Abstract
BACKGROUND:
Growing evidence supports the notion that the onset of tumorigenesis could occur through cancer stem cells (CSCs). These tumour cells show low proliferative rates, high self-renewal capacity, propensity to differentiate into active proliferating tumour cells & resistance to chemoradiotherapy thus, possibly causing local recurrences & metastasis formation. CD44 has been used as a marker to isolate CSCs from colorectal carcinoma (CRC).
AIM:
To investigate the immunohistochemical expression of cancer stem cells marker (CD44) in CRC and correlate its expression with the clinicopathological aspects, TNM staging and modified Dukes’ classification.
MATERIALS AND METHODS:
Tumour biopsies from colectomy specimens of 60 patients with CRC were stained with hematoxylin-eosin for histological evaluation then immunostained with monoclonal antibodies against CD44 which was detected in term of negative or positive expression.
RESULTS:
CD44 was demonstrated in 58.3% (35/60) of cases and showed statistically significant correlation with tumour site and histological type (p-value < 0.05). However, CD44 showed statistically insignificant inverse correlation with tumour invasiveness (T), lymph node status (N), grade, TNM stage grouping and modified Dukes’ classification, while it was directly correlated with distant metastasis (M) (p-value > 0.05). Chi-square /Fisher exact test proportion independence and the p-value are set significant at 0.05 level.
CONCLUSION:
the CD44 rate of expression is higher in the colon than rectum and in adenocarcinoma than mucinous and undifferentiated carcinoma. CD44 showed statistically insignificant relation with T, N, M, grade, TNM stage grouping and modified Dukes’ classification.
Keywords: Cancer stem cells, CD44, Colorectal carcinoma, Metastasis
Introduction
Colorectal cancer (CRC) is the third most common type of cancer [1]. Although the median overall survival of patients with metastatic colorectal cancer has increased from 12 months to approximately 24 months over the past decade as a result of an improvement in systemic therapies including new chemotherapeutic agents, the 5-year survival is still pessimistic [2].
A growing body of evidence supports the notion that only a small subset of cells within a solid tumour has ‘stem-like’ characteristics. These tumor-initiating cells, or cancer stem cells (CSCs), distinct from non-malignant stem cells, show low proliferative rates, high self-renewal capacity, propensity to differentiate into active proliferating tumour cells, and resistance to chemotherapy or radiation [3]. Notably, owing to their high expression of DNA repair mechanisms, detoxifying enzymes, such as aldehyde dehydrogenase-1 (ALDH1), and molecular pumps, CSCs might survive radiochemotherapy; thus, possibly causing local recurrences and metastasis formation despite treatment [4].
Despite the potentially high clinical relevance of CSCs, little is known about the prognostic value of the expression of putative CSC markers in colorectal cancers. Contradictory findings have been reported about the association between the expression of CD44 and tumour progression [5].
Material and Methods
A total of 60 stored, formalin fixed, paraffin embedded tumour biopsies from colectomy specimens of patients with colorectal cancer were collected from Kasr El Aini Hospital and multiple private laboratories with the permission of the head of these labs, the specimens were anonymous for confidentiality and replaced by numbers.
The site of the tumour was classified into the right colon (cecum, ascending colon, hepatic flexure and transverse colon), left colon (splenic flexure, descending colon and sigmoid) and rectum, while the size of the tumour was calculated as the length of the largest diameter. Size and site, as well as age and sex, were obtained from the pathology reports of the patients. Undifferentiated carcinoma cases were documented immunohistochemically from where the cases were recruited.
The tumour extension into other organs, distant metastasis if present and the lymph node status were also obtained from the diagnosis present in the pathology reports (clinical data of distant metastasis in other organs were also obtained from the patient’s sheet).
The paraffin blocks of the tumour were serially sectioned at 4 μm thickness. Afterwards, they were stained with routine hematoxylin-eosin stain for pathological examination and morphologic classification of the colorectal cancer according to the recommendations of the World Health Organization [6] including histological types, subtypes, tumor grade, depth of tumor invasion, perineural invasion and lymphovascular emboli while staging was performed using modified Dukes’ classification of the disease [7], and TNM staging system [8] for each case.
Paraffin section from each case was processed for immunostaining using CD44 Std. / HCAM AB-4 (0.7 ml. of antibody prediluted 0.05 mol/L Tris-HCl, pH 7.6 containing stabilising protein and 0.015 mol/L sodium azide – Thermo Fisher Scientific. UK) and Econo Tek HRP Anti-Polyvalent (DAP) ready-to-use (Scy Tek Laboratories inc. USA) detection system.
CD44 stained sections were examined at high power for immunohistochemical expression and were divided into negative (no immunoreactivity in any cells) and positive (membrane and/or cytoplasm immunoreactivity present) [9].
The antibody labels approximately 90% of all lymphocytes, both T cells and B cells [10], were positively stained lymphocytes were used as an internal positive control.
In colorectal cancer, metastasis was almost exclusively a property of the CSCs that exhibited long-term self-renewing capacity [11]. So we used to divide the histological types, tumour grade and invasiveness (T) into metastatic and non-metastatic groups to correlate them with the rate of CD44 expression.
Statistical methods
SPSS version 18.0 was used for data management. Mean and standard deviation described quantitative data and non-parametric t-test compared two independent groups and non-parametric ANOVA compared more than two groups. Chi-square /Fisher exact test proportion independence and the p-value are set significant at 0.05 level.
Results
The characteristics of the study population are shown in Table 1. The subjects consisted of 29 males (48.3%) and 31 females (51.7%), with a mean age of 54.86 years (median, 56 years; range, 25–75 years) and a mean tumour size of 6.25 cm (median, 6 cm; range, 2–16 cm). Most of the cases were adenocarcinoma (75%) followed by mucinous carcinoma (21.7%) and undifferentiated carcinoma (3.3%). The tumour was distributed regarding the site; (75%) in the colon while (25%) of the cases were found in the rectum. The TNM staging is applied according to the American Joint Committee on Cancer (AJCC) and the International Union for Cancer Control (UICC) where it encodes the extent of the primary tumor (T), regional lymph nodes (N), and distant metastases (M) 3, 17, 31, and 9 patients had stage I–IV cancers, respectively [8]. According to Modified Dukes’ classification [7] cases in this study are classified into 20 cases (33.3%) B, 31 cases (51.7%) stage C and 9 cases (15%) stage D with no cases of stage A.
Table 1.
Description of set of patients and CD44 rate of expression in the study
| No. (%) | CD44 expression | P-value | |||
|---|---|---|---|---|---|
| +ve no. (%) | -ve no. (%) | ||||
| Patients | 60 | 25 (41.7%) | 35 (58.3%) | ||
| Gender: | |||||
| Male | 29 (48.3%) | 16 (55.2%) | 13 (4.48%) | 0.63 | |
| Female | 31 (51.7%) | 19 (61.3%) | 12 (38.7%) | ||
| Age: | |||||
| Range | 25-75 yrs | 0.07 | |||
| Mean | 54.867 | 57.086 | 51.760 | ||
| Std. deviation | 11.0415 | 8.8695 | 13.0776 | ||
| Tumour site: | |||||
| Colon | 45 (75%) | 30 (66.7%) | 15 (33.3%) | 0.02* | |
| Rectum | 15 (25%) | 5 (33.3%) | 10 (66.7%) | ||
| Tumour size: | |||||
| Range | 2-16 cm. | 0.29 | |||
| Mean | 6.250 | 5.929 | 6.700 | ||
| Std. deviation | 2.5669 | 2.1287 | 3.0687 | ||
| Histological type: | |||||
| Adenocarcinoma | 45 (75%) | 31 (68.9%) | 14 (31.1%) | 0.01* | |
| Mucinous | 13 (21.7%) | 4 (30.8%) | 9 (69.2%) | ||
| Undifferentiated | 2 (3.3%) | 0 (0%) | 2 (100%) | ||
| Adenocarcinoma: ** | 45 (75%) | 31 (68.9%) | 14 (31.1%) | 1.0 | |
| Metastatic | 8 | 6 (75%) | 2 (25%) | ||
| Non metastatic | 37 | 25 (67.6%) | 12 (32.4%) | ||
| Mucinous: ** | 13 (21.7%) | 4 (30.8%) | 9 (69.2%) | 0.38 | |
| Metastatic | 1 | 1 (100%) | 0 (0%) | ||
| Non metastatic | 12 | 3 (25%) | 9 (75%) | ||
| Tumor grade: ** | |||||
| Grade 1 | 0 (0%) | 0 (0%) | 0 (0%) | 0.10 | |
| Grade 2 | 53 (83.3%) | 33 (62.3%) | 20 (37.7%) | ||
| Grade 3 | 7 (11.7%) | 2 (28.6%) | 5 (71.4%) | ||
| Grade 2: ** | 53 (83.3%) | 33 (62.3%) | 20 (37.7%) | 0.7 | |
| Metastatic | 8 | 6 (75%) | 2 (25%) | ||
| Non metastatic | 45 | 27 (60%) | 18 (40%) | ||
| Grade 3: ** | 7 (11.7%) | 2 (28.6%) | 5 (71.4%) | 0.29 | |
| Metastatic | 1 | 1 (100%) | 0 (0%) | ||
| Non metastatic | 6 | 1 (16.7%) | 5 (83.3%) | ||
| Tumor extent (T): | |||||
| T1 | T1+T2 | 1 (1.7%) | 7 (77.8%) | 2 (22.2%) | 0.52 |
| T2 | 8 (13.3%) | ||||
| T3 | T3 | 45 (75%) | 25 (55.6%) | 20 (44.4%) | |
| T4a | T4a+T4b | 3 (5%) | 3 (50%) | 3 (50%) | |
| T4b | 3 (5%) | ||||
| T1+T2: ** | 9 (15%) | 7 (77.8%) | 2 (22.2%) | 1.0 | |
| Metastatic | 1 | 1 (100%) | 0 (0%) | ||
| Non metastatic | 8 | 6 (75%) | 2 (25%) | ||
| T3: ** | 45 (75%) | 25 (55.6%) | 20 (44.4%) | 0.36 | |
| Metastatic | 5 | 4 (80%) | 1 (20%) | ||
| Non metastatic | 40 | 21 (52.5%) | 19 (47.5%) | ||
| T4a+T4b: * | 6 (10%) | 3 (50%) | 3 (50%) | 1.0 | |
| Metastatic | 3 | 2 (66.7%) | 1 (33.3%) | ||
| Non metastatic | 3 | 1 (33.3%) | 2 (66.7%) | ||
| Lymph node status (N): | |||||
| N0 | N0 | 22 (36.7%) | 15 (68.2%) | 7 (31.8%) | 0.55 |
| N1a | N1a+N1b | 15 (25%) | 12 (60%) | 8 (40%) | |
| N1b | 5 (8.3%) | ||||
| N2a | N2a+N2b | 11 (18.3 %) | 8 (44.4%) | 10 (55.6%) | |
| N2b | 7 (11.7%) | ||||
| Distant metastasis (M): | |||||
| M0 | 51 (85%) | 29 (56.9%) | 22 (43.1%) | 0.72 | |
| M1a | 4 (6.7%) | 6 (75%) | 3 (25%) | ||
| M1b | 5 (8.3%) | ||||
| Stage grouping: | |||||
| I | 3 (5%) | 2 (66.7%) | 1 (33.3%) | 0.78 | |
| II | 17 (28.4%) | 11 (64.7%) | 6 (35.3%) | ||
| III | 31 (51.6%) | 16 (51.6%) | 15 (48.4%) | ||
| IV | 9 (15%) | 6 (66.7%) | 3 (33.3%) | ||
| Modified Dukes’: | |||||
| Stage A | 0 (0%) | 0 (0%) | 0 (0%) | 0.55 | |
| Stage B | 20 (33.3%) | 13 (65%) | 7 (35%) | ||
| Stage C | 31 (51.7%) | 16 (49.6%) | 15 (50.4%) | ||
| Stage D | 9 (15%) | 6 (66.7%) | 3 (33.3%) | ||
| Perineural invasion: | |||||
| Absent | 52 (86.7%) | ||||
| Present | 8 (13.3%) | ||||
| Lympho-vascular emboli: | |||||
| Absent | 58 (96.7%) | ||||
| Present | 2 (3.3%) | ||||
statistically significant p-value;
pathological parameters such as the grade, histological types and invasiveness (T) of the tumour were classified into metastatic and non-metastatic groups, the metastatic groups showed a higher rate of CD44 expression than non-metastatic ones.
Expression of CD44s in primary tumour was demonstrated in 58.3% (35/60). The relationships between tumour CD44s expression and the clinicopathological features of CRC are summarised in Table 1.
The characteristics of the study population are shown in Table 1. The subjects consisted of 29 males (48.3%) and 31 females (51.7%), with a mean age of 54.86 years (median, 56 years; range, 25–75 years) and a mean tumour size of 6.25 cm (median, 6 cm; range, 2–16 cm). Most of the cases were adenocarcinoma (75%) followed by mucinous carcinoma (21.7%) and undifferentiated carcinoma (3.3%). The tumour was distributed regarding the site; (75%) in the colon while (25%) of the cases were found in the rectum. The TNM staging is applied according to the American Joint Committee on Cancer (AJCC) and the International Union for Cancer Control (UICC) where it encodes the extent of the primary tumor (T), regional lymph nodes (N), and distant metastases (M) 3, 17, 31, and 9 patients had stage I–IV cancers, respectively [8].
According to Modified Dukes’ classification [7] cases in this study are classified into 20 cases (33.3%) B, 31 cases (51.7%) stage C and 9 cases (15%) stage D with no cases of stage A.
Expression of CD44s in primary tumour was demonstrated in 58.3% (35/60). The relationship between tumour CD44s expression and the clinicopathological features of CRC is summarised in Table 1.
Figure 1.
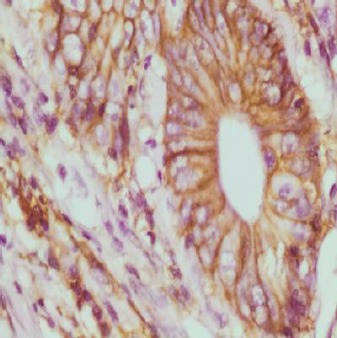
Adenocarcinoma GII with strong CD44 expression; membranous and focal cytoplasmic staining (x400)
CD44 showed a higher rate of expression in colon rather than rectal cases (p-value 0.02) and a higher rate of expression in adenocarcinoma cases than mucinous and undifferentiated carcinoma cases (p-value 0.01). The correlation was statistically significant by Chi-square /Fisher exact test proportion independence (p-value is set significant at 0.05 level).
Figure 2.
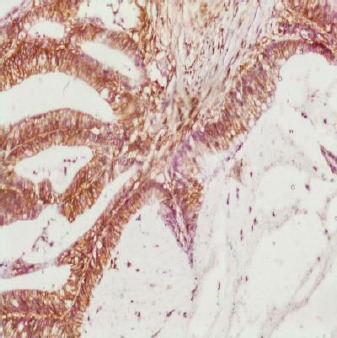
Mucinous carcinoma GII with strong CD44 expression and evident lymphocyte staining as internal control (x100)
The CD44 rate of expression showed statistically insignificant correlation with distant metastasis (M) and the metastatic group of all parameters including grade, histological types and invasiveness. Whereas it showed statistically insignificant inverse correlation with the grade, invasiveness (T), lymph node status (N), TNM stage grouping and modified Dukes’ staging (except for stage IV and stage D respectively which represent the metastatic stage) (p-value > 0.05).
Figure 3.
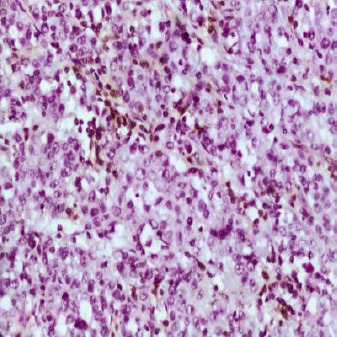
Undifferentiated carcinoma showing negative CD44 expression with focal staining of the lymphocytes which acts as internal control (x400)
No association was detected between CD44 expression and age, gender, tumour size, perineural invasion or lymphovascular invasion (p-value > 0.05).
Discussion
Cancer-related death from colorectal cancer is usually due to the development of distant metastasis. Approximately 70% of all patients diagnosed with CRC undergo potentially curative surgery, but half of those present with or develop advanced local disease or metastases [12]. Although several prognostic factors exist, including clinical staging classification, a more specific recognition marker for CRC with high metastatic potential would provide useful information for evaluating adjuvant therapies [13].
The concept of the contribution of colorectal cancer stem cells to tumour development is widely accepted, but the relation of individual CSC markers expression to disease prognosis is still not completely clear [14].
The relation between the site of the primary tumor and the rate of CD44 expression was statistically significant, where rectal tumors showed lower rate of CD44 expression (33.3%) than colonic tumors (66.7%) with slightly higher rate of expression in the right colon (70.8%) than in the left colon (61.9%) which is consistent with the results of [15] who found lower rate of CD44 expression in rectum (44.4%) than colon (64.4%). However, other studies showed higher rate of CD44 expression in rectum (48%) than colon (21.2%) [16], rectum (53.8%) and colon (30.8%) [13] and in rectum and left colon (53.8%) each then right colon (45.2%) (Al-Maghraby et al., 2012) [17].
The rate of CD44 expression varied according to histological type where there was statistically significant correlation between the rate of CD44 expression and the histological type. Adenocarcinoma cases showed the highest rate of CD44 expression (68.9%), followed by mucinous carcinoma (30.8%) then undifferentiated carcinoma which showed no CD44 expression. While other studies found a higher rate of CD44 expression in mucinous carcinoma (61.1%) than adenocarcinoma (49.5%) [18] and also higher in mucinous carcinoma (85.7%) than adenocarcinoma (55.9%) [15].
The role of CD44 as CSC marker itself is under debate, where Liu et al. (2014) [19] stated that CD44 is of functional importance for cancer initiation, progression, maintenance of the properties of CSC. While Pitule et al. (2014) [14] suggested that the use of CD44 as a single prognostic marker of CRC behaviour is impossible. Moreover Galizia et al. (2012) [20] results showed that evaluation of combined CD133/CD44 expression could be useful to identify putative colorectal CSCs than CD133 and CD44 individually which failed to identify colorectal CSCs.
The inconsistency and controversy of results from various studies correlating CD44 immunoexpression in relation to clinicopathological parameters may be related to technical factors, such as the type of antibody, differences in immunostaining method, type of tissue blocks, immunostaining scoring method and the cut-off for negative/positive or low/high [17].
We can also suggest another cause of controversy of results; which is the different functions of CD44 that may be presented in some tumours and absent in others. This suggestion is supported by Gunthert (1996) [21] who mentioned that during the malignant transformation process, and depending on the origin, the cell either gains or loses its ability to express some forms of this adhesion molecule. Thus, in human malignancies, it seems that the staining pattern for CD44 isoforms differs between the different tumour types, supporting the concept that the growth advantage for cancer cells contributed by CD44 depends on the cellular background.
A limitation of this study was the small scale of cases and unspecified selection of single parameter such as; studying CD44 expression in relation to recurrent cases, certain histological type, grade or stage (whether of invasiveness, lymph node status or metastasis) or even in relation to age, sex or site individually.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. https://doi.org/10.1002/ijc.25516 . PMid: 21351269. [DOI] [PubMed] [Google Scholar]
- 2.Chun-Yan Li, Bao-Xiu Li, Yi Liang, Rui-Qing Peng, Ya Ding, Da-Zhi Xu, Xin Zhang, Zhi-Zhong Pan, De-Sen Wan, Yi-Xin Zeng, Xiao-Feng Zhu, Xiao-Shi Zhang. A Higher percentage of CD133+cells is associated with poor prognosis in colon carcinoma patients with stage IIIB. Journal of Translational Medicine. 2009;7:56. doi: 10.1186/1479-5876-7-56. https://doi.org/10.1186/1479-5876-7-56 . PMid: 19583834. PMCid: PMC2715381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boman BM, Wicha MS. Cancer Stem Cells: A Step toward the Cure. American Society of Clinical Oncology. 2008;26(17):2795–2799. doi: 10.1200/JCO.2008.17.7436. https://doi.org/10.1200/JCO.2008.17.7436 . PMid: 18539956. [DOI] [PubMed] [Google Scholar]
- 4.Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumor-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–823. doi: 10.1038/nrd2137. https://doi.org/10.1038/nrd2137 . PMid: 19794444. [DOI] [PubMed] [Google Scholar]
- 5.Lugli A, Iezzi G, Hostettler I, Muraro MG, Mele V, Tornillo L, Carafa V, Spagnoli G, Terracciano L, Zlobec I. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer. 2010;103:382–390. doi: 10.1038/sj.bjc.6605762. https://doi.org/10.1038/sj.bjc.6605762 . PMid: 20606680. PMCid: PMC2920016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton SR, Bosman FT, Boffetta P. Carcinoma of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumors of the Digestive System. 4th edition. Lyon: IARC Press; 2010. pp. 132–146. [Google Scholar]
- 7.Dukes CE, Bussey HJR, Lamb GW. The examination and classification of operation specimens of intestinal cancer. Bull INT Assoc Med Mus. 1947;27:55–65. [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Handbook. 7th edition. New York: Springer; 2010. pp. 173–206. [Google Scholar]
- 9.Carr NJ, Emory TS, Sobin LH. Epithelial neoplasms of the appendix and colorectum. An analysis of cell proliferation, apoptosis, and expression of p53, CD44, and bcl-2. Arch Pathol Lab Med. 2002;126:837–41. doi: 10.5858/2002-126-0837-ENOTAA. PMid: 12088454. [DOI] [PubMed] [Google Scholar]
- 10.Stoll M, Dalchau R, Schmidt RE. N6 Cluster report: CD44. In: Knapp W, Dörken B, Gilks WR, Rieber EP, Schmidt RE, Stein H, et al., editors. Leukocyte typing IV. White cell differentiation antigens. Proceedings of the 4th International Workshop and Conference 1989 Feb 21-25. Vienna, Austria. Oxford, New York, Tokyo: Oxford University Press; 1989. pp. 619–22. [Google Scholar]
- 11.Dieter SM, Ball CR, Hoffmann CM, Nowrouzi A, Herbst F, Zavidij O, Abel U, Arens A, Weichert W, Brand K, et al. Distinct types of tumor-initiating cells from human colon cancer tumors and metastases. Cell Stem Cell. 2011;9:357–365. doi: 10.1016/j.stem.2011.08.010. https://doi.org/10.1016/j.stem.2011.08.010. PMid: 21982235. [DOI] [PubMed] [Google Scholar]
- 12.Huh JW, Kim HR, Kim YJ, Lee JH, Park YS, Cho SH, Joo JK. Expression of standard CD44 in human colorectal carcinoma: association with prognosis. Pathol Int. 2009;59:241–246. doi: 10.1111/j.1440-1827.2009.02357.x. https://doi.org/10.1111/j.1440-1827.2009.02357.x . PMid: 19351367. [DOI] [PubMed] [Google Scholar]
- 13.Kunimura T, Yoshida T, Sugiyama T, Morohoshi T. The relationships between loss of standard CD44 expression and lymph node, liver metastasis in T3 colorectal carcinoma. J Gastrointest Canc. 2009;40:115–118. doi: 10.1007/s12029-009-9100-0. https://doi.org/10.1007/s12029-009-9100-0. PMid: 19937401. [DOI] [PubMed] [Google Scholar]
- 14.Pitule P, Cedikova M, Daum O, Vojtisek J, Vycital O, Hosek P, Treska V, Hes O, Kralickova M, Liska V. Immunohistochemical Detection of Cancer Stem Cell Related Markers CD44 and CD133 in Metastatic Colorectal Cancer Patients. BioMed Research International 2014. 2014 doi: 10.1155/2014/432139. Article ID 432139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Jiang B, Wang Z, Liu M, Yang H, Xing J, Zhang C, Yao Z, Zhang N, Cui M, Su X. Combined preoperative CEA and CD44v6 improves prognostic value in patients with stage I and stage II colorectal cancer. Clin Transl Oncol. 2014;16(3):285–292. doi: 10.1007/s12094-013-1069-2. https://doi.org/10.1007/s12094-013-1069-2 . PMid: 23860725. [DOI] [PubMed] [Google Scholar]
- 16.Khoursheed M, Mathew TC, Makar PR, Sonia L, Abul H, Asfar S, AL-Sayer H, Dashti HM, AL-Bader A. Expression of CD44s in Human Colorectal Cancer. Pathology Oncology Research. 2002;8(3):170–174. doi: 10.1007/BF03032390. https://doi.org/10.1007/BF03032390. PMid: 12515996. [DOI] [PubMed] [Google Scholar]
- 17.AL-Magrabi J, G0maa W, Bumieda A, AL-Qahtani M, AL-Ahwal M. Decreased Immunoexpression of Standard Form of CD44 Is an Independent Favourable Predictor of Nodal Metastasis in Colorectal Carcinoma. Anticancer Research. 2012;32:3455–3462. PMid: 22843930. [PubMed] [Google Scholar]
- 18.Peng J, Cai S, Lu H, Cai G, Lian P, Guan Z, Wang M, Xu Y. Predicting prognosis of rectal cancer patients with total mesorectal excision using molecular markers. World J Gastroenterol. 2007;13(21):3009–3015. doi: 10.3748/wjg.v13.i21.3009. https://doi.org/10.3748/wjg.v13.i21.3009 . PMid: 17589956. PMCid: PMC4171158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D, Sun J, Zhu J, Zhou H, Zhang X, Zhang Y. Expression and clinical significance of colorectal cancer stem cell marker EpCAMhigh/CD44+in colorectal cancer. Oncology Letters. 2014;7:1544–1548. doi: 10.3892/ol.2014.1907. https://doi.org/10.3892/ol.2014.1907 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galizia G, Gemei M, Del Vecchio L, Zamboli A, Di Noto R, Mirabelli P, Salvatore F, Castellano P, Orditura M, De Vita F, Pinto M, Pignatelli C, Lieto E. Combined CD133/CD44 Expression as a Prognostic Indicator of Disease-Free Survival in Patients With Colorectal Cancer. Arch Surg. 2012;147(1):18–24. doi: 10.1001/archsurg.2011.795. https://doi.org/10.1001/archsurg.2011.795 . PMid: 22250106. [DOI] [PubMed] [Google Scholar]
- 21.Gunthert U. CD44 in malignant disorders. Curr Top Microbiol Immunol. 1996;213(1):271–285. https://doi.org/10.1007/978-3-642-61107-0_16 . PMid: 8814992. [PubMed] [Google Scholar]


