Abstract
BACKGROUND:
Many studies have indicated that the incidence of serious diabetic complications may be reduced through strict glycemic control. A low glycemic index diet is one tool to improve insulin resistance and improve glycemic control in type 2 diabetes mellitus (T2DM).
AIM:
The objective was to study the effect of pseudocereals-based breakfasts (quinoa and buckwheat) on glucose variations at first meal (breakfast) and second meal (standardised lunch) in healthy and diabetic subjects.
SUBJECTS AND METHODS:
Twelve healthy subjects and 12 patients with Type 2 DM (not- insulin dependent) were recruited in the study. Subjects were provided with quinoa and buckwheat breakfast meals. A standardised lunch was provided 4 h after breakfast. Postprandial blood glucose response after breakfast and the second meal effect was measured in healthy and diabetic subjects. Incremental area under the curve (IAUC) values for glucose was measured in response to the breakfast and lunch. The glycemic index of the 2 pseudocereals-based test breakfasts was determined. A white wheat bread (WWB) was served as a reference breakfast meal.
RESULTS:
In post-breakfast analyses, healthy subjects showed that buckwheat meal had significantly lower IAUC values for blood glucose compared to WWB reference meal (P < 0.001) while quinoa meal showed no significance. In diabetic subjects, buckwheat and quinoa meals had significantly lower IAUC values for blood glucose compared to WWB reference meal (P < 0.001 and P < 0.05 respectively). Blood glucose concentrations started to decline gradually for the quinoa and buckwheat but not for WWB in all healthy and diabetic subjects and returned to near-fasting baseline levels by 210 min. Post-lunch analyses indicated higher IAUC for the two breakfast types in healthy and diabetic subjects. In addition, the quinoa and buckwheat breakfast meals were followed by a significantly flatter blood glucose response to the second meal for the period between 270 and 330 min. At the end of the second meal period, values were below or near-fasting baseline levels in the breakfast period. The blood glucose concentration after consuming quinoa meal showed a high peak at 30 min similar to that of WWB reference meal. This peak resulted in a high glycemic index (GI) for quinoa (89.4). The GI of buckwheat recorded a low value (26.8).
CONCLUSION:
The two studied pseudocereals; quinoa and buckwheat have high potential to improve glucose tolerance at the first and second meal (lunch) and are recommended to be introduced in our daily diet for healthy and diabetic subjects.
Keywords: Quinoa, buckwheat, white wheat bread (WWB), incremental area under the curve (IAUC), plasma glucose response, glycemic index (GI)
Introduction
Diabetes mellitus is a progressive chronic disease associated with microvascular and macrovascular complications [1]. Postprandial hyperglycemia is a major risk factor for cardiovascular disease and increased cardiovascular morbidity and mortality in diabetic subjects [2]. Many studies have stated the incidence of serious diabetic complications may be reduced through strict glycemic control [3]. A low glycemic index diet is one tool to improve insulin resistance and improve glycemic control in type 2 diabetes mellitus (T2DM) [4].
Although the ability of certain pseudocereals to lower postprandial glycemia is reported [5], no studies have been conducted on the effect of pseudocereals on the subsequent meal. The second meal effect is the ability of grains to reduce postprandial glycemia not only after a meal at which they were taken but also at a subsequent meal in the day. This effect is beneficial for blood glucose control in diabetic patients and also causes an unanticipated decrease in insulin demands at the subsequent meal [6].
The second meal effect occurs when the glycemic index (GI) of one meal influences the glycemic response to a second meal [7]. A low glycemic index breakfast has been shown to lower the postprandial glucose response to lunch [8]. The second meal effect is important for individuals with type 2 diabetes and for healthy individuals trying to control their glucose excursions as well. Jenkins et al. [9] determined that the Staub-Traugott effect (the second meal effect), lasted from breakfast to lunch (a 4 hour period) by showing that slow digesting carbohydrate breakfast meals improved glucose tolerance during a subsequent lunch. Nowadays, the second meal effect is beginning to be taken into consideration.
Quinoa and Buckwheat are grains which belong to the “pseudocereals” category. They are characterised by an excellent nutrient profile [10] and absence of gluten proteins (prolamines) found in commonly used cereal grains such as wheat, barley, and oats [11]. Pseudocereals are essentially starchy crops, rich in essential amino acids, fatty acids, minerals, and vitamins that make them an alternative to traditional nutrient starch based gluten-free foods. Pseudocereals are starchy seeds as cereals but they are not members of the grass family.
Buckwheat (Fagopyrum esculentum Moench) is denominated as ‘king of the healing grains’. It is a rich source of essential amino acids, fibres, minerals, antioxidants, folate, omega-3 fatty acids, potassium, B vitamins [12]. Buckwheat has also been suggested as a low GI grain ingredient in some designed foods [13, 14, 15].
Buckwheat grains contain numerous nutraceutical compounds as catechins, rutin, and many other polyphenols [16]. These functional components are shown to influence positively on the variations in blood glucose levels, on hypertension, and dyslipidemia [17]. Moreover, buckwheat grains are a rich source of soluble and total dietary fibre dietary fibre and are useful in lowering the incidence of obesity and diabetes [18].
Quinoa (Chenopodium quinoa Willd) is an ancient seed cultivated in the Andean region [19]. It is characterised by its high nutritional properties [20]. Quinoa seeds are rich in proteins having a well-balanced content of essential amino acids when compared to those of common cereals [21]. Quinoa contains significant amounts of phytochemicals including saponins, flavonoids, phytosterols, phenolic acids, fat-soluble vitamins, fatty acids, trace elements and minerals which have many advantageous biochemical effects [22]. Studies on the hypoglycemic effects of Quinoa in vivo are rare. A previous study recommended quinoa to be used as an alternative to commonly used grains in the production of cereal-based gluten-free products with a low GI [23].
The aim of this study was to determine the postprandial glycemic response after consuming breakfast meal containing quinoa or buckwheat as compared to the white wheat bread reference meal in healthy and diabetic subjects. In addition, the effect of these breakfast meals on postprandial glycemic response after a second meal (lunch) was studied and glycemic indexes of the previously mentioned grains were calculated.
Subjects and Methods
Subjects
During this randomised prospective study, healthy and diabetic subjects consumed three breakfast and lunch meal combinations for a period of three weeks. Twelve healthy subjects and 12 patients with Type 2 DM (non-insulin dependent) were recruited in the study. The 12 healthy subjects were six women and six men with a mean age of 30 years (range, 22–40) and normal body mass index (mean BMI of 21.6 ± 1.5 kg/m^2). The 12 patients with Type 2 DM were seven women and five men with a mean age of 55 years (range, 40–68) and body mass index of 24 ± 1.6 kg/m^2. Their mean duration of diabetes was 10 years. Before the start of the experiment, fasting blood glucose of diabetic subjects was monitored for a minimum period of 6 months. The average value of fasting blood glucose was 126 mg/dL. Patients controlled their diabetes by taking oral hypoglycemic agents beside their normal daily diet; they have not been diagnosed with other chronic diseases and were taking breakfast regularly. On the day of the experiment, patients consumed tested and reference breakfasts and lunch with no oral hypoglycemic agents. The volunteers were enrolled for this study in the National Research Centre (NRC), Cairo, Egypt, after a written informed consent was obtained from each of them. Participants were non-smokers and were not exercising on a regular basis.
Methods
The effect of two pseudocereals-based test breakfasts and white wheat bread (WWB) served as the reference meal was evaluated. Blood glucose response after breakfast and after the second meal was measured in healthy and diabetic subjects.
Subjects were provided with the test breakfast meals. Two of the meals were composed primarily of two pseudocereals either quinoa or buckwheat; the third meal was the reference white wheat bread WWB. The food items contained in the breakfast control and test meals are provided in Table 1. Quinoa seeds were bought from our local market; while buckwheat seeds imported from Russia, were purchased from Dubai market, United Arab of Emirates. The two tested boiled pseudocereals (quinoa; buckwheat) and the WWB reference breakfast meals were served with butter and cheese to balance the fat and protein contents of the meals. In addition, 100 mL milk (3% fat), 150 mL water and 150 mL tea were provided with each meal. The size of all meals corresponded to 50 g available carbohydrates, 20 g protein, and 9.6 g fat and provided 1533 kJ.
Table 1.
Composition of the breakfast meals
| Breakfast | WWB | Quinoa | Buckwheat | Cottage cheese | Butter | Milk | Water | Tea |
|---|---|---|---|---|---|---|---|---|
| g | g | g | g | g | mL | mL | mL | |
| WWB meal | 125 | - | - | 40 | 7 | 100 | 150 | 150 |
| Quinoa meal | - | 80 | - | 50 | 1.5 | 100 | 150 | 150 |
| Buckwheatmeal | - | - | 67 | 75 | 5 | 100 | 150 | 150 |
WWB, white wheat bread (reference meal).
Blood glucose concentration readings were taken every 30 min using Accu-Check Active meter (Roche Diagnostics GmbH, Germany) [24].
White wheat bread WWB
WWB made from 100% refined wheat flour (extraction 72%) was used as the reference food. The bread was made from 300 g white-wheat flour, 200 ml. water, 3 g dry yeast and 3 g salt. Each dough was proofed for 60 minutes to allow fermentation, then flattened and cut into round pieces of 15 cm diameter and 0.2 cm thickness, and a second proofing was made for 30 minutes. WWB was baked in an oven at 250° C. WWB was served as the reference meal.
Second-meal study
Four hours after consuming the test and reference breakfast meals, healthy and diabetic subjects were served a second standardised high-GI meal. This meal consisted of 100 g deep- fried meatballs, 250 g mashed potatoes, and 60 g canned sweet corn (El Tahya, Cairo, Egypt). Lunch meal provided 2384.88 kJ. In addition, 250 mL water was served with each meal. Both breakfast and lunch meals provided approximately 45% of a total daily energy intake.
Estimation of blood glucose response after breakfast and second-meal
Subjects were requested to fast overnight for 10–12 hours. Capillary blood sample was obtained by a finger prick using a monoejector Lancet device (Accu-ChekSoftclix). Blood samples were taken before breakfast meals to determine fasting blood glucose concentrations (0 min). Postprandial blood samples were taken at 30, 60, 90, 120, 150, 180 and 210 min after the breakfast. In addition, blood samples were taken for glucose determination immediately before the second meal (240 min, ie, 4 h after breakfast considered as 0 times for the second meal) and at 270, 300, 330, 360 and 390 min post-lunch. The first drop of blood was placed onto the strip and readings were taken (within 5-10 sec) and recorded.
Calculation of Glycemic index (GI)
The GIs of quinoa and buckwheat were determined after the test meals had been served to healthy subjects. The WWB reference meal and the test meals were taken by the subjects in random order after an overnight fast and were separated by a washout period for the duration of one week. The meals were served at the same time in the morning and subjects were asked to consume all meals within 10-12 min.
The Incremental areas under curves (IAUC) of test meals and reference meal of each individual were calculated. The GI for the test meals was then calculated by dividing the value of glucose IAUC of the test meal by that of the reference meal for the same individual ignoring any area beneath the baseline [25] multiplied by 100 as shown in the following equation:
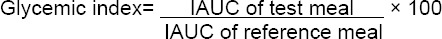
Statistical analysis
Differences between fasting and postprandial glucose concentrations were determined and incremental area under the curve (IAUC) calculations were completed using the AutoCAD program. The IAUC for blood glucose was calculated between 0–60, 0–120, 0–180 and 0–210 minutes postprandial for all participants for breakfast, and 240-300, 240-360, 240–390 min for lunch. Paired t- test were used to identify differences between quinoa, buckwheat, and the WWB reference meal. P-Value < 0.05 indicated a statistically significant difference for all tests. All continuous variable data are reported as the mean ± standard error. Statistical Package for the Social Sciences software (SPSS) for windows (SPSS Inc., Chicago, IL, version 17.0) was used for the statistical analysis.
Results
First meal responses in healthy and diabetic subjects
Glucose tolerance curves:
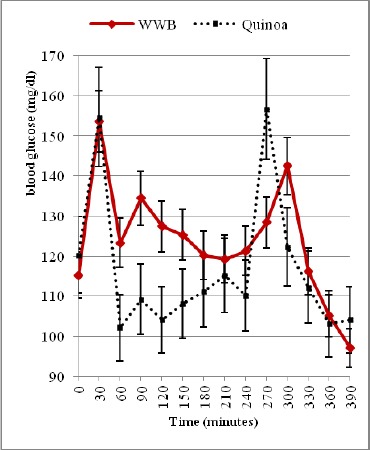
The blood glucose tolerance curves are shown in (Fig. 1 & 2) and (Fig. 3 & 4) for healthy and diabetic subjects after consumption of three breakfast meals. By comparison with the WWB reference meal, the blood glucose response curves and the peak rise of blood glucose were significantly reduced after consumption of buckwheat meal (p < 0.001). No significant differences were observed in the peak rise of blood glucose after consumption of quinoa meal as compared to the WWB reference meal. In healthy subjects, there was a quick rise in blood glucose concentrations (mean ± SD) and peaked at 30 min for the WWB reference, quinoa and buckwheat meals (152 ± 18.5 mg/dL, 153 ± 16.6 mg/dL, 124 ± 13.1 mg/dL respectively).
Figure 1.
Mean capillary blood glucose conc. during the first and second meal following ingestion of WWB or Quinoa breakfast in healthy subjects
Figure 2.
Mean capillary blood glucose conc. during the first and second meal following ingestion of WWB or Buckwheat breakfast in healthy subjects
Figure 3.
Mean capillary blood glucose conc. during the first and second meal following ingestion of WWB or Quinoa breakfast in diabetic subjects
Figure 4.
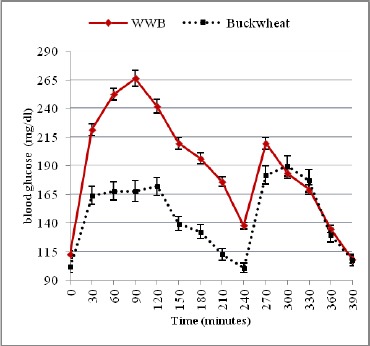
Mean capillary blood glucose concentration during the first and second meal following ingestion of WWB or Buckwheat breakfast in diabetic subjects
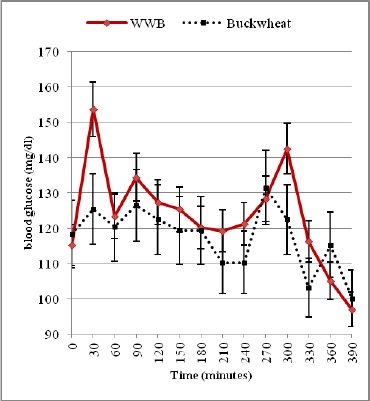
Otherwise, in diabetic subjects, blood glucose concentration peaked at 90 min (263 ± 14.8 mg/dL) for the WWB reference meal, while that of quinoa and buckwheat meal peaked at 60 min (264 ± 24.6 mg/dL) and at 120 min (174 ± 13.5 mg/dL) respectively. Blood glucose concentrations started to decline gradually in all healthy and diabetic subjects and returned to near-fasting baseline levels by 210 min (Fig. 1 & 2, 3 & 4). In contrast, diabetic subjects consuming the WWB reference meal had higher blood glucose concentrations at 210 min (174 ± 9.8 mg/dL) than that of the baseline level (112 ± 6.3 mg/dL).
Glucose incremental area under the curve (IAUC)
The first and second meal glucose IAUC of the reference and the two test meals are shown in Table 2. The percent change is given by the following formula:
Table 2.
Postprandial blood glucose incremental area under the curve (IAUCs) values after the reference white wheat bread, quinoa and buckwheat breakfast meals and the following second meal in healthy and diabetic subjects (n = 12)
| Time | WWB Mean ± SE | QUINOA Mean ± SE | BUCKWHEAT Mean ± SE | *P - value | ||
|---|---|---|---|---|---|---|
| I | II | III | a | b | ||
| HEALTHY | 0-60 min | 1257 ± 14 | 1625 ± 28 | 241 ± 5 | <0.05 | <0.001 |
| 0-120 min | 2123 ± 37 | 1912 ± 43 | 569 ± 10 | NS | <0.001 | |
| 0-180 min | 2681 ± 46 | 2307 ± 39 | 683 ± 12 | NS | <0.001 | |
| 0-210 min | 2857 ± 50 | 2703 ± 47 | 679 ± 15 | NS | <0.001 | |
| 240-300 min | 527 ± 12 | 1566 ± 35 | 809 ± 14 | <0.001 | <0.05 | |
| 240-360 min | 789 ± 14 | 1783 ± 40 | 927 ± 21 | <0.001 | NS | |
| 240-390 min | 791 ± 25 | 1803 ± 31 | 939 ± 16 | <0.001 | NS | |
| DIABETICS | 0-60 min | 5373 ± 157 | 5423 ± 93 | 2891 ± 49 | NS | <0.001 |
| 0-120 min | 13613 ± 176 | 12892 ± 225 | 7000 ± 157 | NS | <0.001 | |
| 0-180 min | 19864 ± 208 | 14889 ± 333 | 9764 ± 171 | <0.05 | <0.001 | |
| 0-210 min | 22021 ± 230 | 15041 ± 263 | 10262 ± 175 | <0.05 | <0.001 | |
| 240-300 min | 2801 ± 29 | 3715 ± 63 | 3857 ± 67 | <0.05 | <0.05 | |
| 240-360 min | 4363 ± 46 | 7978 ± 178 | 7445 ± 127 | <0.05 | <0.05 | |
| 240-390 min | 4363 ± 46 | 8519 ± 149 | 8023 ± 179 | <0.05 | <0.05 | |
A = I vsII, b = I vs III.
P < 0.05 (significant), P < 0.001 (highly significant), p > 0.05 (not significant).
Percent change = (Reference mean- Test mean) ÷ Reference meal × 100
In healthy subjects, the greatest reduction in the breakfast periods glucose IAUC at 0–60 min, 0–120 min, 0–180 min and 0–210 min was achieved with buckwheat meal which recorded 80.8%, 73.2%, 74.5% and 76.2% respectively lower values than that after the WWB reference meal (p<0.001).
The breakfast glucose IAUC of quinoa meal was not significantly different from the WWB reference meal, although it was reduced at 0–120 min, 0–180 min and 0–210 min by 9.9%, 14.0% and 5.4% respectively. However, at 0–60 min, the breakfast glucose IAUC of quinoa meal was 29.3% greater than that produced by the WWB reference meal and the difference was statistically significant (p < 0.05).
In diabetic subjects, the greatest reduction in the breakfast periods glucose IAUC at 0–60 min, 0–120 min, 0–180 min and 0–210 min was achieved with buckwheat meal which recorded 46.2 %, 48.6%, 50.8% and 53.4% respectively lower values than that after the WWB reference meal (p < 0.001).
No significant differences were observed in the breakfast glucose IAUC of quinoa meal at 0–60 and at 0–120 min that was reduced by 1% and 5.3%, while at 0–180 min and 0–210 min, reduction were 25.0% and 31.7% respectively lower values than that after the WWB reference meal (p < 0.05).
Second meal responses in healthy and diabetic subjects
Glucose tolerance curves:
Blood glucose concentrations determined immediately before and after the second meal are shown in (Fig.1 & 2) and in (Fig. 3 & 4) for healthy and diabetic subjects. From these figures, it is shown that the blood glucose concentrations just before the second meal (at 240 min) were significantly lower after the quinoa and buckwheat breakfast meals than after the WWB reference meal in healthy and diabetic subjects. These concentrations were below the breakfast fasting baseline values.
There was a quick rise in blood glucose concentrations that peaked at 270 min for the quinoa and buckwheat meals (155 ± 18.7 mg/dL and 130 ± 9.5 mg/dL) in healthy subjects after lunch. Otherwise, in diabetic subjects, blood glucose concentrations peaked at 300 min (198 ± 16.8 mg/dL and 192 ± 14.9 mg/dL). Glucose values during the second meal period were fairly flat for quinoa and buckwheat breakfast meals between 270 and 330 min (ie. 30-90 min. after consumption of the lunch).
At the end of the second meal period, values were below or near-fasting baseline levels in the breakfast period (Fig. 1-4) in healthy and diabetic subjects.
Glucose incremental area under the curve (IAUC)
The second meal IAUC calculations began at 240 min. In healthy subjects, the second meal glucose IAUC at 240–300 min, 240–360 min and 240–390 min that followed the buckwheat breakfast meal recorded higher values than that after the WWB reference meal by 53.5% (p < 0.05) for the first period of time, while it showed no significant difference for the other periods of time (17.5% and 18.7% respectively). High significant differences were observed in the second-meal glucose IAUC of quinoa breakfast meal at 240–300 min, 240–360 min, 240–390 min, and values were 197.2%, 126.0% and 127.9% respectively greater than that after the WWB reference meal (p < 0.001) (Table 2).
On the other hand, in diabetic subjects, the second meal glucose IAUC values at 240–300 min, 240–360 min, 240–390 min after the buckwheat and quinoa breakfast meal produced both a significantly higher glucose area than that after the WWB reference meal (by 37.7%, 70.6% and 83.9% for the former; and 32.6%, 82.9% and 95.3% for the later (p < 0.05) (Table 2).
Glycemic indexes of the test breakfast meals
The GIs of all the test breakfast meals are given in Table 3. The GI of the buckwheat meal (GI: 26.8) was significantly lower than that of the WWB reference meal (GI:100) (p < 0.001). The GI for the quinoa meal (GI:89.4) was not significantly different from that of the WWB meal (GI:100).
Table 3.
Glycemic Index of the reference and test meals (per 50 g carbohydrates portion)
| Breakfast meals | Glycemic Index (Mean ± SE) |
|---|---|
| WWB | 100.0a± 2.0 |
| Quinoa | 89.4b± 1.9 |
| Buckwheat | 26.8c± 1.5 |
Values in the same column with different superscript letters are significantly different (P < 0.05).
Discussion
In this study, the effect of quinoa and buckwheat meals on glycemic response of healthy and diabetic subjects has been investigated. This study was designed to test the first meal response of a pseudocereal breakfast meal containing either quinoa or buckwheat and to determine their residual effects after the second meal of high glycemic index lunch. The goal of this study was to maintain blood glucose levels close to normal baseline as possible without risking hypoglycemia.
After the first meal, postprandial glucose excursions and IAUC values were lower for the quinoa meal compared to the control WWB reference meal, while the lowest postprandial glucose variations and IAUC were observed for the buckwheat meal. Previous studies have similarly reported that buckwheat significantly lowered plasma glucose responses as compared with the refined wheat flour bread [26].
For diabetics, the blood glucose excursions’ recovery periods returning to the baseline level are longer than those for healthy subjects [27]. In other words, diabetic subjects had a slower rate of glucose clearance in the blood, while healthy subjects had better blood glucose clearance following ingestion of meals. This concept is consistent with the present results showing that concentrations of blood glucose after consuming quinoa and buckwheat meal breakfasts steadily began to decline to below baseline levels by 120 min in healthy and by 210 min in diabetic subjects (Fig. 1-3). Meanwhile, concentrations of blood glucose after consuming WWB reference meal began to decline, and returned to a value slightly higher than baseline level in healthy subjects, while in diabetic subjects, a high blood glucose response was recorded and blood glucose failed to return to the baseline value by 210 min (Fig. 1-3). Postprandial glycemia for type 2 diabetes vary widely depending on the type and the amount of carbohydrate consumed [28].
In this study, peak blood glucose response after consuming buckwheat meal was lower than that of quinoa and WWB reference meal in both healthy and diabetic subjects (Fig. 2-4). It must be noted that in diabetic subjects, blood glucose peak was observed at 60 min (264 mg/dl) and at 90 min (263 mg/dl) after the consumption of quinoa and WWB reference meal respectively. The buckwheat meal resulted in a lower blood glucose peak at 120 min (174 mg/dl) than did the quinoa, or WWB reference meal. These results may be attributed to the variability in two polysaccharides: amylose and amylopectin of quinoa, wheat and buckwheat. Information in the literature suggests that considerable variability exists in the amylose content of quinoa (3% - 20%) [29] which is considered to be lower than in cereals as wheat (20%-30%) [30]. Furthermore, amylose content of buckwheat starch granules recorded the highest values and fluctuates between 15% and 52% and has higher resistant starch content [31].
Several studies have shown that the amylose content was negatively correlated with the onset of gelatinization [32]; the degree of gelatinization is greater when the amylose content is low and vice-versa. This phenomenon leads to that amylose will be more susceptible to be hydrolyzed by alpha-amylase (starch digestive enzymes) and become glucose resulting in an increase in blood sugar levels [33]. In other words, starches with low amylose level have higher glycemic indexes [34]. Inversely, starches with a higher amylose content, as in buckwheat, will be less susceptible to gelatinization, amylose retrogrades more rapidly than amylopectin after cooling, and forms an amylase-resistant crystal structure (resistant starch) leading to reduction in the rate of digestion [35], that is slowing the breaking down into glucose, which makes the low glycemic index. Such previous studies are in accordance with our results as quinoa recorded an early postprandial blood glucose peak (60 min) and a high glycemic index 89, while buckwheat recorded postprandial blood glucose peak later (120 min) and a low glycemic index 26.8. In an In vitro study by Wolter et al [36], it was reported that quinoa bread showed highest predicted GI (95) as compared to WWB (GI = 100). Other studies made in individuals with celiac disease showed that quinoa has a glycemic index slightly lower than that of gluten-free bread and pasta [37]. All these results were found to be similar to the present study.
Despite the high GI of quinoa and early high blood glucose peak (peak at 30 min in healthy and at 60 min in diabetic subjects) [38], concentrations of blood glucose after consuming quinoa meal breakfasts steadily began to decline and returned to below baseline levels. This could be explained by the fact that quinoa contains considerably a high content of health-beneficial phytochemicals including vitamin E, iron, zinc and magnesium contents, as well as saponins, phenolics and phytosterols [39]. These bioactive compounds may attenuate carbohydrate metabolism and hyperglycemia; improve pancreatic β-cell function and insulin release [40]. Abugoch found antioxidants capacity compounds such as polyphenols, phytosterols, and flavonoids in grains of quinoa [41]. These compounds may be related to the effects of the reduction in postprandial glucose levels in the individuals tested, suggesting an improvement in insulin action and pancreatic function.
A second-meal effect in the form of a reduced glucose response to a high GI lunch was also shown after quinoa and buckwheat breakfast meal.
The results of the present study add to evidence that quinoa and buckwheat breakfast meals improve glucose tolerance at a subsequent high glycemic second meal as compared to WWB reference meal in healthy and diabetic subjects. Before lunch, at 240 min, blood glucose concentrations for quinoa and buckwheat were below fasting baseline values than in breakfast period. In contrast, blood glucose concentrations in subjects consuming WWB reference meal recorded a value higher than the fasting baseline values in breakfast period.
Furthermore, in diabetic subjects it is clear that quinoa and buckwheat breakfast meal improved postprandial glycemia resulting in a flattened postprandial curve (270; 300; 330 min), indicating modulation in insulin response to the glucose released into blood, and prevention of rebound hypoglycemia. Consumption of quinoa meal has been shown to improve glucose tolerance at the subsequent meal. This effect may be attributed to the fact that quinoa contains Alpha-glucosidase inhibitors which act at the small intestine’s brush border, inactivating the enzyme responsible for breaking down complex carbohydrates (slowing digestion and absorption of high GI meal lunch), prolonging glucose absorption, and flattening the postprandial glycemic curve [42] (low glucose peak 198, plateau during postprandial glucose at 270; 300; 330 min).
Results of the present study confirmed that buckwheat breakfast meal lowered first and second blood glucose response in healthy and diabetic subjects. In healthy subjects, buckwheat starch was so slowly digested that it failed to increase postprandial blood glucose concentrations to a measurable extent. In diabetic subjects, a relevant control of the glucose release in the first two hours was observed, resulting in a flattening of the glucose curve followed by a slow decrease of blood glucose concentration then returning to the pre-prandial level. Previous studies suggested that the delay of the absorption rate of the carbohydrate component is possibly due to the high viscous fibre content and the high ratio of amylose starch to amylopectin that induced reductions in both postprandial glucose and insulin responses [43]. The high amount of resistant starch content in buckwheat has been identified as a key contributing factor to its low GI characteristics. The amylase enzyme is unable to break down this form of starch, therefore it passes undigested throughout the body and may be used as a nutrient source for gut microflora and providing a mechanism for blood glucose control [44].
Another proposed mechanism involved in buckwheat’s glucose lowering abilities is its high content of D-chiro-inositol known as fagopyritols (D-CI is a rare isomer of vitamin B8 that is naturally found in many grains). Buckwheat containing the second high concentrations next to mung beans [45]. Fagopyritols are structurally similar to a galactosamine derivative of D-chiro-inositol, a putative insulin mediator that facilitates a decrease in blood glucose concentrations [46]. Furthermore, buckwheat grain contains D-fagomine, a natural glucose analogue that is not digestible. This substance is a simple soluble fibre that controls the postprandial glycemic response by delaying the absorption of carbohydrates in the small intestine delivering these nutrients to the large intestine, improving the assimilation of the nutrients and also maintaining a healthy digestive gut system. These findings support a mechanism related to a slow rate of glucose delivery to the blood. In addition to a prolonged digestive phase is a prolonged suppression of plasma fatty acids, which has been shown to be associated with improved response to insulin action [47].
At the end of the second meal period, values were near or below fasting baseline levels as in the first meal period for all breakfast types.
This study showed that despite quinoa has a high glycemic index, blood glucose after consuming quinoa meal breakfasts steadily began to decline and return to below baseline levels. Quinoa meal also improves glucose tolerance after the second meal. The low-glycemic index breakfast meal containing buckwheat has a remarkable impact on glycemic control as it improves glucose tolerance after the first and second meal in healthy and diabetic subjects. Both pseudocereals quinoa and buckwheat improve glycemic control after the second meal presented by the flattened glucose curve (270; 300; 330 min), and glucose levels returned to near or below fasting baseline levels as in the first meal period preventing rebound hypoglycemia.
In summary, quinoa and buckwheat pseudocereals could be considered as a new source of specific foods with potential health benefits to improve first and second-meal glucose excursions in healthy and diabetic subjects. In addition, it should be noted that despite the fact that quinoa can help to improve the management of Type 2 DM as it shows sustainable blood glucose response after the first and second meal, it must be consumed in a measured amount as blood glucose level peaked early at 60 min in diabetic subjects. This phenomenon must be considered and needs more investigations.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546–551. doi: 10.4103/2230-8210.183480. http://dx.doi.org/10.4103/2230-8210.183480 . PMid: 27366724. PMCid: PMC4911847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Ca-izo-Gómez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. 2014;5(4):444–470. doi: 10.4239/wjd.v5.i4.444. http://dx.doi.org/10.4239/wjd.v5.i4.444 . PMid: 25126392. PMCid: PMC4127581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghannadi S, Amouzegar A, Amiri P, Karbalaeifar R, Tahmasebinejad Z, Kazempour-Ardebili S. Evaluating the Effect of Knowledge, Attitude, and Practice on Self-Management in Type 2 Diabetic Patients on Dialysis. J Diabetes Res. 2016;2016:1–7. doi: 10.1155/2016/3730875. http://dx.doi.org/10.1155/2016/3730875 . PMid: 27478845. PMCid: PMC4958437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood RJ, Fernandez ML. Carbohydrate-restricted versus low-glycemic-index diets for the treatment of insulin resistance and metabolic syndrome. Nutr Rev. 2009;67(3):179–183. doi: 10.1111/j.1753-4887.2009.00186.x. http://dx.doi.org/10.1111/j.1753-4887.2009.00186.x . PMid: 19239633. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez KA. Observations regarding consumption of Peruvian native grains (Quinoa, Amaranth and Kaiwa), weight status, and perceptions of potential risk factors, warning signs and symptoms of type 2 diabetes among Peruvian adults: a case study.”. PhD diss. 2012. [Last accessed on 2016 Aug 23]. Available from: http://drum.lib.umd.edu/handle/1903/12830 .
- 6.Higgins JA. Whole grains, legumes, and the subsequent meal effect: implications for blood glucose control and the role of fermentation. J Nutr Metab. 2012;2012:829238. doi: 10.1155/2012/829238. http://dx.doi.org/10.1155/2012/829238 . PMid: 22132324. PMCid: PMC3205742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher JA, Perfield JW, II, Thyfault JP, Rector RS. The Second Meal Effect and Its Influence on Glycemia. Journal of Nutritional Disorders & Therapy 2012. 2012 [Google Scholar]
- 8.Kaur B, Ranawana V, Teh AL, Henry CK. The impact of a low glycemic index (GI) breakfast and snack on daily blood glucose profiles and food intake in young Chinese adult males. J Clin Transl Endocrinol. 2015;2(3):92–98. doi: 10.1016/j.jcte.2015.05.002. http://dx.doi.org/10.1016/j.jcte.2015.05.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkins DJ, Wolever TM, Taylor RH, Griffiths C, Krzeminska K, Lawrie JA, et al. Slow release dietary carbohydrate improves second meal tolerance. Am J Clin Nutr. 1982;35:1339–1346. doi: 10.1093/ajcn/35.6.1339. PMid: 6282105. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez-Jubete L, Arendt EK, Gallagher E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci Technol. 2010;21(2):106–13. http://dx.doi.org/10.1016/j.tifs.2009.10.014 . [Google Scholar]
- 11.Mota C, Santos M, Mauro R, Samman N, Matos AS, Torres D, et al. Protein content and amino acids profile of pseudocereals. Food Chem. 2016;193:55–61. doi: 10.1016/j.foodchem.2014.11.043. http://dx.doi.org/10.1016/j.foodchem.2014.11.043 . PMid: 26433287. [DOI] [PubMed] [Google Scholar]
- 12.Ravichand DM. Neutraceuticals: Role of natural molecules in pharmacotherapy. Int J Pharm Biosci. 2015;6:444–55. [Google Scholar]
- 13.Skrabanja V, LiljebergElmståhl HG, Kreft I, Björck IM. Nutritional properties of starch in buckwheat products: studies in vitro and in vivo. J Agric Food Chem. 2001;49(1):490–6. doi: 10.1021/jf000779w. http://dx.doi.org/10.1021/jf000779w . PMid: 11170616. [DOI] [PubMed] [Google Scholar]
- 14.Shakib MCR, Gabrial SGN, Gabrial GN. Buckwheat consumption improved lipid profile, fasting and postprandial blood glucose in hyper-cholesterolemic and type 2 diabetic patients. Int J Acad Res. 2011;3(4):132–139. [Google Scholar]
- 15.Vujić L, Čepo DV, Šebečić B, Dragojević IV. Effects of pseudocereals, legumes and inulin addition on selected nutritional properties and glycemic index of whole grain wheat-based biscuits. J Food Nutr Res. 2014;53:152–161. [Google Scholar]
- 16.Giménez-Bastida JA, Zieliński H. Buckwheat as a functional food and its effects on health. J Agric Food Chem. 2015;63(36):7896–913. doi: 10.1021/acs.jafc.5b02498. http://dx.doi.org/10.1021/acs.jafc.5b02498 . PMid: 26270637. [DOI] [PubMed] [Google Scholar]
- 17.Chopra N, Dhillon B, Puri S. Formulation of Buckwheat Cookies and their Nutritional, Physical, Sensory and Microbiological Analysis. IJABR. 2014;5(3):381–387. [Google Scholar]
- 18.Christa K, Soral-Śmietana M. Buckwheat grains and buckwheat products–nutritional and prophylactic value of their components–a review. Czech J Food Sci. 2008;26(3):153–162. [Google Scholar]
- 19.Coral LLT, Cusimamani EF. An Andean Ancient Crop, Chenopodium quinoa Willd: A Review. Agricultura Tropica et Subtropica. 2014;47(4):142–146. [Google Scholar]
- 20.James LE. Quinoa (Chenopodium quinoa Willd.). composition, chemistry, nutritional, and functional properties. Adv Food Nutr Res. 2009;58:1–31. doi: 10.1016/S1043-4526(09)58001-1. http://dx.doi.org/10.1016/S1043-4526(09)58001-1 . [DOI] [PubMed] [Google Scholar]
- 21.Bhargava A, Shukla S, Ohri D. Chenopodium quinoa--An Indian perspective. Ind Crops Prod. 2006;23:73–87. http://dx.doi.org/10.1016/j.indcrop.2005.04.002 . [Google Scholar]
- 22.Paśko P, Zagrodzki P, Bartoń H, Chłopicka J, Gorinstein S. Effect of quinoa seeds (Chenopodium quinoa) in diet on some biochemical parameters and essential elements in blood of high fructose-fed rats. Plant Foods Hum Nutr. 2010;65(4):333–338. doi: 10.1007/s11130-010-0197-x. http://dx.doi.org/10.1007/s11130-010-0197-x . PMid: 21104320. PMCid: PMC2998641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capriles VD, dos Santos FG, Arêas JA. Gluten-free breadmaking: Improving nutritional and bioactive compounds. J Cereal Sci. 2016;67:83–91. http://dx.doi.org/10.1016/j.jcs.2015.08.005 . [Google Scholar]
- 24.Hettiarachi UPK, Ekanyake S, Welihinda J. How accurate is glucometer in determining glycemic index? Int Food Res J. 2012;19(4):1511–1516. [Google Scholar]
- 25.FAO/WHO. Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation. FAO Food Nutr Pap. 1998;66:1–140. PMid: 9743703. [PubMed] [Google Scholar]
- 26.Su-Que L, Ya-Ning M, Xing-Pu L, Ye-Lun Z, Guang-Yao S, Hui-Juan M. Effect of consumption of micronutrient enriched wheat steamed bread on postprandial plasma glucose in healthy and type 2 diabetic subjects. Nutrition J. 2013;12:64. doi: 10.1186/1475-2891-12-64. http://dx.doi.org/10.1186/1475-2891-12-64 . PMid: 23680007. PMCid: PMC3679746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsin-i Wu. A case study of type 2 diabetes self-management. Biomed Eng Online. 2005;4:4. doi: 10.1186/1475-925X-4-4. http://dx.doi.org/10.1186/1475-925X-4-4 . PMid: 15644138. PMCid: PMC546416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blaak EE, Antoine J-M, Benton D, et al. Impact of postprandial glycaemia on health and prevention of disease. Obes Rev. 2012;13(10):923–984. doi: 10.1111/j.1467-789X.2012.01011.x. http://dx.doi.org/10.1111/j.1467-789X.2012.01011.x . PMid: 22780564. PMCid: PMC3494382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown DC, Cepeda-Cornejo V, Maughan PJ, Jellen EN. Characterization of the granule-bound starch synthase I gene in Chenopodium. Plant Genome. 2015;8:1–12. doi: 10.3835/plantgenome2014.09.0051. [DOI] [PubMed] [Google Scholar]
- 30.Hallström E, Sestili F, Lafiandra D, et al. A novel wheat variety with elevated content of amylose increases resistant starch formation and may beneficially influence glycaemia in healthy subjects. Food Nutr Res. 2011;55:7074. doi: 10.3402/fnr.v55i0.7074. http://dx.doi.org/10.3402/fnr.v55i0.7074 . PMid: 21876685. PMCid: PMC3162347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sindhu R, Khatkar BS. Composition and Functional Properties of Common Buckwheat (Fagopyrum Esculentum Moench) Flour and Starch. IJIRAS. 2016;3(7):154–159. [Google Scholar]
- 32.Alcázar-Alay SC, Meireles MAA. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci Technol. (Campinas) 2015;35(2):215–236. http://dx.doi.org/10.1590/1678-457X.6749 . [Google Scholar]
- 33.Boers HM, Seijen ten Hoorn J, Mela DJ. A systematic review of the influence of rice characteristics and processing methods on postprandial glycaemic and insulinaemic responses. Br J Nutr. 2015;114(7):1035–1045. doi: 10.1017/S0007114515001841. http://dx.doi.org/10.1017/S0007114515001841 . PMid: 26310311. PMCid: PMC4579564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis B, Melina V. Becoming Vegan: Comprehensive Edition – The Complete Reference to Plant Based Nutrition”. The Book Publishing Company; 2014. [Google Scholar]
- 35.Pandey S, Senthil A, Fatema K. Effect of Hydrothermal Treatment on the Nutritional and Functional Properties of Husked and Dehusked Buckwheat. J Food Process Technol. 2015;6:461. [Google Scholar]
- 36.Wolter A. Fundamental studies of sourdoughs fermented with Weissellacibaria and Lactobacillus plantarum: influence on baking characteristics, sensory profiles and in vitro starch digestibility of gluten free breads. PhD Thesis, University College Cork. 2013. [Last accessed on 2016 Aug 23]. Available from: http://hdl.handle.net/10468/1478 .
- 37.Berti C, Riso P, Monti LD, Porrini M. In vitro starch digestibility and in vivo glucose response of gluten free foods and their counterparts. Eur J Nutr. 2004;43:198–204. doi: 10.1007/s00394-004-0459-1. http://dx.doi.org/10.1007/s00394-004-0459-1 . PMid: 15309439. [DOI] [PubMed] [Google Scholar]
- 38.Mithila MV, Khanum F. Effectual comparison of quinoa and amaranth supplemented diets in controlling appetite;a biochemical study in rats. J Food Sci Technol. 2015;52(10):6735–6741. doi: 10.1007/s13197-014-1691-1. http://dx.doi.org/10.1007/s13197-014-1691-1 . PMid: 26396423. PMCid: PMC4573157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graf BL, Rojas-Silva P, Rojo L E, Delatorre-Herrera J, Baldeón ME, Raskin I. Innovations in Health Value and Functional Food Development of Quinoa (Chenopodium quinoa Willd.) Compr Rev Food Sci Food Saf. 2015;14:431–445. doi: 10.1111/1541-4337.12135. http://dx.doi.org/10.1111/1541-4337.12135 . PMid: 27453695. PMCid: PMC4957693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirmiran P, Bahadoran Z, Azizi F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J Diabetes. 2014;5(3):267–281. doi: 10.4239/wjd.v5.i3.267. http://dx.doi.org/10.4239/wjd.v5.i3.267 . PMid: 24936248. PMCid: PMC4058731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abugoch James LE. Quinoa (Chenopodium quinoa Willd.). Composition, chemistry, nutritional and functional properties. Adv Food Nutr Res. 2009;58:1–31. doi: 10.1016/S1043-4526(09)58001-1. http://dx.doi.org/10.1016/S1043-4526(09)58001-1 . [DOI] [PubMed] [Google Scholar]
- 42.Ranilla LG, Apostolidis E, Genovese MI, Lajolo FM, Shetty K. Evalution of indigenous grains from the Peruvian Andean region for antidiabetes and antihypertension potential using in vitro methods. J Med Food. 2009;12(4):704–713. doi: 10.1089/jmf.2008.0122. http://dx.doi.org/10.1089/jmf.2008.0122 . PMid: 19735168. [DOI] [PubMed] [Google Scholar]
- 43.Augustin LS, Chiavaroli L, Campbell J, Ezatagha A, Jenkins AL, Esfahani A, et al. Post-prandial glucose and insulin responses of hummus alone or combined with a carbohydrate food: a dose–response study. Nutr J. 2016;15:13. doi: 10.1186/s12937-016-0129-1. http://dx.doi.org/10.1186/s12937-016-0129-1 . PMid: 26818604. PMCid: PMC4730744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stirling H. The effect of buckwheat and couscous on food intake and satiety in male adults. Diss. Mount Saint Vincent University. 2016. [Last accessed on 2016 Aug 23]. Available from: http://dc.msvu.ca.8080/xmlui/handle/10587/1747 .
- 45.Kawa JM, Taylor CG, Przybylski R. Buckwheat Concentrate Reduces Serum Glucose in Streptozotocin-Diabetic Rats. J Agric Food Chem. 2003;51(25):7287–7291. doi: 10.1021/jf0302153. http://dx.doi.org/10.1021/jf0302153 . PMid: 14640572. [DOI] [PubMed] [Google Scholar]
- 46.Jing R, Li HQ, Hu CL, Jiang YP, Qin LP, Zheng CJ. Phytochemical and Pharmacological Profiles of Three Fagopyrum Buckwheats. Int J Mol Sci. 2016;17(4):589. doi: 10.3390/ijms17040589. http://dx.doi.org/10.3390/ijms17040589 . PMid: 27104519. PMCid: PMC4849043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borel A-L, Boulet G, Nazare J-A, Smith J, Alméras N, Tremblay A, et al. Improved Plasma FFA/Insulin Homeostasis Is Independently Associated With Improved Glucose Tolerance After a 1-Year Lifestyle Intervention in Viscerally Obese Men. Diabetes Care. 2013;36(10):3254–3261. doi: 10.2337/dc12-2353. http://dx.doi.org/10.2337/dc12-2353 . PMid: 23695818. PMCid: PMC3781540. [DOI] [PMC free article] [PubMed] [Google Scholar]


