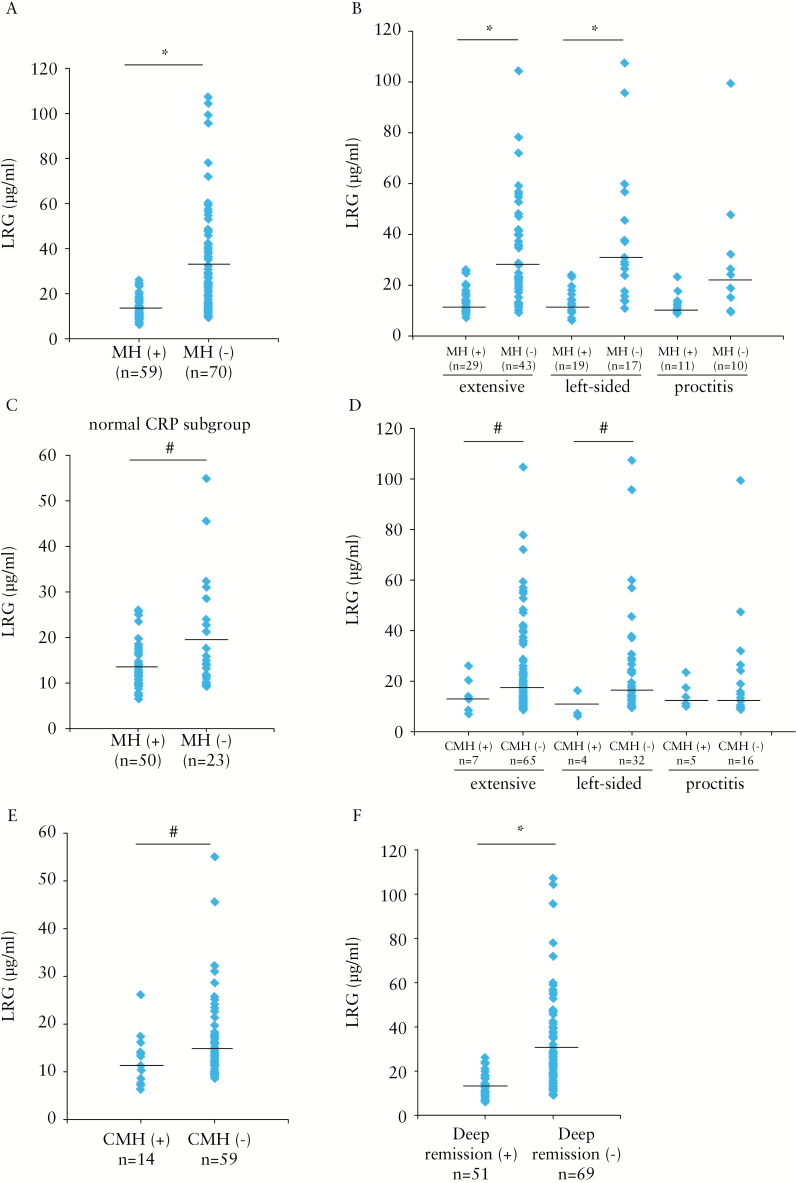
We also found that serum LRG levels were significantly higher in patients without mucosal healing than in those who achieved mucosal healing [Figure 3A]. In addition, serum LRG levels were significantly higher in endoscopically active patients with UC having extensive and left-sided colitis than in those with mucosal healing, as shown in Figure 3B. The difference in LRG levels also tended to be higher in endoscopically active patients with proctitis than in those with mucosal healing [p = 0.09]. Moreover, among patients with UC showing normal CRP levels, serum LRG levels were significantly higher in those without mucosal healing than in those with mucosal healing [Figure 3C].
Figure 3.
Serum LRG levels are increased in endoscopically active patients with ulcerative colitis [UC]. [A] Serum LRG levels in 129 patients with UC (59 patients with mucosal healing [MH] [Matts’ grade of 1 or 2) and 70 patients in endoscopically active stage [Matts’ grade of 3 or 4] are shown. [B] Serum LRG levels in patients with UC categorised according to disease location are shown. Mucosal healing patients (MH[+]): extensive colitis [n = 29], left-sided colitis [n = 19], and proctitis [n = 11]. Non-mucosal healing patients (MH[−]): extensive colitis [n = 43], left-sided colitis [n = 17], and proctitis [n = 10]. [C] Serum LRG levels in UC patients with normal C-reactive protein [CRP] levels [≤ 0.2mg/dl] are shown. Patients with UC were divided into MH[+] [n = 50] and MH[−] [n = 23] in two groups. [D] Serum LRG levels in patients with UC categorised according to disease location and complete mucosal healing [CMH] are shown. In CMH[+] patients, Matts’ grade of 1: extensive colitis [n = 7], left-sided colitis [n = 4], and proctitis [n = 5]. In patients with no CMH (CMH[−]), Matts’ grade of 2 to 4: extensive colitis [n = 65], left-sided colitis [n = 32], and proctitis [n = 16]. [E] Serum levels of LRG in UC patients with normal CRP levels [≤ 0.2mg/dl] are shown. Patients with UC were categorised into CMH[+] [n = 14] and CMH[−] [n = 59] in two groups. [F] Serum LRG levels in 51 UC patients with deep remission and 69 patients without deep remission are shown; #p < 0.05, *p < 0.01 by the Mann–Whitney U test.
Recent comprehensive analyses indicated that achieving CMH was more critical to avoid disease recurrence.26 We therefore assessed whether LRG could detect CMH in patients with UC by defining CMH as Matts’ grade of 1. In patients with extensive colitis and left-side colitis, LRG levels were significantly higher in those without CMH than in those with CMH [Figure 3D]. Similar to that observed for mucosal healing [Figure 3C], in patients with normal CRP levels, LRG was significantly increased in patients without CMH compared with that in patients with established CMH [Figure 3E]. Moreover, when we defined deep remission as the presence of both clinical remission and mucosal healing, we observed that LRG levels were also significantly higher in those without deep remission than in those with deep remission [Figure 3F]. These data strongly indicated that LRG accurately detected both clinical and endoscopic activities in patients with UC.
3.3. Efficacy of LRG as a serological marker for evaluation of mucosal healing in patients with UC
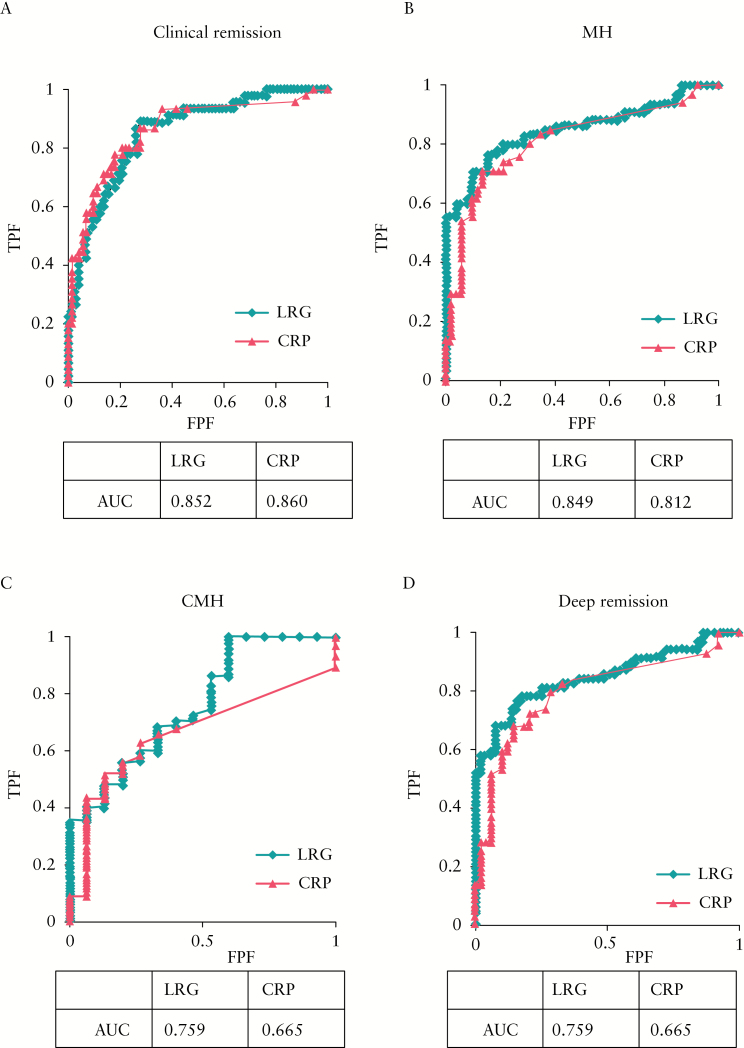
Given the potential role of LRG as a biomarker for clinical and endoscopic disease in UC, we next investigated its diagnostic accuracy in detecting mucosal healing in comparison with CRP. We compared the sensitivity and specificity of LRG with those of CRP by ROC curve and AUC analyses. As shown in Figure 4A, the AUC was similar between LRG and CRP in determining clinical remission by CAI; however, the AUC for LRG was higher than that for CRP for the determination of endoscopic mucosal healing [Figure 4B]. Moreover, the AUC was higher for LRG than CRP for the determination of CMH [Figure 4C] and deep remission [Figure 4D]. Collectively, these results demonstrated that, compared with CRP, LRG had higher sensitivity and specificity in determining mucosal healing in patients with UC. We further analysed the factors associated with the increase in LRG by using univariate followed by multivariate logistic regression analyses. We found that mucosal healing and CRP were independently associated with increase in LRG [Supplementary Table 1, available as Supplementary data at ECCO-JCC online]. We also performed histological analysis for 45 patients. Furthermore, we obtained similar results with endoscopic analysis, showing that LRG levels in patients with histological healing are significantly lower than those in patients without histological healing [Supplementary Figure 1, available as Supplementary data at ECCO-JCC online]. These results strengthen our evidence that LRG is a biomarker for reflecting mucosal healing.
Figure 4.
LRG better differentiates mucosal healing of patients with ulcerative colitis [UC] than C-reactive protein [CRP] does. The receiver operating curves [ROCs] for LRG and CRP levels in discrimination of clinical remission [A], mucosal healing [MH] [B], complete mucosal healing [CMH] [C], and deep remission [D] of patients with UC are shown. The values of area under the ROC curve [AUC] are also shown.
3.4. LRG as a biomarker to monitor mucosal activity during the clinical course of patients with UC
We next sought to determine whether changes in LRG levels reflected changes in endoscopic mucosal status during the course of disease in UC, by serial LRG measurements at two times in a subset of patients: when the disease was endoscopically active [Matts’ grade of 3 or 4] and when the patient achieved mucosal healing [Matts’ grade of 1 or 2]. A total of 38 patients were included in this analysis, and the median time interval between the two measurements was 396 [range, 13–2142] days. LRG levels were significantly higher at the time of endoscopically active disease than at the time of mucosal healing [Figure 5]. Analysis of the relationship between the percentage decrease of LRG levels and establishment of mucosal healing in these longitudinally followed patients showed that none of the patients whose LRG levels were decreased more than 30% [0/17 patients] showed endoscopic worsening. In contrast, 71.4% patients [15/21 patients] whose LRG levels did decrease less than 30% exhibited endoscopic worsening. These results suggested serum LRG as a novel biomarker of mucosal status during the disease course of patients with UC.
Figure 5.
LRG levels are increased in association with mucosal activity during the clinical disease course in patients with UC. Serial LRG levels in patients in relation to the mucosal status are shown. Mucosal healing [(MH[+]) was defined as a Matts’ grade of 1 or 2 [left lane], and non-mucosal healing (MH[−]) was defined as an endoscopically active status with a Matts’ grade of 3 or 4. LRG levels were significantly higher in patients during endoscopically active disease (MH[−]) than those during mucosal healing (MH[+]); **p < 0.001 by paired t test.
4. Discussion
We have previously reported increased serum LRG levels in IBD, particularly in clinically active patients. This study clearly demonstrated that serum LRG levels were significantly elevated in endoscopically active patients with UC in comparison with those with mucosal healing. We also demonstrated serum LRG as a novel disease-monitoring marker that successfully detected endoscopic mucosal activity in patients with UC, and as a novel surrogate marker of endoscopic inflammation in patients with normal serum CRP levels.
CRP is a widely used serum biomarker for the detection and monitoring of many inflammatory diseases. CRP also reflects inflammation in IBD, particularly in CD that shows transmural disease phenotype, and was reported as a biomarker for the detection of clinical and endoscopic activities.4 CRP synthesis in the liver is induced by circulating IL-6. We previously showed that LRG levels were correlated with clinical disease activity even in clinically active patients with CD showing normal CRP levels.7 Serum CRP may be a less effective biomarker in UC, performing as an indicator of superficial inflammation limited to the mucosa, and may not show systemic response related to circulating IL-6.4 In this study, we also showed that LRG levels were increased even in clinically and/or endoscopically active patients with UC showing normal CRP levels. These results indicated that LRG was a useful biomarker for detecting IL-6-independent inflammation that may not be detectable by CRP. We previously showed that LRG expression in intestinal epithelial cells was induced not only by IL-6 but also by TNF-α and IL-22, all of which were increased in sera of patients with UC.10 LRG expression in intestinal epithelial cells that is induced by these cytokines might be a mechanism of the observed stronger association of mucosal inflammation with LRG levels than with CRP.
In the current study, we clearly demonstrated that the endoscopic activity could be accurately monitored by serial measurements of LRG in patients with UC. Due to the relapsing and remitting course of UC, frequent monitoring is occasionally necessary particularly after starting new therapy. Periodic monitoring is also necessary to avoid persistent chronic inflammation that can cause colorectal cancer. In addition to serum CRP, recent reports showed that faecal calprotectin was an effective biomarker for mucosal healing;27 however, faecal samples have inherent associated problems such as limited quantitative capability and cumbersome sampling, which are not significant concerns for serum samples. Accurate detection of mucosal inflammation by a serum biomarker will facilitate the avoidance of frequent endoscopic procedures in patients. Our findings also showed that none of the UC patients with more than 30% decrease in LRG levels showed endoscopic worsening, supporting LRG as a reliable and beneficial monitoring marker to avoid frequent endoscopic evaluation and to efficiently assess therapeutic outcomes.
Recent reports showed that CMH with an MES of 0 was superior to MES of 1 in efficacy to predict recurrence.20,28 However, there are currently no surrogate biomarkers that can distinguish MES of 0 and 1, which are equivalent to the Matts’ grade of 1 and 2. In the present study, we showed significant differences in the serum levels of LRG between patients with CMH [Matts’ grade 1] and patients without CMH [Figure 3D and E]. As the MES is more commonly used than the Matts’ grade for assessing mucosal status, further analysis using both MES and Matts’ grade is necessary to determine if CMH can be detected by changes in LRG levels. Moreover, an improved system with higher sensitivity is currently being developed, which will be critical in more precisely evaluating the extent and level of mucosal healing.
The current study has several limitations. First, this is a retrospective study with a small sample size, and patient backgrounds and treatments were heterogeneous. Second, analysis of the endoscopic activity was not investigated in a blinded fashion by multiple clinicians and was not performed with the more commonly used scoring systems such as the MES21 or the UC-EIS.29 Third, association of LRG with faecal calprotectin30 was not investigated. Finally, the intervals in serial measurements of LRG were inconsistent across patients. Future studies are needed to determine the efficacy of LRG for UC in clinical treatment.
In conclusion, serum LRG is potentially more effective than CRP as a biomarker for detecting endoscopic activity in patients with UC.
Funding
This research was supported by the Research on Practical Research Project for Rare/Intractable Diseases from Japan Agency for Medical Research and Development [15ek0109045h0002]. This research was also supported by Eidia Co. Ltd.
Conflict of Interest
None.
Supplementary Data
Supplementary data are available at ECCO-JCC online
Supplementary Material
Acknowledgments
We gratefully acknowledge the commitment of participating patients for their invaluable contribution to this research.
References
- 1. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 2012;61:1619–35. [DOI] [PubMed] [Google Scholar]
- 2. Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, et al. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol 2010;7:15–29. [DOI] [PubMed] [Google Scholar]
- 3. Toniatti C, Arcone R, Majello B, et al. Regulation of the human C-reactive protein gene, a major marker of inflammation and cancer. Mol Biol Med 1990;7:199–212. [PubMed] [Google Scholar]
- 4. Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis 2004;10:661–5. [DOI] [PubMed] [Google Scholar]
- 5. Jones J, Loftus EV, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2008;6:1218–24. [DOI] [PubMed] [Google Scholar]
- 6. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 2006;55:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serada S, Fujimoto M, Ogata A, et al. iTRAQ-based proteomic identification of leucine-rich alpha-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann Rheum Dis 2010;69:770–4. [DOI] [PubMed] [Google Scholar]
- 8. Haupt H, Baudner S. (Isolation and characterization of an unknown, leucine-rich 3.1-S-alpha2-glycoprotein from human serum [author’s translation]). Hoppe Seylers Z Physiol Chem 1977;358:639–46. [PubMed] [Google Scholar]
- 9. Takahashi N, Takahashi Y, Putnam FW. Periodicity of leucine and tandem repetition of a 24-amino acid segment in the primary structure of leucine-rich alpha 2-glycoprotein of human serum. Proc Natl Acad Sci U S A 1985;82:1906–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serada S, Fujimoto M, Terabe F, et al. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm Bowel Dis 2012;18:2169–79. [DOI] [PubMed] [Google Scholar]
- 11. Wang X, Abraham S, McKenzie JAG, et al. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature 2013;499:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watson CJ, Ledwidge MT, Phelan D, et al. Proteomic analysis of coronary sinus serum reveals leucine-rich α2-glycoprotein as a novel biomarker of ventricular dysfunction and heart failure. Circ Heart Fail 2011;4:188–97. [DOI] [PubMed] [Google Scholar]
- 13. Furukawa K, Kawamoto K, Eguchi H, et al. Clinicopathological significance of leucine-rich α2-glycoprotein-1 in sera of patients with pancreatic cancer. Pancreas 2015;44:93–98. [DOI] [PubMed] [Google Scholar]
- 14. Podolsky DK. Inflammatory bowel disease [1]. N Engl J Med 1991;325:928–37. [DOI] [PubMed] [Google Scholar]
- 15. Podolsky DK. Inflammatory bowel disease [2]. N Engl J Med 1991;325:1008–16. [DOI] [PubMed] [Google Scholar]
- 16. Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rachmilewitz D. Coated mesalazine [5-aminosalicylic acid] versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ 1989;298:82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matts SG. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q J Med 1961;30:393–407. [PubMed] [Google Scholar]
- 19. Fukuba N, Ishihara S, Tada Y, et al. Prevalence of irritable bowel syndrome-like symptoms in ulcerative colitis patients with clinical and endoscopic evidence of remission: prospective multicenter study. Scand J Gastroenterol 2014;49:674–80. [DOI] [PubMed] [Google Scholar]
- 20. Nakarai A, Kato J, Hiraoka S, et al. Prognosis of ulcerative colitis differs between patients with complete and partial mucosal healing, which can be predicted from the platelet count. World J Gastroenterol 2014;20:18367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- 22. Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000;47:404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zenlea T, Yee EU, Let R, et al. Histology grade is independently associated with relapse risk in patients with ulcerative colitis in clinical remission: A prospective study. Am J Gastroenterol 2016;111:685–90. [DOI] [PubMed] [Google Scholar]
- 24. Fujimoto M, Serada S, Suzuki K, et al. Leucine-rich α2 -glycoprotein as a potential biomarker for joint inflammation during anti-interleukin-6 biologic therapy in rheumatoid arthritis. Arthritis Rheumatol 2015;67:2056–60. [DOI] [PubMed] [Google Scholar]
- 25. Beck JR, Shultz EK. The use of relative operating characteristic [ROC] curves in test performance evaluation. Arch Pathol Lab Med 1986;110:13–20. [PubMed] [Google Scholar]
- 26. Naganuma M, Aoyama N, Suzuki Y, et al. Twice-daily budesonide 2-mg foam induces complete mucosal healing in patients with distal ulcerative colitis. J Crohns Colitis 2016;10:828––36.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Falvey JD, Hoskin T, Meijer B, et al. Disease activity assessment in IBD: clinical indices and biomarkers fail to predict endoscopic remission. Inflamm Bowel Dis 2015;21:824–31. [DOI] [PubMed] [Google Scholar]
- 28. Yokoyama K, Kobayashi K, Mukae M, et al. Clinical study of the relation between mucosal healing and long-term outcomes in ulcerative colitis. Gastroenterol Res Pract 2013;2013:192794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Travis SP, Schnell D, Krzeski P, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity [UCEIS]. Gut 2012;61:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tibble JA, Sigthorsson G, Bridger S, et al. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 2000;119:15–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.