Abstract
Extracellular adherence protein (Eap) from Staphylococcus aureus inhibits the adherence of neutrophils to nonstimulated and tumor necrosis factor alpha-stimulated endothelial cells in both static adhesion assays and flow adhesion assays. Consequently, Eap also impaired their transendothelial migration. During an S. aureus infection, Eap may thus serve to reduce inflammation by inhibiting neutrophil adhesion and extravasation.
Staphylococcus aureus is one of the most common agents causing bacterial infections in humans and animals. The clinical manifestations of S. aureus infections range from mild wound infections to more life-threatening infections, such as endocarditis, osteomyelitis, and septic shock (14). S. aureus produces a wide range of extracellular matrix binding proteins, which are proposed to contribute to successful colonization and persistence at various sites in the host. Extracellular adherence protein (Eap) is an extracellular protein of S. aureus. Eap binds to many plasma proteins, including fibrinogen, fibronectin, and prothrombin (16). It has also been shown that Eap enhances the binding and internalization of S. aureus into eukaryotic cells (9, 12). In addition, Eap plays a role as an immunomodulating protein (1, 13). Chavakis et al. found a strong interaction of Eap and the cell adhesion molecule ICAM-1 (intercellular adhesion molecule 1) in vitro (1). No interaction could be seen between Eap and its ligands, Mac-1 and LFA-1 (lymphocyte function-associated antigen-1), expressed on leukocytes (1). During the infectious process, inflammatory stimuli activate vascular endothelial cells to express adhesion molecules and chemokines that physically engage circulating leukocytes. A coordinated sequence of adhesion and locomotion steps, including (i) leukocyte rolling, (ii) cell activation, (iii) firm cell adhesion, and (iv) transendothelial migration, requires that adhesion receptors on leukocytes and endothelial cells are up-regulated and activated (20). The binding of Eap to ICAM-1 suggests that Eap may inhibit the binding of leukocytes to endothelial cells and thereby inhibit the extravasation of leukocytes from the bloodstream into the site of infection (2). In this study, we show that Eap from S. aureus inhibits neutrophil binding to, and migration across, the endothelium in vitro. In addition, the inhibiting effect exerted by Eap was dose dependent and of the same magnitude as the blocking effect elicited by antibodies against ICAM-1.
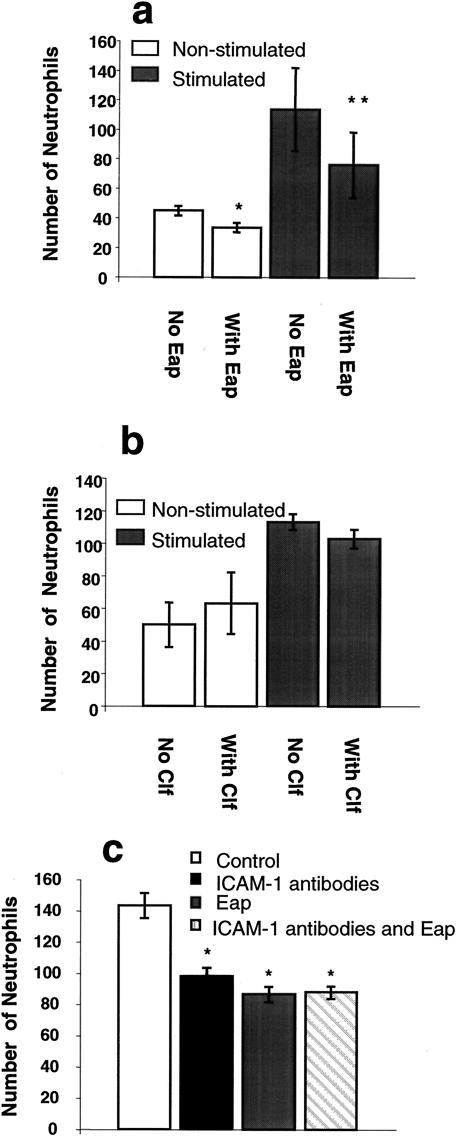
To determine the effects of Eap on the adhesion of neutrophils to nonstimulated or tumor necrosis factor alpha (TNF-α)-stimulated endothelial cells, static and flow adhesion assays were performed as described previously (5). Human aortic endothelial cells (HAECs; Clonetics, Walkersville, Md.) were cultured in EBM-2 medium supplemented according to the supplier (Clonetics). Human neutrophils were isolated as described previously (5) and used within 4 h of isolation. To assess whether Eap affects the static adhesion of neutrophils, a static adhesion assay was performed. Neutrophils were diluted to 5 × 105 cells/ml in AIM-V (GIBCO, BRL, Life Technologies, Paisley, Scotland), 2 ml of cell suspension was added to confluent monolayers of HAECs, and the cells were incubated for 5 min at 37°C. Prior to the assay, endothelial cells were incubated in medium alone (nonstimulated) or stimulated with recombinant TNF-α (20 ng/ml; R&D Systems, Abingdon, United Kingdom) for 6 h at 37°C. After 4 h of incubation, Eap (final concentration, 30 μg/ml) was added and allowed to interact with the cells for 2 h. To estimate the optimal inhibiting concentration of Eap, a dose-response analysis was performed using 0 to 60 μg of Eap/ml. The maximum effect was obtained with 30 μg/ml (data not shown). Eap was purified from S. aureus supernatants by using affinity chromatography, followed by ion-exchange chromatography, as described previously (16). Clumping factor (Clf), a fibrinogen-binding protein from S. aureus, was used as a control protein (final concentration, 20 μg/ml) (6, 15). Nonadherent neutrophils were carefully washed away, and the cells in 10 visual fields for each well were microscopically examined and counted. Regardless of the condition of the HAECs, Eap significantly (P < 0.05) inhibited binding of neutrophils to endothelial cells under static conditions (Fig. 1a). As expected, neutrophil adhesion to nonactivated HAECs was lower than that to HAECs activated with TNF-α. The reduction of neutrophil binding exerted by Eap was more pronounced on activated than on nonactivated endothelial cells (Fig. 1a). The presence of Clf did not significantly inhibit the binding of neutrophils to endothelial cells (Fig. 1b).
FIG. 1.
Static adhesion assay. Neutrophils (1 × 106 cells) were added to confluent monolayers of endothelium and allowed to adhere for 5 min at 37°C. (a and b) Endothelial cells were treated at 37°C with medium alone (white bars) or TNF-α (shaded bars) for 6 h prior to the assay. After 4 h, Eap (final concentration, 30 μg/ml) was added to some wells and further incubated for 2 h (a). Clf from S. aureus (final concentration, 20 μg/ml) was used as a control protein (b). In the antibody-blocking assay (c), all endothelial cells were treated at 37°C with TNF-α for 6 h prior to the assay. After 4 h, some wells were preincubated with ICAM-1 antibodies for 20 min. Eap (final concentration, 30 μg/ml) was added to some wells and further incubated for2 h. Cells in 10 visual fields were counted for each well. The data are presented as the mean ± the standard error of the mean (SEM) of results from five experiments (a), three experiments (b), or four experiments (c). Statistical significance was determined by Student's t test. *, P < 0.05; **, P < 0.01.
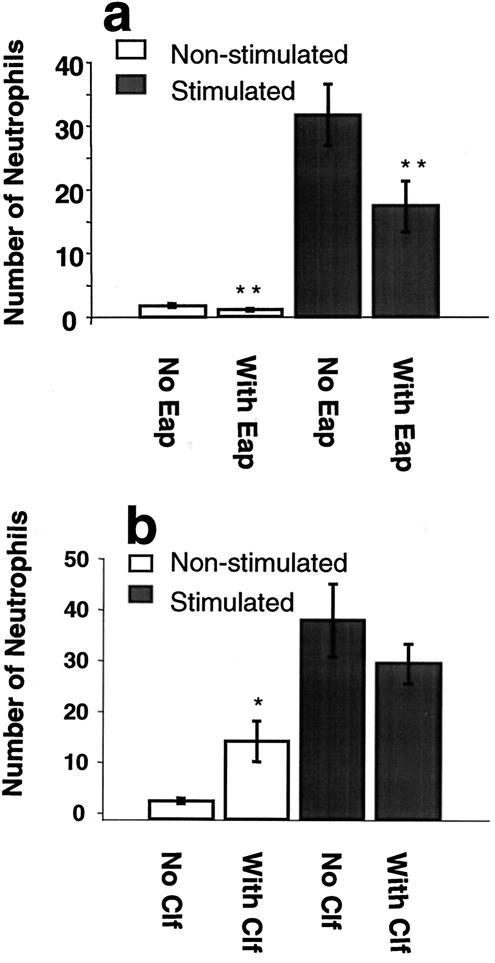
In order to investigate whether Eap mediated all its blocking effect on neutrophil adhesion to the endothelium via ICAM-1 binding (2), the blocking effect of Eap alone was compared to that of anti-ICAM-1 antibodies alone or anti-ICAM-1 antibodies in combination with Eap in a static adhesion experiment. Only TNF-α-stimulated endothelium was used in this experiment, and after 4 h of TNF-α treatment, anti-human ICAM-1 immunoglobulin G antibodies (final concentration, 8 μg/ml; R&D Systems) were added for 20 min. A blocking effect was elicited by the ICAM-1 antibodies (P < 0.05), in accordance with other studies in which ICAM-1 antibodies have been used (7). The blocking effects of Eap and ICAM-1 antibodies were of similar magnitude, and no additive or synergistic effects on blocking could be detected when antibodies and Eap were used together (P < 0.05) (Fig. 1c). In order to investigate whether Eap also affected the adherence of neutrophils under conditions of physiological flow, neutrophils (5 × 105 cells/ml) were perfused through a flow chamber (Glycotech, Rockville, Md.) over confluent monolayers of HAECs at 1 dyne/cm2 at 37°C. The endothelium was treated as described above, and cells in 10 visual fields were counted for each well. In the flow adhesion assay, Eap also significantly (P < 0.01) inhibited neutrophil adhesion, both to nonstimulated and to TNF-α-stimulated endothelium (Fig. 2a). The presence of Clf (final concentration, 20 μg/ml) in the flow assay did not significantly affect the binding of neutrophils to stimulated endothelial cells (Fig. 2b). In contrast, neutrophils bound significantly better to nonstimulated endothelial cells in the presence of Clf than in the absence of Clf (Fig. 2b). Further investigations to explain the molecular events behind this observation are under way.
FIG. 2.
Flow adhesion assay. Human neutrophils (5 × 105 cells/ml) were perfused over confluent monolayers of HAECs at 1 dyne/cm2 for 15 min at 37°C. Endothelial cells were treated at 37°C with medium alone (white bars) or with TNF-α (shaded bars) for 6 h prior to the assay. After 4 h of incubation, Eap (final concentration, 30 μg/ml) was added to some wells, and the wells were further incubated for 2 h (a). Clf (final concentration, 20 μg/ml) was used as a control protein (b). Ten visual fields were counted for each well. The data are presented as the mean ± the SEM of results from six experiments. Statistical significance was determined by Student's t test. *, P < 0.05; **, P < 0.01.
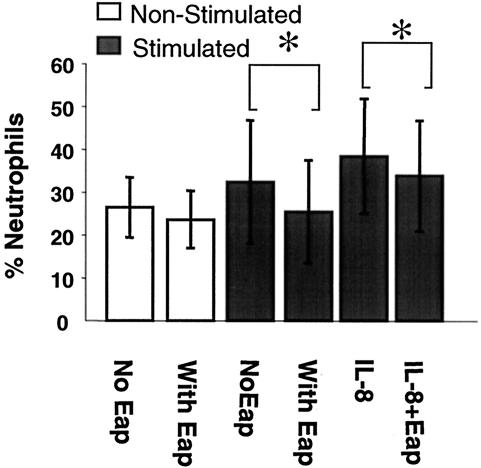
The effect of Eap on neutrophil transendothelial migration was investigated in a migration assay performed as previously described (10). HAECs were cultured on gelatin-coated filters (Transwell; Costar, Cambridge, Mass.) for at least 5 days to reach confluence and were stimulated as described above. Freshly isolated neutrophils were labeled with 10 μM Calcein-AM (Molecular Probes Inc., Eugene, Ore.) in Krebs Ringer glucose for 20 min at 37°C. Labeled neutrophils (2 × 106 cells) were added to the upper compartment of the Transwell and allowed to migrate through the endothelium for 2 h at 37°C. As an attractant, 50 ng of recombinant human interleukin-8 (IL-8; R&D Systems)/ml in culture medium was added to the lower compartment. Medium alone was added as a control for random migration. After migration, 0.1% Triton X-100 (Sigma Chemical Co., St. Louis, Mo.) was added to each well to release Calcein from cells that had migrated. The concentration of Calcein in each well was measured by a Fluor-S Max MultiImager (Bio-Rad Laboratories, Hercules, Calif.). The value for maximal migration was obtained by measuring the fluorescence of cells directly added to the lower well. The percentage of migration was obtained by dividing migration by maximal migration. In the migration assay, we observed that Eap inhibited the migration of neutrophils across stimulated endothelium in the presence or absence of human IL-8 (P < 0.05) (Fig. 3). However, no effect of Eap on the migration across nonstimulated endothelial cells was detected. Eap thus blocks only the transendothelial migration which is dependent on stimulation and the expression of ICAM-1 (10).
FIG. 3.
Migration assay. Human neutrophils (2 × 106 cells) were allowed to migrate across monolayers of HAECs for 2 h at 37°C. Endothelial cells were treated at 37°C with medium alone (white bars) or TNF-α (shaded bars) for 6 h prior to the assay. After 4 h of incubation, Eap (final concentration, 30 μg/ml) was added to some wells, and the wells were further incubated for 2 h. As an attractant, recombinant human IL-8 in culture medium was added to the lower compartment. The value for maximal migration was obtained by measuring the fluorescence of cells directly added to the lower compartment of the Transwell plate. The percentage migration was obtained by dividing the observed migration by maximal migration. The data are presented as the mean ± the SEM of results from five experiments. Statistical significance was determined by Student's t test. *, P < 0.05.
Our present study demonstrates that Eap from S. aureus inhibits the binding of neutrophils to the endothelium under static and dynamic flow conditions and in an in vitro model also inhibits transendothelial migration. We furthermore showed that the blocking effect of Eap was of the same magnitude as the blocking effect of human ICAM-1 antibodies, suggesting that Eap exerts its effect solely by ICAM-1 binding.
Eap is claimed to be an immunomodulating protein (13). In a previous paper, it was shown that the interaction of Eap with ICAM-1 inhibited the binding of leukocytes to the endothelial cells and that thereby leukocyte extravasation into the site of infection was reduced in an in vivo model (1). Furthermore, Lee et al. showed that the presence of Eap (also termed Map and p70) interfered with T-cell proliferation, leading to chronic manifestations of the infection, such as arthritis, osteomyelitis, and abscess formation, in an animal model (13). These two studies complement each other in the sense that T-cell proliferation is dependent on the interaction of T cells with antigen-presenting cells, and ICAM-1 plays a pivotal role in the conjugation of these two cell types (3, 11). We were able to show step by step that Eap has a direct impact on the binding of neutrophils to endothelial cells. Eap was able to block neutrophil binding to endothelial cells at all levels of static adherence (Fig. 1a) and all conditions of flow (Fig. 2a). As a consequence, the transendothelial migration of neutrophils, a step essential for the first line of defense against infections, was also inhibited (Fig. 3).
The immunomodulating properties of Eap from S. aureus are not unique for this protein. Chemotaxis-inhibitory protein of S. aureus (CHIPS) is an exoprotein which has been shown to inhibit the responses of neutrophils and monocytes to chemoattractants, such as C5a and formylated peptides, by binding to the corresponding receptors on the host cells (4, 17). Both of these chemoattractants have been shown to induce infiltration, trafficking, and homing of leukocytes to the site of infection. Furthermore, several other bacterial species also influence the immune system, either by increasing transendothelial migration through an up-regulation of adhesion molecules, such as VCAM-1 and ICAM-1, on endothelial cells (Salmonella enterica serovar Typhimurium, Streptococcus mutans, and Borrelia burgdorferi) (8, 19, 21) or by blocking migration through binding to Mac-1 on leukocytes, as has been seen with the adhesin filamentous hemagglutinin from Bordetella pertussis (18). The reduction of neutrophil transendothelial migration demonstrated in this study is probably the result of an inhibitory effect of Eap on firm adhesion of neutrophils to ICAM-1. We found that antibodies against ICAM-1 had the same blocking effect as Eap and that there was no additive effect when both antibodies and Eap were used. This result further confirmed previous observations that Eap's inhibitory effect is solely ICAM-1 dependent (Fig. 1c) (1). The inhibitory effect elicited by Eap on the migration of neutrophils across endothelial cells in vivo may give S. aureus anti-inflammatory characteristics. The presence of Eap during an S. aureus infection and its effect on transendothelial migration and antigen presentation to T cells will weaken the protective cellular immunity, which is the main mechanism used by the host to clear bacterial infections.
Acknowledgments
This work was supported by grants from the Swedish Research Council (to J.-I.F. and J.H.) and from Biostapro AB. J.H. holds a position within the program Glycoconjugates in Biological Systems financed by the Swedish Foundation for Strategic Research.
Editor: J. D. Clements
REFERENCES
- 1.Chavakis, T., M. Hussain, S. M. Kanse, G. Peters, R. G. Bretzel, J.-I. Flock, M. Herrmann, and K. T. Preissner. 2002. Staphylococcus aureus extracellular adherence protein serves as anti-inflammatory factor by inhibiting the recruitment of host leukocytes. Nat. Med. 8:687-693. [DOI] [PubMed] [Google Scholar]
- 2.Chavakis, T., K. T. Preissner, and S. Santoso. 2002. Leukocyte trans-endothelial migration: JAMs add new pieces to the puzzle. Thromb. Haemostasis 89:13-17. [PubMed] [Google Scholar]
- 3.Dang, L. H., M. T. Michalek, F. Takei, B. Benaceraff, and K. L. Rock. 1990. Role of ICAM-1 in antigen presentation demonstrated by ICAM-1 defective mutants. J. Immunol. 144:4082-4091. [PubMed] [Google Scholar]
- 4.de Haas, C. J. C., K. E. Veldkamp, A. Peschel, F. Weerkamp, W. J. B. van Wamel, E. C. J. M. Heezius, M. J. J. G. Poppelier, K. P. M. Van Kessel, and J. A. G. Van Strijp. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 199:687-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrnfelt, C., L. Serrander, and J. Holgersson. 2003. Porcine endothelium activated by anti-alpha-GAL antibody binding mediates increased human neutrophil adhesion under flow. Transplantation 76:1112-1119. [DOI] [PubMed] [Google Scholar]
- 6.Eidhin, D. N., S. Perkins, P. Francois, P. Vaudaux, M. Höök, and T. J. Foster. 1998. Clumping factor B (Clf B), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 7.Ellerbroek, P. M., A. I. M. Hoepelman, F. Wolbers, J. J. Zwaginga, and F. E. J. Coenjaerts. 2002. Cryptococcal glucuronoxylomannan inhibits adhesion of neutrophils to stimulated endothelium in vitro by affecting both neutrophils and endothelial cells. Infect. Immun. 70:4762-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galdiero, M., A. Folgore, M. Molitierno, and R. Greco. 1999. Porins and lipopolysaccharide (LPS) from Salmonella typhimurium induce leucocyte transmigration through human endothelial cells in vitro. Clin. Exp. Immunol. 116:453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haggar, A., M. Hussain, H. Lönnies, M. Herrmann, A. Norrby-Teglund, and J.-I. Flock. 2003. Extracellular adherence protein from Staphylococcus aureus enhances internalization into eukaryotic cells. Infect. Immun. 71:2310-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauzenberger, E., D. Hauzenberger, K. Hultenby, and J. Holgersson. 2000. Porcine endothelium supports transendothelial migration of human leukocyte subpopulations: anti-porcine vascular cell adhesion molecule antibodies as species-specific blockers of transendothelial monocyte and natural killer cell migration. Transplantation 69:1837-1849. [DOI] [PubMed] [Google Scholar]
- 11.Hogg, N., P. A. Bates, and J. Harvey. 1991. Structure and function of intercellular adhesion molecule-1. Chem. Immunol. 50:98-115. [PubMed] [Google Scholar]
- 12.Hussain, M., A. Haggar, C. Heilmann, G. Peters, J.-I. Flock, and M. Herrmann. 2002. Insertional inactivation of eap in Staphylococcus aureus strain Newman confers reduced staphylococcal binding to fibroblasts. Infect. Immun. 70:2933-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, L. Y., Y. J. Miyamoto, W. McIntyre, M. Höök, K. W. McCrea, D. McDevitt, and E. L. Brown. 2002. The Staphylococcus aureus Map protein is an immunomodulator that interferes with T cell-mediated response. J. Clin. Investig. 110:1461-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 15.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 16.Palma, M., A. Haggar, and J.-I. Flock. 1999. Adherence of Staphylococcus aureus is enhanced by an endogenous secreted protein with broad binding activity. J. Bacteriol. 181:2840-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postma, B., M. J. J. G. Poppelier, J. C. van Galen, E. R. Prossnitz, J. A. G. Van Strijp, C. J. C. de Haas, and K. P. M. Van Kessel. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the C5a and formylated peptide receptor. J. Immunol. 172:6994-7001. [DOI] [PubMed] [Google Scholar]
- 18.Rozdzinski, E., J. Sandros, M. van der Flier, A. Young, B. Spellerberg, C. Bhattacharyya, J. Straub, G. Musso, S. Putney, R. Starzyk, and E. Tuomanen. 1995. Inhibition of leukocyte-endothelial cell interactions and inflammation by peptides from a bacterial adhesin which mimic coagulation factor X. J. Clin. Investig. 95:1078-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sellati, T. J., L. D. Abrescia, J. D. Radolf, and M. B. Furie. 1996. Outer surface lipoproteins of Borrelia burgdorferi activate vascular endothelium in vitro. Infect. Immun. 64:3180-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Springer, T. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: a multistep paradigm. Cell 76:301-314. [DOI] [PubMed] [Google Scholar]
- 21.Vernier-Georgenthum, A., S. al-Okla, B. Gourieux, J. Klein, and D. Wachsmann. 1998. Protein I/II of oral viridans streptococci increases expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Cell. Immunol. 187:145-150. [DOI] [PubMed] [Google Scholar]