Abstract
Background and Aims:
To assess golimumab pharmacokinetics [PK] and exposure-response [ER] in adults with moderate-to-severe ulcerative colitis [UC] from the Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment [PURSUIT] studies.
Methods:
We analysed golimumab PK and ER data of patients with moderate-to-severe UC from the PURSUIT-subcutaneous induction [N = 1064] and maintenance [N = 464] studies. Induction analyses evaluated serum golimumab concentration [SGC] and efficacy data through Week [wk] 6 following subcutaneous doses at wk0 and wk2; maintenance analyses assessed data through wk54 following 4-weekly dosing. ER relationships were assessed using trend, logistic regression, and receiver-operating-characteristic curve analyses.
Results:
Median SGCs peaked at induction wk2 for golimumab 100/50mg, 200/100mg, and 400/200mg. Wk6 median SGCs were 0.78, 1.78, and 4.01 μg/ml, respectively. SGCs were sustained, reaching steady state approximately 8wks after golimumab maintenance commenced [wk14 of golimumab] regardless of induction dose. Median trough SGCs from maintenance wks8–44 ranged from 0.69 to 0.83 µg/ml [50 mg] and 1.33–1.58 µg/ml [100 mg]. SGCs were approximately dose proportional, and higher SGCs were associated with higher efficacy response rates during induction and maintenance. Factors associated with golimumab exposure were body weight, antibody-to-golimumab status, serum albumin, alkaline phosphatase, faecal markers, C-reactive protein, and pancolitis. SGCs of 2.5 µg/ml [induction wk6] and 1.4 µg/ml [maintenance steady-state trough] are potential target concentrations. Immunomodulators had no apparent impact on SGC with golimumab 100mg, whereas drug levels were slightly higher with golimumab 50mg with vs without immunomodulators.
Conclusions:
SGCs are approximately dose proportional, and a positive SGC-efficacy relationship exists during induction/maintenance golimumab treatment of adult UC patients. Optimal SGC targets require validation in prospective studies.
Keywords: Ulcerative colitis, anti-tumour necrosis factor, pharmacokinetics
1. Introduction
The introduction and effectiveness of biologic therapies in treating ulcerative colitis [UC], including those directed against tumour necrosis factor-α [TNFα], has led to substantial changes in UC treatment strategies and goals, as demonstrated by evolving endpoints in clinical trials and targets used in clinical practice. One of the concepts that has gained acceptance is that therapeutic drug monitoring and dose optimisation of anti-TNF agents can maximise treatment efficacy, as opposed to previous goals centred on adjustment of therapy based on the signs and symptoms of the disease. The research behind such a paradigm shift has evaluated correlations between anti-TNF agent trough concentrations, anti-drug antibodies, and clinical outcomes. Results of such evaluations have shown that detectable, as opposed to undetectable, infliximab trough concentrations are associated with significantly higher clinical and endoscopic response and remission rates.1 Similarly, an infliximab trough concentration exceeding 2 µg/ml, regardless of antibody-to-infliximab status, was associated with a significantly higher rate of corticosteroid-free clinical remission when compared with lower trough concentrations; higher response rates were sustained for approximately 20 months.2 To add more specificity to these findings, post hoc analyses of the ACT 1 and ACT 2 trials, which evaluated the anti-TNF agent infliximab in patients with UC, indicated that approximate serum infliximab concentrations of 41 µg/ml at induction Week 8 and 3.7 µg/ml at maintenance steady state were associated with optimal outcomes in patients with UC.3 Likewise, distinct trough concentrations of adalimumab have been associated with efficacy outcomes in patients with inflammatory bowel disease [IBD],4 particularly in patients with Crohn’s disease.5
In 2013, the United States Food and Drug Administration approved golimumab, a human monoclonal anti-TNF agent, for the treatment of patients with moderate-to-severe UC, largely based on the results of the Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment [PURSUIT], including the PURSUIT-subcutaneous induction [PURSUIT-SC; NCT00487539]6 and PURSUIT maintenance [PURSUIT-M; NCT00488631]7 trials. In these randomised, double-blind, placebo-controlled trials, treatment with subcutaneous [SC] golimumab induced clinical response, remission, and mucosal healing, and increased quality of life in larger percentages of patients with active UC than did placebo, and continued golimumab in patients who responded to induction therapy maintained clinical response through Week 54 [golimumab 50 or 100 mg] and achieved clinical remission and mucosal healing at Weeks 30 and 54 [golimumab 100 mg].6,7 In a recently published small observational study of patients with moderate-to severe UC, golimumab concentrations appeared to be associated with clinical response, as median serum golimumab concentrations [SGCs] were significantly higher in partial clinical responders versus nonresponders.8 We now report on golimumab pharmacokinetics [PK] and exposure-response [ER] relationships using data derived from the large PURSUIT-SC induction and maintenance studies in UC, which to our knowledge is the most comprehensive PK and ER evaluation of golimumab in UC.
2. Patients and Methods
2.1. Patients and study design
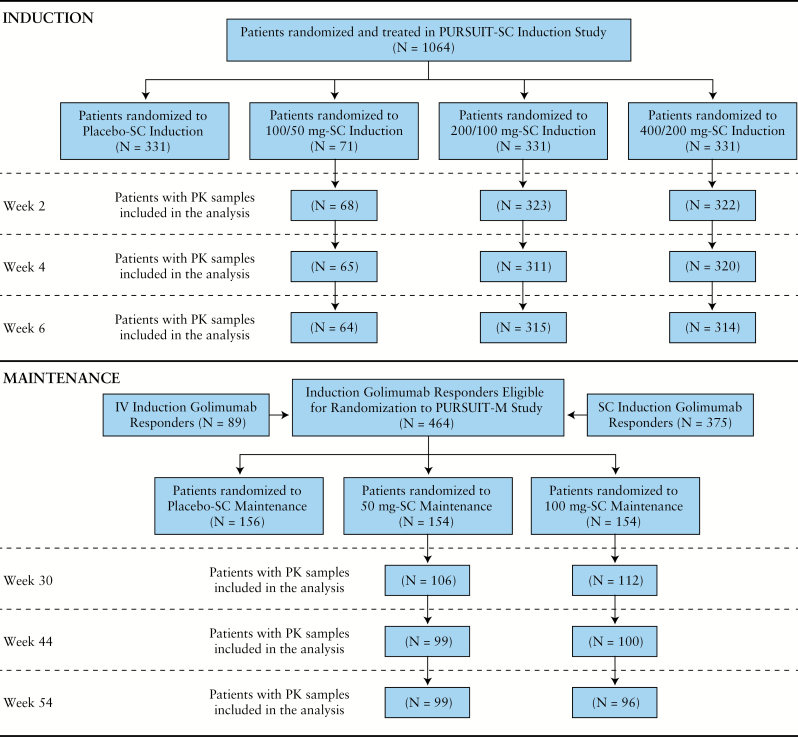
Details of the PURSUIT-SC [NCT00487539]6 and PURSUIT-M [NCT00488631]7 trials have been reported. The PURSUIT-SC trial comprised an integrated Phase 2 SC dose-finding phase followed by a Phase 3 confirmatory phase. UC patients [N = 1064] with Mayo scores of 6–12 inclusive, including endoscopic subscore ≥ 2, were randomised to receive placebo/placebo [n = 331], golimumab 100mg/50mg [before Phase 3 dose selection only, n = 71], golimumab 200mg/100mg [n = 331], or golimumab 400mg/200mg [n = 331] at induction Weeks 0 and 2, respectively. Patients from the PURSUIT- SC and the PURSUIT-intravenous [PURSUIT-IV; NCT00488774] induction studies who responded to induction therapy with golimumab [n = 464] were assigned randomly in the PURSUIT-M study to receive placebo [n = 156] or injections of 50mg [n = 154] or 100mg [n = 154] golimumab every 4 weeks through Week 52. Patients with available SGC data at the time points of interest in PURSUIT-SC, as well as golimumab induction responders with available SGC data in PURSUIT-M, were the focus of the present PK and ER analyses for induction and maintenance, respectively. A patient disposition flow chart showing patients contributing data at various time points is shown in Figure 1.
Figure 1.
Patient disposition throughout the Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment [PURSUIT] among 1064 patients who had serum golimumab concentration and efficacy outcome data suitable for analysis. IV, intravenous; M, maintenance; PK, pharmacokinetic; SC, subcutaneous.
2.2. Study evaluations and analyses
Serum golimumab concentrations were measured in blood samples collected at scheduled visits through Week 6 of induction treatment and through Week 54 of maintenance treatment [Table 1], using a validated electrochemiluminescent assay with a lowest quantifiable concentration of 0.039 µg/ml.9 The presence of antibodies to golimumab was determined using a validated bridging immunoassay, in which golimumab was used to capture and then detect induced immune responses to golimumab. Samples for the evaluation of antibodies to golimumab were obtained at baseline and Week 6 of the induction study, and at Week 30 and Week 54 of the maintenance study. In addition, blood samples were collected for the evaluation of SGCs and anti-golimumab antibodies from patients who discontinued study agent administration or who lost response. Patients were classified as positive if antibodies were detected at any time in their serum sample, or negative otherwise. Of note, the presence of golimumab in serum samples can interfere with the detection of antibodies to golimumab with this assay.
Table 1.
Pharmacokinetic blood sampling times for the Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment [PURSUIT] trials.
| Visits scheduled through induction Week 6 of PURSUIT-SC | Visits scheduled through maintenance Week 54 of PURSUIT-M | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week | 0 & 2 | 4 & 6 | 0 | 4, 8, & 12 | 16, 20, & 24 | 28 | 30 | 36 | 44 | 54 |
| Sample obtained | Xa | X | Xa,b | Xa | Xa,c | Xa | X | Xa | Xa | X |
aSample obtained before golimumab administration.
bSample at maintenance Week 0 is the same as sample at induction Week 6.
cIn addition to predose sample obtained at Week 20, a sample was randomly obtained at any time between maintenance Weeks 16–24.
M, maintenance; PURSUIT, Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment; SC, subcutaneous.
To assess disease activity, Mayo scores10 were calculated. Clinical response was defined as a decrease from baseline in the Mayo score ≥ 30% and ≥ 3 points, accompanied by either a rectal bleeding subscore of 0 or 1 or a decrease from baseline in the rectal bleeding subscore ≥ 1. Clinical remission was defined as a Mayo score ≤ 2 points, with no individual subscore > 1, and mucosal healing was defined as a Mayo endoscopy subscore of 0 or 1.11,12 Intrinsic and extrinsic patient factors, including concomitant immunomodulator use, were recorded throughout the study.
The relationships between SGCs and efficacy outcomes during induction and maintenance were analysed separately. For induction, the primary focus was on the relationships between SGCs and induction efficacy outcomes including clinical response, clinical remission, and mucosal healing, all at Week 6. Similarly, steady-state trough SGCs [at Week 44] during maintenance and SGCs at Week 30 and Week 54 were correlated with maintenance efficacy outcomes including maintaining clinical response through Week 54, achieving and sustaining clinical remission at both Week 30 and Week 54, and achieving and sustaining mucosal healing at both Week 30 and Week 54. In addition, the prognostic value of earlier SGCs on subsequent induction and maintenance efficacy outcomes was evaluated.
2.3. Statistical analysis
All SGC data were summarized through the use of descriptive statistics. Missing SGC data were not imputed. Serum golimumab concentration data were compared between patients achieving and not achieving the specified efficacy outcomes, using a two-sided Wilcoxon–Mann–Whitney, two-sample, rank-sum test. The presence of a trend in the proportion of patients with a given clinical efficacy outcome was evaluated across SGC quartiles using a one-sided Cochrane–Armitage trend test. The Fisher’s exact and Kruskal-Wallis tests were used to compare categorical and continuous variables across SGC quartiles, respectively. Associations between SGCs and efficacy outcomes, including the impact of patient factors, were evaluated using multivariable logistic regression modelling. Thresholds of SGCs for efficacy outcomes were determined using receiver-operating-characteristics [ROC] curve analysis.
3. Results
3.1. Baseline patient characteristics and patient disposition
Among the 1064 PURSUIT-SC patients [Figure 1], baseline demographic and other characteristics were representative of an adult population with moderately-to-severely active UC [Table 2].
Table 2.
Baseline patient characteristics among 1064 PURSUIT-SC patients.
| Characteristic | Median [range] or proportion |
|---|---|
| Male | 56.0% |
| Age, years | 38.0 [18.0 – 78.0] |
| Body weight, kg | 72.0 [33.0 – 149.7] |
| Disease duration, years | 4.2 [0.1 – 48.3] |
| C-reactive protein, mg/l | 4.8 [0.1 – 258.0] |
| Log-transformed faecal calprotectin, µg/kg | 2.9 [1.1 – 4.5] |
| Log transformed fecaal lactoferrin, µg/ml | 2.3 [-0.6 – 3.7] |
| Mayo score | 8.0 [5.0 – 12.0] |
| Serum albumin, g/dl | 4.2 [2.1 – 5.5] |
| Concomitant azathioprine/6-mercaptopurine/methotrexate | 32.4% |
| Concomitant corticosteroid use | 42.8% |
| Extent of disease [pancolitis] | 42.1% |
| Smoking history [yes] | 35.2% |
PURSUIT, Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment; SC, subcutaneous.
3.2. Golimumab exposure through induction Week 6 and through maintenance Week 54
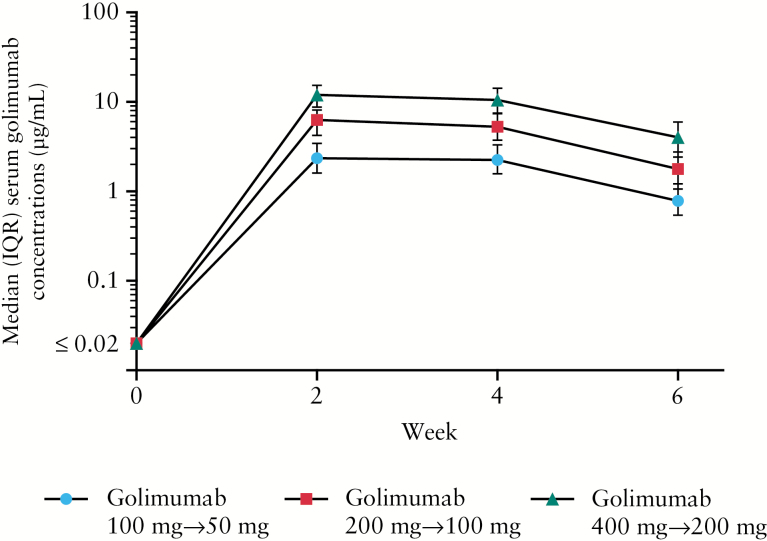
Median SGCs peaked at induction Week 2 across all SC dose groups, with concentrations of 2.34 μg/ml, 6.27 μg/ml and 11.95 μg/ml for the 100mg/50mg, 200mg/100mg, and 400mg/200mg induction dose groups, respectively. Respective median SGCs at Week 6 were 0.78 μg/ml, 1.78 μg/ml, and 4.01 μg/ml. Thus, SGCs were approximately dose proportional during induction therapy [Figure 2].
Figure 2.
Median serum golimumab concentrations illustrating dose proportionality over time during the induction phase of PURSUIT. IQR, interquartile range; PURSUIT, the Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment.
Serum golimumab concentrations, which were also approximately dose proportional during maintenance therapy, were sustained, reaching steady state approximately 8 weeks after the start of SC golimumab maintenance [or 14 weeks after initiation of golimumab induction therapy], regardless of the SC induction dose. Median pre-administration [trough] SGCs from maintenance Weeks 8 to 44 ranged from 0.69 to 0.83 μg/ml in the golimumab 50mg and from 1.33 to 1.58 μg/ml in the golimumab 100mg maintenance groups. Median SGCs at Week 30 and Week 54 of maintenance [2 weeks postdose for both visits] were 1.73 μg/ml and 1.81 μg/ml, respectively, in the golimumab 50mg group, and 3.81 μg/ml and 3.52 μg/ml, respectively, in the golimumab 100mg group.
3.3. Relationships between serum golimumab concentrations and patient factors
During induction, patients with SGCs in the lowest quartile were more likely to weigh more, have a lower serum albumin concentration, have higher concentrations of faecal biomarkers [calprotectin and lactoferrin] and serum C-reactive protein [CRP], and have higher incidences of antibodies to golimumab and pancolitis. Similarly, during maintenance, patients in the lowest SGC quartile were heavier, with a higher incidence of antibodies to golimumab, lower serum albumin levels, and higher serum concentrations of CRP and alkaline phosphatase [Table 3].
Table 3.
Summary of patient characteristics at baseline by SGC quartiles at Week 6 of induction and Week 44 of maintenance among patients treated with golimumab in the PURSUIT studies.
| Characteristic | All | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-Valuea |
|---|---|---|---|---|---|---|
| Induction Week 6, N | 693 | 173 | 173 | 173 | 174 | |
| Age [years] | ||||||
| Mean [SD] | 40.5 [13.5] | 41.5 [13.5] | 39.1 [13.3] | 41.0 [14.2] | 40.5 [13.1] | |
| Median [IQR] | 39.0 [30.0, 50.0] | 41.0 [31.0, 52.0] | 37.0 [28.0, 49.0] | 40.0 [28.0, 52.0] | 39.0 [31.0, 49.0] | 0.404 |
| Range | 18.0 – 78.0 | 18.0 – 78.0 | 18.0 – 71.0 | 19.0 – 72.0 | 18.0 –77.0 | |
| Body weight [kg] | ||||||
| Mean [SD] | 74.5 [18.2] | 79.0 [19.5] | 74.9 [16.7] | 74.3 [18.6] | 69.8 [16.7] | |
| Median [IQR] | 73.1 [61.0, 85.0] | 78.0 [63.5, 90.0] | 75.0 [62.6, 84.0] | 73.0 [60.0, 83.6] | 68.2 [56.8, 77.5] | < 0.001 |
| Range | 33.0 – 149.0 | 40.0 – 137.8 | 33.0 – 123.0 | 35.0 – 149.7 | 37.4 – 121.2 | |
| Mayo score | ||||||
| Mean [SD] | 8.5 [1.5] | 8.7 [1.5] | 8.5 [1.5] | 8.6 [1.5] | 8.3 [1.4] | |
| Median [IQR] | 8 [7.0, 10.0] | 9.0 [8.0, 10.0] | 9.0 [7.0, 10.0] | 8.0 [8.0, 10.0] | 8.0 [7.0, 9.0] | 0.055 |
| Range | 5.0 – 12.0 | 5.0 – 12.0 | 6.0 – 12.0 | 6.0 – 12.0 | 6.0 – 12.0 | |
| Disease duration from time of diagnosis [years] | ||||||
| Mean [SD] | 6.5 [6.4] | 5.8 [6.1] | 6.8 [6.4] | 6.8 [7.1] | 6.7 [6.0] | 0.205 |
| Median [IQR] | 4.4 [2.0, 9.0] | 4.0 [1.7, 7.3] | 5.0 [2.0, 10.0] | 4.4 [2.1, 8.8] | 4.0 [2.2, 10.1] | |
| Range | 0.1 – 41.7 | 0.1 – 37.5 | 0.1 – 33.8 | 0.1 – 41.7 | 0.2 – 33.5 | |
| Log faecal calprotectin | ||||||
| Mean [SD] | 2.9 [0.7] | 3.1 [0.7] | 2.9 [0.6] | 2.9 [0.7] | 2.7 [0.7] | |
| Median [IQR] | 2.9 [2.6, 3.3] | 3.1 [2.8, 3.4] | 3.0 [2.6, 3.2] | 2.9 [2.6, 3.3] | 2.8 [2.4, 3.2] | < 0.001 |
| Range | 1.1 – 4.5 | 1.1 – 4.5 | 1.1 – 4.2 | 1.1 – 4.2 | 1.1 – 4.4 | |
| Log faecal lactoferrin | ||||||
| Mean [SD] | 2.1 [0.8] | 2.3 [0.8] | 2.1 [0.8] | 2.1 [0.8] | 1.9 [0.8] | |
| Median [IQR] | 2.3 [1.8, 2.7] | 2.5 [2.0, 2.8] | 2.3 [1.8, 2.7] | 2.2 [1.9, 2.7] | 2.0 [1.4, 2.6] | < 0.001 |
| Range | -0.6 – 3.5 | -0.6 – 3.5 | -0.6 – 3.3 | -0.6 – 3.3 | -0.6 – 3.3 | |
| Albumin [g/dl] | ||||||
| Mean [SD] | 4.2 [0.4] | 4.0 [0.5] | 4.2 [0.4] | 4.2 [0.4] | 4.3 [0.4] | |
| Median [IQR] | 4.2 [3.9, 4.5] | 4.0 [3.7, 4.3] | 4.3 [4.0, 4.5] | 4.3 [4.0, 4.5] | 4.4 [4.1, 4.6] | < 0.001 |
| Range | 2.5 – 5.3 | 2.5 – 5.3 | 2.6 – 5.3 | 2.7 – 5.2 | 3.0 – 5.3 | |
| CRP [ mg/l] | ||||||
| Mean [SD] | 11.3 [17.9] | 16.3 [18.9] | 10.7 [21.2] | 11.1 [17.5] | 7.0 [11.8] | |
| Median [IQR] | 4.9 [1.7, 13.3] | 9.8 [3.8, 20.0] | 5.5 [1.8, 12.6] | 4.4 [1.9, 11.7] | 2.9 [0.8, 7.7] | < 0.001 |
| Range | 0.1 – 240.0 | 0.1 – 94.7 | 0.1 – 240.0 | 0.1 – 98.9 | 0.1 – 73.3 | |
| Alkaline phosphatase [IU/l] | ||||||
| Mean [SD] | 73.1 [26.3] | 75.8 [26.5] | 77.2 [32.2] | 72.4 [24.5] | 67.2 [19.4] | |
| Median [IQR] | 69.0 [57.0, 84.0] | 72.0 [59.0, 86.0] | 71.0 [60.0, 88.0] | 69.0 [55.0, 86.0] | 64.0 [55.0, 76.0] | 0.002 |
| Range | 19.0 – 359.0 | 30.0 – 242.0 | 29.0 – 359.0 | 26.0 – 167.0 | 19.0 – 156.0 | |
| Gender [male], % | 57.3 | 61.3 | 61.3 | 54.9 | 51.7 | 0.185 |
| Antibody-to-golimumab status [positive], % | 2.9 | 6.9 | 1.7 | 1.2 | 1.7 | 0.010 |
| Extent of disease [pancolitis], % | 41.9 | 55.5 | 39.9 | 35.8 | 36.2 | < 0.001 |
| Immunomodulator use [yes], % | 32.5 | 36.4 | 33.5 | 28.9 | 31.0 | 0.481 |
| Corticosteroid use [yes], % | 44.4 | 47.4 | 43,9 | 42.8 | 43.7 | 0.835 |
| Smoking history [yes], % | 37.4 | 41.6 | 35.8 | 35.8 | 36.2 | 0.627 |
| Maintenance week 44, N | 199 | 49 | 50 | 50 | 50 | |
| Age [years] | ||||||
| Mean[SD] | 40.1 [13.1] | 44.2 [14.2] | 39.7 [12.4] | 38.2 [13.1] | 38.4 [12.2] | |
| Median [IQR] | 39.0 [30.0, 49.0] | 44.0 [33.0, 55.0] | 39.5 [30.0, 49.0] | 36.5 [27.0, 48.0] | 37.0 [30.0, 48.0] | 0.148 |
| Range | 18.0 -74.0 | 20.0 – 74.0 | 18.0 -63.0 | 19.0 – 72.0 | 18.0 – 64.0 | |
| Body weight [kg] | ||||||
| Mean [SD] | 72.7 [18.1] | 76.4 [22.7] | 76.4 [13.9] | 73.1 [19.0] | 65.2 [13.3] | |
| Median [IQR] | 72.0 [59.0, 83.6] | 74.5 [62.7, 84.0] | 75.0 [66.5, 88.0] | 70.3 [58.0, 85.0] | 65.0 [55.0, 74.1] | 0.003 |
| Range | 35.0 – 137.8 | 35.0 – 137.0 | 49.9 – 102.0 | 42.0 – 116.4 | 37.4 - 93.5 | |
| Mayo score | ||||||
| Mean [SD] | 8.3 [1.4] | 8.3 [1.5] | 8.1 [1.2] | 8.1 [1.4] | 8.4 [1.4] | |
| Median [IQR] | 8.0 [7.0, 9.0] | 8.0 [7.0, 9.0] | 8.0 [7.0, 9.0] | 8.0 [7.0, 9.0] | 8.0 [7.0, 9.0] | 0.564 |
| Range | 6.0 – 12.0 | 6.0 – 11.0 | 6.0 – 11.0 | 6.0 – 11.0 | 6.0 – 12.0 | |
| Disease duration from time of diagnosis [years] | ||||||
| Mean [SD] | 7.3 [7.2] | 8.6 [8.4] | 6.6 [6.3] | 6.4 [6.9] | 7.5 [7.0] | |
| Median [IQR] | 5.3 [2.3, 10.0] | 7.2 [2.7, 11.3] | 5.4 [2.1, 9.0] | 4.5 [2.8, 7.3] | 4.7 [2.3, 10.5] | 0.644 |
| Range | 0.1 – 41.7 | 0.2 – 37.7 | 0.1 – 35.9 | 0.3 – 41.7 | 0.4 – 33.5 | |
| Log faecal calprotectin | ||||||
| Mean [SD] | 2.7 [0.7] | 2.7 [0.6] | 2.7 [0.8] | 2.6 [0.8] | 2.8 [0.7] | |
| Median [IQR] | 2.8 [2.3, 3.1] | 2.8 [2.3, 3.0] | 2.8 [2.3, 3.3] | 2.8 [2.4, 3.1] | 2.8 [2.3, 3.3] | 0.872 |
| Range | 1.1 – 4.3 | 1.1 – 4.3 | 1.1 – 4.1 | 1.1 – 4.2 | 1.1 – 4.1 | |
| Log faecal lactoferrin | ||||||
| Mean [SD] | 1.9 [0.9] | 1.9 [0.9] | 1.9 [1.0] | 1.9 [0.9] | 1.9 [0.9] | |
| Median [IQR] | 2.1 [1.4, 2.6] | 2.0 [1.6, 2.5] | 2.3 [1.3, 2.7] | 2.2 [1.6, 2.6] | 1.9 [1.4, 2.6] | 0.893 |
| Range | -0.6 – 3.2 | -0.2 – 2.9 | -0.6 – 3.2 | -0.6 – 3.2 | -0.6 – 3.2 | |
| Albumin [g/dl] | ||||||
| Mean [SD] | 4.3 [0.4] | 4.1 [0.4] | 4.3 [0.4] | 4.4 [0.4] | 4.3 [0.4] | |
| Median [IQR] | 4.3 [4.0, 4.5] | 4.1 [3.9, 4.4] | 4.3 [4.1, 4.6] | 4.4 [4.2, 4.7] | 4.3 [4.0, 4.6] | 0.004 |
| Range | 2.7 – 5.3 | 3.3 – 5.0 | 3.5 – 5.3 | 2.7 – 5.2 | 3.3 – 5.1 | |
| CRP [ mg/l] | ||||||
| Mean [SD] | 6.9 [10.7] | 8.6 [9.0] | 4.2 [4.9] | 7.9 [10.4] | 6.6 [15.4] | |
| Median [IQR] | 3.5 [1.0, 8.2] | 5.8 [2.2, 12.5] | 2.5 [1.2, 5.6] | 3.6 [0.7, 10.5] | 1.9 [0.8, 5.4] | 0.014 |
| Range | 0.1 – 89.1 | 0.1 – 38.2 | 0.1 – 27.1 | 0.1 – 48.6 | 0.1 – 89.1 | |
| Alkaline phosphatase [IU/l] | ||||||
| Mean [SD] | 73.0 [30.8] | 85.8 [44.7] | 73.5 [25.7] | 66.4 [22.7] | 66.3 [20.9] | |
| Median [IQR] | 67.0 [55.0, 83.0] | 76.0 [58.0, 93.0] | 66.5 [58.0, 90.0] | 64.0 [52.0, 76.0] | 66.5 [57.0, 72.0] | 0.041 |
| Range | 19.0 – 287.0 | 41.0 - 287.0 | 32.0 – 156.0 | 19.0 – 137.0 | 25.0 – 131.0 | |
| Gender [male], % | 56.3 | 59.2 | 58.0 | 58.0 | 50.0 | 0.784 |
| Antibody-to-golimumab status [positive] % | 2.0 | 8.2 | 0.0 | 0.0 | 0.0 | 0.003 |
| Extent of disease [pancolitis], % | 43.2 | 55.1 | 44.0 | 42.0 | 32.0 | 0.143 |
| Immunomodulator use [yes], % | 29.7 | 22.5 | 24.0 | 40.0 | 32.0 | 0.211 |
| Smoking history [yes], % | 30.2 | 30.6 | 28.0 | 36.0 | 26.0 | 0.734 |
a p-values are based on a comparison of the proportions for discrete variables [Fisher’s exact test] or median values for continuous variables [Kruskal-Wallis test], across the four data quartiles.
CRP, C-reactive protein; IQR, interquartile range; PURSUIT, Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment; SD, standard deviation; SGC, serum golimumab concentration.
Approximately 30% of patients were receiving immunosuppressives, including azathioprine, 6-mercaptopurine, or methotrexate, in PURSUIT. In general, the use of immunomodulators had no apparent impact on median steady-state SGCs in the golimumab 100mg group, while median steady-state SGCs were slightly higher among patients receiving golimumab 50mg in combination with immunomodulators versus without immunomodulators [Table S1, available as Supplementary data at ECCO-JCC online].
The overall incidence of antibodies to golimumab from induction Week 0 through maintenance Week 54 was low [approximately 3%]. Of note, the incidence of antibodies to golimumab in patients receiving concomitant immunomodulators was lower than in patients not receiving immunomodulators [1.5% vs 3.5%, respectively]. Although there was no apparent impact of antibodies to golimumab on efficacy during induction, a numerically lower proportion of patients who were positive for antibodies to golimumab achieved maintenance efficacy outcomes compared with those who tested negative for antibodies. This difference did not reach statistical significance [Table S2, available as Supplementary data at ECCO-JCC online].
3.4. Exposure-response: serum golimumab concentrations and efficacy outcomes
Median SGCs were higher among patients achieving efficacy outcomes compared with those not achieving those outcomes [Table 4]. When assessed by the degree of efficacy, trough SGCs were higher in patients attaining the more robust endpoint of clinical remission compared with those achieving only clinical response or those not in response [Table S3, available as Supplementary data at ECCO-JCC online]. In addition, SGCs in patients who attained a Mayo endoscopic subscore of 0 were numerically higher than in patients who attained a Mayo endoscopic subscore of 1; however, the difference did not consistently reach statistical significance [Table S3].
Table 4.
Median SGCs at Week 6 and at Week 44 among patients who did and did not achieve clinical response, clinical remission, and mucosal healing endpoints in the PURSUIT studies.
| Induction efficacy outcomes at Week 6 | Week 6 SGC [µg/ml] | p-Value | |
|---|---|---|---|
| Achieved | Not Achieved | ||
| Clinical responsea | 2.96 [n = 373] |
1.55 [n = 320] |
< 0.001 |
| Mucosal healingb | 3.14 [n = 315] |
1.70 [n = 378] |
< 0.001 |
| Clinical remissionc | 3.14 [n = 134] |
2.13 [n = 559] |
< 0.001 |
| Maintenance efficacy outcomes | Week 44 SGC [µg/ml] | p-Value | |
| Achieved | Not achieved | ||
| Clinical responsea through Week 54 | 1.17 [n = 142] |
0.83 [n = 57] |
0.003 |
| Mucosal healingb at Week 30 and Week 54 | 1.22 [n = 124] |
0.83 [n = 75] |
0.005 |
| Clinical remissionc at Week 30 and Week 54 | 1.50 [n = 74] |
0.87 [n = 125] |
< 0.001 |
aClinical response was defined as a decrease from baseline in the Mayo score ≥ 30% and ≥ 3 points, accompanied by either a rectal bleeding subscore of 0 or 1 or a decrease from baseline in the rectal bleeding subscore ≥ 1.
bMucosal healing was defined as a Mayo endoscopy subscore of 0 or 1.
cClinical remission was defined as a Mayo score ≤ 2 points, with no individual subscore > 1.
PURSUIT, Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment; SGC, serum golimumab concentration.
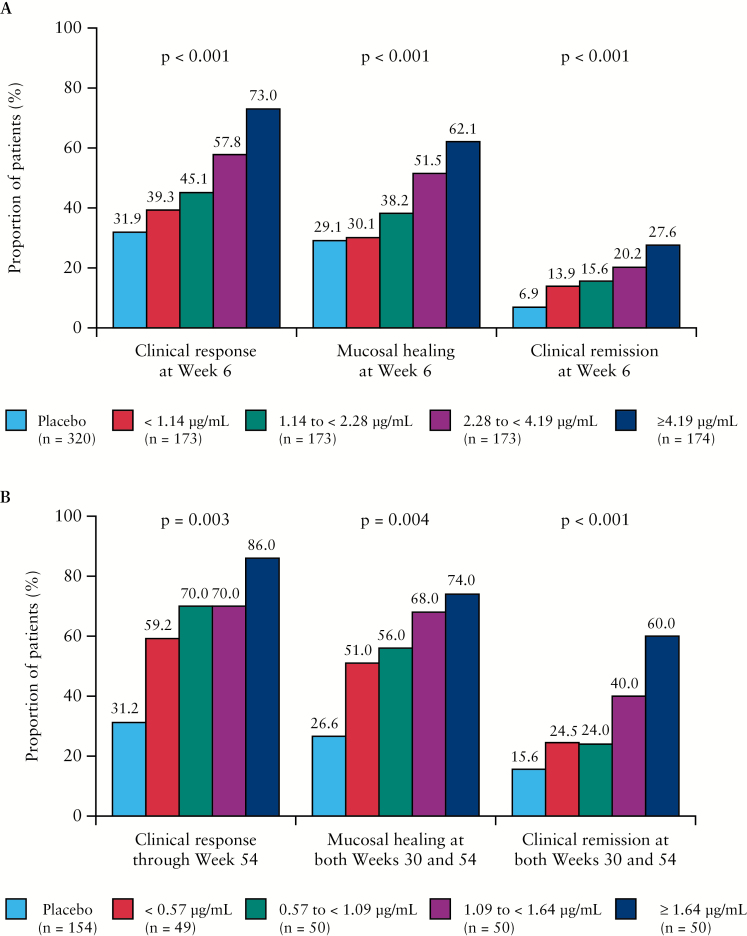
When assessed by SGC quartiles, the proportions of patients achieving efficacy outcomes generally increased with increasing golimumab concentrations. Patients with SGCs in the lowest quartile consistently showed lower rates of clinical response, clinical remission, and mucosal healing, with rates of success sometimes approaching those observed in patients assigned to placebo, during both induction [Figure 3A] and maintenance [Figure 3B]. Of note, when ER relationships were assessed by dose group, despite having higher SGCs, the proportions of patients achieving clinical response in the lowest SGC quartile of the higher dose group was not greater than the proportion in the lowest quartile of the lower dose group. This observation is illustrated in Figure S1A and B, available as Supplementary data at ECCO-JCC online for the endpoints of clinical response at Week 6 [induction] and clinical remission at both Weeks 30 and 54 [maintenance], respectively.
Figure 3.
Proportions of patients achieving clinical response, mucosal healing, and clinical remission during induction by SGC quartile at Week 6 [A], and during maintenance by SGC quartile at Week 44 [B], in PURSUIT. PURSUIT, Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment; SGC, serum golimumab concentration.
Multivariable logistic regression analyses were performed to identify factors associated with the golimumab ER relationship in UC patients during induction and maintenance treatment. The final logistic regression model for induction showed that only higher SGC at Week 6 and female gender were statistically significant predictors of all efficacy outcomes assessed at Week 6, i.e. clinical response, remission, and mucosal healing. Non-use of corticosteroids at baseline predicted clinical response and mucosal healing but not clinical remission. A lower baseline Mayo score was associated with a higher probability of mucosal healing and clinical remission but not clinical response. Also, a higher baseline albumin concentration was predictive of mucosal healing, but not clinical response or remission, whereas a lower baseline calprotectin level was predictive of only clinical response [Table S4, available as Supplementary data at ECCO-JCC online]. With respect to maintenance outcomes, only steady-state trough SGC was statistically significant for the endpoints of clinical response through Week 54, clinical remission at both Week 30 and Week 54, and mucosal healing at both Week 30 and Week 54 in the multivariable model. A lower endoscopic score at the start of induction also was associated with a greater likelihood of clinical remission and mucosal healing during maintenance [Table S4].
To assess if SGCs measured at earlier time points were predictive of future outcomes, the associations between SGC at Week 2 and at Week 4, and efficacy outcomes at Week 6, were evaluated. Both Week 2 and Week 4 SGCs were predictive of clinical response and mucosal healing, but only Week 4 SGC was predictive of clinical remission at Week 6 [Table S5, available as Supplementary data at ECCO-JCC online].
With respect to maintenance golimumab treatment, similar analyses showed there was no trend between SGC at Week 6 and efficacy outcomes at Week 30 and/or Week 54. Higher steady-state trough concentrations obtained earlier in maintenance treatment [e.g. at Week 28], however, were indicative of future efficacy outcomes [Table S6, available as Supplementary data at ECCO-JCC online].
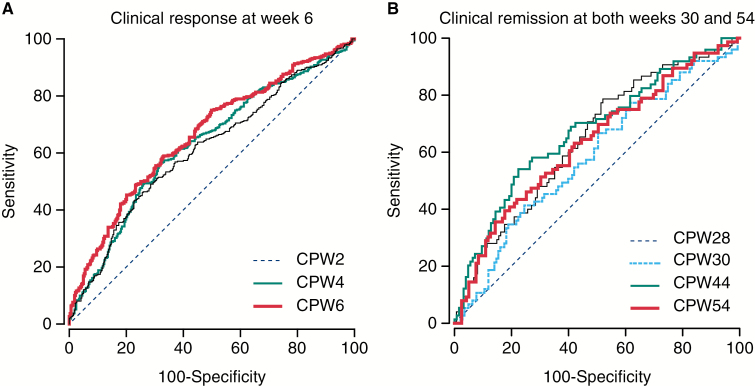
To identify optimal SGC thresholds associated with clinical improvement in UC patients, ROC curves were generated for efficacy endpoints during both the induction and maintenance treatment periods. The ROC curves for the endpoints of clinical response at induction Week 6 and clinical remission at maintenance Weeks 30 and 54 are shown in Figure 4A and B, respectively.
Figure 4.
Receiver-operating-characteristic curve analysis of optimal SGC thresholds associated with clinical response at Week 6 [A] and clinical remission at Weeks 30 and 54 [B] of the PURSUIT studies. CPW2/4/6/28/30/44/54, concentration at Week 2/4/6/28/30/44/54; PURSUIT, Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment; SGC, serum golimumab concentration.
Optimal SGC thresholds and ROC estimates are presented for key efficacy endpoints in Table 5. Serum golimumab concentrations of 2.5 µg/ml at Week 6 during induction and 1.4 µg/ml at Week 44 [steady-state trough] during maintenance are estimated to be desirable concentration targets for attainment of optimal clinical outcomes.
Table 5.
Optimal SGC thresholds and ROC curve analysis estimates for key efficacy endpoints in PURSUIT.
| Efficacy end point | SGC time point | ROC analysis metric | Estimate |
|---|---|---|---|
| Clinical responsea during | Week 2 | Threshold, µg/ml | 8.9 |
| induction at Week 6 | Sensitivity | 49.1 | |
| Specificity | 71.5 | ||
| PPV | 65.4 | ||
| NPV | 56.1 | ||
| Week 4 | Threshold, µg/ml | 7.4 | |
| Sensitivity | 56.6 | ||
| Specificity | 68.3 | ||
| PPV | 58.0 | ||
| NPV | 67.1 | ||
| Week 6 | Threshold, µg/ml | 2.5 | |
| Sensitivity | 59.0 | ||
| Specificity | 67.2 | ||
| PPV | 67.7 | ||
| NPV | 58.4 | ||
| Clinical remissionb at both | Week 28 | Threshold, µg/ml | 0.9 |
| Week 30 and Week 54 of | during maintenance | Sensitivity | 78.7 |
| maintenance | [steady-state trough] | Specificity | 48.0 |
| PPV | 43.4 | ||
| NPV | 81.6 | ||
| Week 30 | Threshold, µg/ml | 3.7 | |
| during maintenance | Sensitivity | 41.3 | |
| Specificity | 75.5 | ||
| PPV | 47.0 | ||
| NPV | 71.1 | ||
| Week 44 | Threshold, µg/ml | 1.4 | |
| during maintenance | Sensitivity | 54.1 | |
| [steady-state trough] | Specificity | 77.6 | |
| PPV | 58.8 | ||
| NPV | 74.0 | ||
| Week 54 | Threshold, µg/ml | 3.7 | |
| during maintenance | Sensitivity | 39.5 | |
| Specificity | 82.4 | ||
| PPV | 58.8 | ||
| NPV | 68.1 |
aClinical response was defined as a decrease from baseline in the Mayo score ≥ 30% and ≥ 3 points, accompanied by either a rectal bleeding subscore of 0 or 1 or a decrease from baseline in the rectal bleeding subscore ≥ 1.
bClinical remission was defined as a Mayo score ≤ 2 points, with no individual subscore > 1.
NPV, negative predictive value; PPV, positive predictive value; PURSUIT, Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment; ROC, receiver operating characteristic; SGCs, serum golimumab concentrations.
4. Discussion
In these analyses of the PURSUIT-SC induction and maintenance data, the PK of golimumab in patients with UC and the association between efficacy outcomes and SGCs were evaluated. Additionally, we aimed to identify SGC thresholds for use in guiding therapeutic decisions when managing patients with UC receiving golimumab treatment. As such, to our knowledge, this is the most comprehensive PK and ER evaluation of golimumab in UC. Understanding golimumab PK characteristics, the relationship between SGC and efficacy outcomes, as well as factors influencing this relationship, is important in the efforts to optimise the efficacy of golimumab therapy. Our analyses show that golimumab exhibits approximately dose-proportional PK behaviour at doses ranging from 50mg to 400mg. These analyses further show strong positive associations between SGCs and several efficacy outcomes during both induction and maintenance golimumab treatment. We also identify golimumab concentration thresholds that may be taken into account as part of an overall evaluation of a patient who is receiving golimumab for the treatment of UC.
Dose proportionality within a therapeutic dose range is a desirable PK characteristic for a drug because it facilitates prediction of the effect of dose adjustment on the systemic drug exposure. In the case of golimumab, this characteristic is particularly useful in that SGCs would be readily predictable following treatment with golimumab [within the aforementioned dose range] in studies involving dose escalation or decrease. The present analysis also identified several patient factors associated with golimumab exposure, namely body weight, antibody-to-golimumab status, serum albumin, alkaline phosphatase, faecal markers, CRP, and pancolitis. In particular, the relationships between some of these factors and SGCs suggest that a higher inflammatory burden may lead to faster elimination of golimumab. These findings are largely consistent with those observed with infliximab in another report.13 The presence of an effect of concomitant immunomodulators on SGCs at the lower maintenance golimumab dose level [50mg every 4 weeks], but not at the higher golimumab dose level [100mg every 4 weeks], indicates that the relationship between immunomodulator use and the PK of an anti-TNF may be dose dependent. This may explain why reports on these relationships with other anti-TNF agents have led to conflicting conclusions. For example in the SONIC trial, patients receiving immunomodulators demonstrated higher infliximab concentrations compared with those who were not receiving immunomodulators.14 Conversely, other reports have shown minimal-to-modest impact of concomitant immunomodulators on infliximab concentrations.15,16 Of note in SONIC, a 5-mg/kg infliximab dose regimen was used, whereas in the ACT and ACCENT I studies, data from patients receiving dose regimens higher than 5mg/kg also were included in the PK analyses.14–16 An inverse association was observed between alkaline phosphatase and serum golimumab levels. Although the reason for this finding is unknown, the correlation is consistent with those observed for CRP, an established marker of inflammation in patients with IBD.
With respect to ER, a positive relationship between SGCs and efficacy outcomes was identified at several time points during both induction and maintenance. There is a growing body of evidence demonstrating that serum concentrations of anti-TNF agents are associated with efficacy outcomes in IBD. As a result, therapeutic drug monitoring of anti-TNF agents is increasingly coming into focus as a desirable strategy in the management of patients with IBD. Although a majority of the reports on the relationship between drug concentration and efficacy have focused on infliximab,1,3,17,18 such associations have also been seen with adalimumab4,5,19 and certolizumab.20 However, these relationships have not been shown to be causal.
Golimumab was first approved for the treatment of UC in adult patients in 2013, and there is considerable clinical interest in determining the value of SGCs in the management of patients with UC. In a recently published, small [n = 21] observational study of patients with moderate-to-severe UC being treated with golimumab in a clinical setting, median SGCs were significantly higher in partial clinical responders than in nonresponders at Week 2 [10.0 vs 7.4 µg/ml, p = 0.035] and Week 6 [5.1 vs 2.1 µg/ml, p = 0.037].8 Although the determination of optimal concentration cut-offs that balance trade-offs between sensitivity and specificity can be challenging for biologics in IBD, 3,17 it is interesting that despite using different PK assays, the authors of this observational study reported a cut-off of 2.6 µg/ml at Week 6 for the association with partial clinical response at Week 14, which is similar to the proposed Week 6 concentration [2.5 µg/ml] threshold for induction in the current analysis. Our analysis, which is based on a much larger dataset, goes further by reporting for the first time several SGC thresholds at different time points during both induction and maintenance therapy, making it the first report identifying SGC thresholds that may be useful in therapeutic drug monitoring of golimumab in the treatment of UC.
In our analyses, we note that patients in the lowest SGC quartiles tended to have the worst efficacy outcomes. As expected, patients in this category were more likely to have factors known to contribute to higher clearance of golimumab, including a higher incidence of immunogenicity, higher body weight, higher inflammatory burden [e.g. higher concentrations of CRP and faecal markers], and low albumin. Given that some patients in the lowest SGC quartiles had poor efficacy outcomes despite higher golimumab doses, these data may indicate a subgroup of patients with UC who may be less responsive to golimumab at the dose regimens evaluated in the PURSUIT study, and potentially require higher golimumab doses or a longer treatment period than those evaluated in the study. These findings are largely consistent with the data from a similar analysis reported for infliximab in the pivotal ACT 1 and ACT 2 studies.3 Also consistent with the infliximab data is the prognostic value of earlier SGCs in predicting later efficacy outcomes. As early as Week 2, SGCs correlated with induction efficacy outcomes at Week 6. Similarly, SGCs measured at Week 28 [steady state] correlated with efficacy outcomes at Week 30 and Week 54. Thus, knowledge of SGCs earlier in the UC treatment interval could provide some insight into the eventual treatment outcome with golimumab. In line with this, SGCs were observed to be lower in patients who needed a dose adjustment than in those who did not require a dose adjustment in the PURSUIT-M study [data on file]. Finally, higher SGCs were observed in patients achieving more stringent efficacy outcomes [e.g. remission], suggesting that the extent of clinical benefit may depend on drug levels.
This study had some limitations. First, the assay used for the analysis of SGCs is not commercially available, leading to some uncertainty about the applicability of the identified thresholds to practice settings if different assays are used. Interestingly, strong correlations were observed when infliximab concentrations were analysed using several different assay formats, suggesting that drug levels of serum anti-TNFs obtained with various assays may be largely comparable.21 Nevertheless, it may be important to cross-validate PK assays used to measure SGCs in various commercial and academic group settings if these thresholds are to find global utility in clinical practice.
Second, whereas the influence of anti-drug antibodies on SGCs in these analyses is unambiguous, the impact on efficacy was less conclusive. In particular, multivariable logistic regression analysis did not identify the presence of anti-drug antibodies as an independent factor for the ER relationship during induction or maintenance, suggesting that the primary impact of these antibodies is on the SGCs. However, it should be noted that for these analyses, the presence of anti-drug antibodies was not detectable in the presence of golimumab. As such, the results on the impact of anti-golimumab antibodies may need to be re-evaluated if a drug-tolerant assay is employed to analyse these data.
Another limitation of these analyses is that the proposed SGC thresholds have not been prospectively validated. Of note, mixed results have been reported in studies that examined infliximab-trough-level-guided therapy.22–25 Thus, it is important to recognise that although these analyses establish a robust association between higher SGCs and an increased likelihood of achieving or maintaining efficacy outcomes in UC, they do not resolve the question about whether patients identified with low concentrations can attain or regain efficacy if their golimumab exposure were to be increased. Addressing this important question requires a prospective trial where SGCs are measured and patients with low drug levels undergo dose titration with the goal of attaining or exceeding the identified thresholds. Another approach to confirm the utility of these thresholds would be to compare patients maintained at or above these thresholds with those who are treated without regard to their SGCs, to evaluate which approach is more likely to result in better patient outcomes. For any of these therapeutic drug monitoring approaches to be applied to golimumab, optimal golimumab target levels need to be identified. The current analyses provide such thresholds during induction and maintenance using data from large randomised clinical trials. Consequently, the identified SGC targets can provide a reliable starting point for future study designs to prospectively evaluate the utility of therapeutic drug monitoring, and to confirm the usefulness of these SGC thresholds in the management of patients with UC.
In conclusion, a positive association between SGCs and efficacy outcomes in patients with UC was confirmed during both induction and maintenance portions of the PURSUIT studies. Factors related to the distribution of SGCs, as well as optimal concentration thresholds for efficacy outcomes in UC, were identified. These data provide a basis for further research aimed towards individualised therapy or therapeutic drug monitoring of golimumab in patients with UC.
Funding
The work was supported by Janssen Research & Development, LLC [Spring House, PA]. This study was designed and conducted by the PURSUIT-SC Steering Committee and Janssen Research & Development, LLC who jointly analysed and interpreted the data and contributed to the manuscript. OJA prepared the first draft of the manuscript.
Conflict of Interest
OJA, ZX, CWM,, RS, HZ, JJ, HZ, and HMD are employees of Janssen Research & Development, LLC. WR has served: as a speaker for Abbott Laboratories, AbbVie, Aesca, Aptalis, Celltrion, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Immundiagnostik, Janssen, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, Schering-Plough, Shire, Takeda, Therakos, Vifor, and Yakult; as a consultant for Abbott Laboratories, AbbVie, Aesca, A mgen, AM Pharma, Astellas, Astra Zeneca, Avaxia, Bioclinica, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Cellerix, Celltrion, Chemocentryx, Covance, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Galapagos, Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestle, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Robarts Clinical Trial, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Vifor, Zyngenia, and 4SC; as an advisory board member for Abbott Laboratories, AbbVie, Aesca, A mgen, AM Pharma, Astellas, Astra Zeneca, Avaxia, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Celltrion, Danone Austria, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestle, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Zyngenia, and 4SC; and has received research funding from Abbott Laboratories, AbbVie, Aesca, Falk Pharma GmbH, Immundiagnostik, Janssen, and MSD. BGF has received consulting fees from AbbVie, Actogenix, Akros, Albireo Pharma, A mgen, Astra Zeneca, Avaxia Biologics Inc., Avir Pharma, Axcan, Baxter Healthcare Corp., Biogen Idec, Boehringer-Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, GiCare Pharma, Gilead, Given Imaging Inc., GSK, Ironwood Pharma, Janssen, Kyowa Kakko Kirin Co Ltd, Lexicon, Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nektar, Nestles, Novonordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Protagonist, Receptos, Salix Pharma, Serono, Shire, Sigmoid Pharma, Synergy Pharma Inc., Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, VHsquared Ltd, Warner-Chilcott, Wyeth, Zealand, Zyngenia; research grants from AbbVie, A mgen, Astra Zeneca, Bristol-Myers Squibb, Janssen, Roche/Genentech, Millennium, Pfizer, Receptos, Santarus, Sanofi, Tillotts, and UCB Pharma; and speaking fees from AbbVie, Janssen, Takeda, Warner-Chilcott, UCB Pharma. Dr Feagan has served as an advisory board member for AbbVie, A mgen, Astra Zeneca, Avaxia Biologics Inc., Bristol-Myers Squibb, Celgene, Elan/Biogen, Ferring, Janssen, Merck, Nestles, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Takeda, Teva, TiGenix, Tillotts Pharma AG, and UCB Pharma and is a board member of Robarts Clinical Trials, Inc. PR has received research funding, and/or served as speaker, consultant, and/or advisory board member for AbbVie, Janssen Research & Development, LLC, Merck Research Laboratories, Merck Serono, UCB Pharma, Millenium/Takeda, Genentech/Hoffman LaRoche, Bristol Myers Squibb, Robarts, Tillotts Pharma, and Medimmune/AstraZeneca/A mgen. WJS has received consulting fees from Abbott, ActoGeniX NV, AGI Therapeutics Inc, Alba Therapeutics Corp, Albireo, Alfa Wasserman, A mgen, AM-Pharma BV, Anaphore, Astellas, Athersys Inc, Atlantic Healthcare Ltd, Aptalis, BioBalance Corp, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Celek Pharmaceuticals, Cellerix SL, Cerimon Pharmaceuticals, ChemoCentryx, CoMentis, Cosmo Technologies, Coronado Biosciences, Cytokine Pharmasciences, Eagle Pharmaceuticals, EnGene Inc, Eli Lilly, Enteromedics, Exagen Diagnostics Inc, Ferring Pharmaceuticals, Flexion Therapeutics Inc, Funxional Therapeutics Ltd, Genzyme Corp, Gilead Sciences, Given Imaging, GlaxoSmithKline, Human Genome Sciences, Ironwood Pharmaceuticals, Janssen Research & Development, LLC; KaloBios Pharmaceuticals, Lexicon Pharmaceuticals, Lycera Corp, Meda Pharmaceuticals, Merck Research Laboratories, Merck Serono, Millenium Pharmaceuticals, Nisshin Kyorin Pharmaceuticals, Novo Nordisk, NPS Pharmaceuticals, Optimer Pharmaceuticals, Orexigen Therapeutics Inc, PDL Biopharma, Pfizer, Procter and Gamble, Prometheus Laboratories, ProtAb Ltd, Purgenesis Technologies Inc, Relypsa Inc, Roche, Salient Pharmaceuticals, Salix Pharmaceuticals, Santarus, Schering Plough, Shire Pharmaceuticals, Sigmoid Pharma Ltd, Sirtris Pharmaceuticals, SLA Pharma UK Ltd, Targacept, Teva Pharmaceuticals, Therakos, Tilliotts Pharma AG, TxCell SA, UCB Pharma, Viamet Pharmaceuticals, Vascular Biogenics Ltd, Warner Chilcott UK Ltd and Wyeth; research grants from Abbott, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Janssen Research & Development, LLC, Millennium Pharmaceuticals, Novartis, Pfizer, Procter and Gamble, Shire Pharmaceuticals and UCB Pharma; payments for lectures/speakers bureau from Abbott, Bristol-Myers Squibb, and Janssen Research & Development, LLC; and holds stock/stock options in Enteromedics.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
Supplementary Material
Acknowledgments
The authors thank Professor Jean-Frederic Colombel for his contribution to the PURSUIT clinical trial programme as a steering committee member and Michelle Perate MS [Janssen Scientific Affairs, LLC] for writing and submission support.
References
- 1. Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010;59:49–54. [DOI] [PubMed] [Google Scholar]
- 2. Murthy S, Kevans D, Seow CH, et al. Association of serum infliximab and antibodies to infliximab to long-term clinical outcome in acute ulcerative colitis. Gastroenterology 2012;142 [Suppl 1]:S-388. [Google Scholar]
- 3. Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014;147:1296–307. [DOI] [PubMed] [Google Scholar]
- 4. Roblin X, Marotte H, Rinaudo M, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014;12:80–4. [DOI] [PubMed] [Google Scholar]
- 5. Paul S, Moreau AC, Del Tedesco E, et al. Pharmacokinetics of adalimumab in inflammatory bowel diseases: a systematic review and meta-analysis. Inflamm Bowel Dis 2014;20:1288–95. [DOI] [PubMed] [Google Scholar]
- 6. Sandborn WJ, Feagan BG, Marano C, et al. ; for the PURSUIT-SC Study Group. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:85–95. [DOI] [PubMed] [Google Scholar]
- 7. Sandborn WJ, Feagan BG, Marano C, et al. ; for the PURSUIT-Maintenance Study Group. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 96–109. [DOI] [PubMed] [Google Scholar]
- 8. Detrez I, Dreesen E, VanStappen T, et al. Variability in golimumab exposure: a ‘real-life’ observational study in active ulcerative colitis. J Crohns Colitis 2016;10:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhuang Y, Xu Z, Frederick B, et al. Golimumab pharmacokinetics after repeated subcutaneous and intravenous administrations in patients with rheumatoid arthritis and the effect of concomitant methotrexate: an open label, randomised study. Clin Ther 2012;34:77–90. [DOI] [PubMed] [Google Scholar]
- 10. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. a randomised study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- 11. D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007;132:763–86. [DOI] [PubMed] [Google Scholar]
- 12. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. [DOI] [PubMed] [Google Scholar]
- 13. Ternant D, Ducourau E, Perdriger A, et al. Relationship between inflammation and infliximab pharmacokinetics in rheumatoid arthritis. Br J Clin Pharmacol 2014;78:118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu Z, Davis HM, Zhou H. Clinical impact of concomitant immunomodulators on biologic therapy: Pharmacokinetics, immunogenicity, efficacy and safety. J Clin Pharmacol 2015;55 [Suppl 3]:S60–74. [DOI] [PubMed] [Google Scholar]
- 15. Fasanmade AA, Adedokun OJ, Blank M, et al. Pharmacokinetic properties of infliximab in children and adults with Crohn’s disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther 2011;33:946–64. [DOI] [PubMed] [Google Scholar]
- 16. Fasanmade AA, Adedokun OJ, Ford J, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol 2009;65:1211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut 2014;63:1721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol 2006;4:1248–54. [DOI] [PubMed] [Google Scholar]
- 19. Chiu YL, Rubin DT, Vermeire S, et al. Serum adalimumab concentration and clinical remission in patients with Crohn’s disease. Inflamm Bowel Dis 2013;19:1112–22. [DOI] [PubMed] [Google Scholar]
- 20. Colombel JF, Sandborn WJ, Allez M, et al. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:423–31. [DOI] [PubMed] [Google Scholar]
- 21. Marini J, Sendecki J, Cornillie F, et al. Comparison of commercially available assays for infliximab concentrations and antibodies to infliximab with assays developed at Janssen and used in clinical studies of Remicade® [infliximab] in IBD patients. United Eur Gastroenterol J 2015;3[5S]:A421. [Google Scholar]
- 22. Steenholdt C, Brynskov J, Thomsen OO, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27. [DOI] [PubMed] [Google Scholar]
- 23. vande Casteele N, Gils A, Ballet V, et al. Randomised controlled trial of drug level versus clinically based dosing of infliximab maintenance therapy in IBD: final results of the TAXIT study. United Eur Gastroenterol J 2013;1[1S]:A1–A134. [Google Scholar]
- 24. Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis 2014;20:1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paul S, Del Tedesco E, Marotte H, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Gastroenterology 2013;144:S-92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.