Abstract
AIM:
To assess the antibacterial and antifungal potentials of different parts of Moringa oleifera plant using different extraction methods in attempts to formulate natural dental remedies from this plant.
MATERIAL AND METHODS:
Three solvents extracts (Ethanol, acetone, and ethyl acetate) of different parts of Egyptian Moringa tree were prepared and tested against oral pathogens: Staphylococcus aureus, Streptococcus mutans, and Candida albicans using disc diffusion method; As well as to incorporate the plant extract to formulate experimental toothpaste and mouthwash. The two dental remedies were assessed against the same microbial strains. Statistical analysis was performed using One-Way ANOVA test to compare the inhibition zone diameter and t-test.
RESULTS:
Ethanol extracts as well as leaves extracts demonstrated the highest significant mean inhibition zone values (P ≤ 0.05) against Staphylococcus aureus and Streptococcus mutans growth. However, all extracts revealed no inhibition zone against Candida albicans. For dental remedies, experimental toothpaste exhibited higher mean inhibition than the mouthwash against Staphylococcus aureus, Streptococcus mutans and only the toothpaste revealed antifungal effect against Candida albicans.
CONCLUSION:
The different extracts of different parts of Moringa showed an antibacterial effect against Staphylococcus aureus and Streptococcus mutans growth. The novel toothpaste of ethanolic leaves extract has antimicrobial and antifungal potential effects all selected strains.
Keywords: Moringa extract, Antimicrobial Activity, Novel experimental tooth paste, mouth wash, (Novel Dental Remedies)
Introduction
Although antibiotics and the chemotherapeutic agents have been used to fight many infectious diseases; none of the available drugs is free from unfavourable effects and limitations. In addition to the development of multi-drug resistant strains of bacteria which is also another growing problem [1-3]. All necessitates the search for alternate therapies to fight infections [4, 5].
Medicinal plants are among the raising therapeutic options since they contained various chemical compounds with antimicrobial effects [6-9]. In developing countries, due to the expensive cost of antibiotics, the use of medicinal plants for treatment of infection is considered the affordable and safer natural treatment modality [10-13].
Moringa oleifera is among the most valuable world’s trees, as almost every part of the Moringa tree can be used for food or has curative characteristics [14, 15]. Remarkably, it was discovered that Moringa oil was used for skin care since ancient Egyptian time’s [16].
Considerable studies have been conducted to evaluate the biologically active substances of varies part of this plants [17-20]. For its great antimicrobial effects, Moringa lately, has been used in different health care products [16]. The extracts of different parts of the tree with different solvents are expected to produce active substances with various antimicrobial effects. Hence, it was interesting to assess the antibacterial and antifungal potentials of different parts of Moringa oleifera plant using different extraction methods in attempts to formulate natural dental remedies from this plant.
Materials and Methods
Collection and extraction of plant parts:
The fresh leaves, roots, and seeds of Moringa Oleifera were supplied by the Egyptian Scientific Society of Moringa farm and identified by Prof. Aboelfetoh Mohamed Abdella, National research centre, Giza, Egypt. The different parts of the plant were washed with distilled water and air-dried at room temperature separately. Then each plant part was cut into small pieces (1-2 cm) to make it suitable for grinding. Fifty grammes of each part of Moringa powder (leaves, roots, seeds, and a mixture of all these parts) were kept soaked for 72 hours in 500 ml of ethanol 95%, acetone and ethyl acetate solvents at room temperature. The prepared mixtures were then magnetically stirred for 24 hours to obtain a homogenous mix and stored for 48 hours at 4°C to allow for extraction of active ingredients. Each of the resultant extracts was filtered using Whatman No. 1 filter paper and concentrated by a rotary vacuum evaporator (Rotary Evaporator HS2001N, ARC Distributors, India) at 40°C to evaporate the solvents used for extraction. The ethanol extraction yields (w/w); (19.38 %, 7.34%, 6.40%, and 12.36%) for leaves, roots, seeds and their mixture respectively, while acetone extraction yields; (15.20%, 5.38%, 4.30%, and 9.32%), and for ethyl acetate extraction yields; ((12.50%, 3.90%, 3.32% and 7.45%).
Microorganisms
Three microorganisms were used to test the antimicrobial potential of the prepared Moringa plant extracts, they were: Staphylococcus aureus (S. aureus) and Streptococcus mutans (S. mutans) and Candida albicans (C. albicans). S. aureus was isolated and serologically identified by dairy microbiological Lab., National Research Center, Giza, Egypt. While S. mutans were isolated from the dental plaque of high caries index patients by swabbing method from the oral cavity of the patients according to Sanchez et al., 2004 [21]. Selective media Mitis salivarius-bacitracin agar (MSB; BD Difco, Paris) was used to isolate and grow S. mutans. Both pathogenic bacteria were routinely transferred into Tryptone Soya broth (TSB; Difco laboratories, Detroit, MI, USA) and incubated at 37°C for 24 hours. C. albicans (ATCC 10231) was provided by the Department of Microbiology, Cairo University and maintained on Sabouraud Liquid Medium (1% peptone, 4% glucose and adjusted to pH 5.8) to grow at a temperature of 35-37°C for 48 hours.
Assessment of antimicrobial activity
A disc diffusion method was used to test the antimicrobial activity of ethanol, acetone, and ethyl acetate extracts of the different plant parts and their mixture on the profile growth of S. aureus and S. mutans and C. albicans. A total of 324 samples were divided into four main groups (n = 81) according to the plant parts (leaves, roots, seeds, and mixture) being tested. Each group was further subdivided into three subgroups according to the type of solvent used (ethanol, acetone, and ethyl acetate). In addition, each subgroup was divided into three smaller groups according to the type of pathogenic bacteria testing (S. aureus, S. mutans and C. albicans).
Two grammes of each prepared extract were dissolved in 5.0 ml of Dimethyl sulfoxide (DMSO) to get 400 mg/ml. A 0.1ml (approximately 109 cells/ml) of each pathogenic bacterium was dispersed on the surface of Tryptone soya agar. While 50 µl of the broth medium containing C. albicans was inoculated in a selective culture medium Sabouraud’s Dextrose Agar (SDA) with the aseptically addition of Streptomycin. Sterile filter paper discs 4 mm in diameter were impregnated with 20 µl of each extract and placed on surface agar inoculated with pathogenic bacteria and fungus (triplicate for each tested microorganisms). Then, the plates were incubated at 35-37°C for 24 hours. At the end of incubation period, the antimicrobial activity of different plant parts extracts was evaluated by determining the diameter of inhibition zones (mm) around each disc of extract solution (Fig.1a, Fig.1b).
Figure 1.
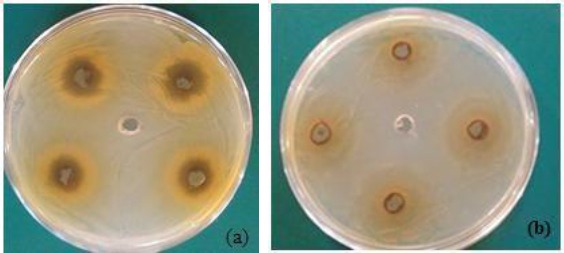
Antibacterial activity of ethanolic extract of Moringa Oleifera leaves against the profile growth of (a) staphylococcus aureus and (b) Streptococcus mutans
Incorporation of Moringa Oleifera leaves into the experimental prepared toothpaste and mouthwash
The ethanol extract of Moringa Oleifera leaves was incorporated in specially prepared experimental Passive toothpaste and mouthwash base formulas with appropriate concentration for each remedy (patent under registration no 2015/2069, Ministry of scientific research, Academy of scientific research & technology, Egypt).
Assessment of antimicrobial Activity of the experimental prepared toothpaste and mouthwash
The antimicrobial activity of experimental toothpaste was screened by modified agar well diffusion method while the mouthwash was determined by disc diffusion method. A total of (144) samples were divided into four main groups (n = 36) according to the active additives incorporated in each dental base formula: A1: The experimental toothpaste base (control), A2: toothpaste with the active additive ethanolic leaves extract, A3: experimental mouths wash base formula, and A4: mouthwash with active ethanolic leaves extract. Each group was further subdivided into three subgroups (C) (n = 12) according to the type of pathogenic bacteria and fungus being tested.
In the well diffusion method, a 50µl of diluted S. mutans and S. aureus were spread on the surface of Tryptone Soya agar plate, as well as 50 µl of the broth medium containing C. albicans was inoculated in a selective culture medium Sabouraud’s Dextrose Agar. A sterile 5 mm cork- borer was used to cut four wells at equidistance in the plate. Similar calculated amount of toothpaste (250 mg) was introduced into the four wells. In disc diffusion method 20µl of mouthwash solution was placed on surface agar plate inoculated with the pathogenic bacteria and fungus (triplicate for each tested microorganisms). The tested plates were incubated at 35-37°C for 24 hours. At the end of incubation period, the antimicrobial activity of experimental toothpaste and mouthwash were evaluated by measuring the mean diameter of the zone of inhibitions in millimetre (Fig. 2a, Fig. 2b, Fig. 3c).
Figure 2.
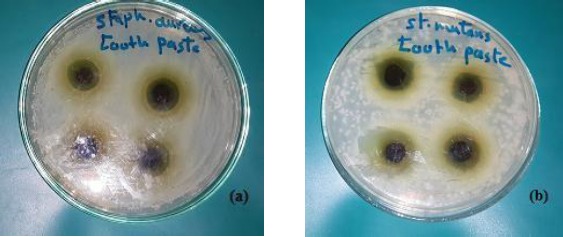
Antibacterial activity of the experimental toothpaste with the active additive Moringa leaves ethanolic extract on the profile growth of (a) staphylococcus aureus and (b) Streptococcus mutans
Figure 3.
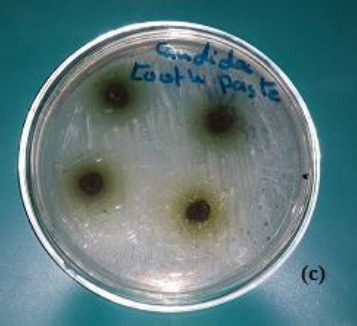
Antifungal activity of experimental toothpaste with the active additive Moringa leaves ethanolic extract on the profile growth of (c) Candida albicans
Statistical analysis
Data were analysed using IBM® SPSS® (SPSS Inc., IBM Corporation, NY, USA) Statistics Version 23 for Windows and then checked for normality using Kolmogorov - Smirnov test. One Way ANOVA test was used to compare between different extracts of Moringa Oleifera plant parts followed by Tukey’s posthoc test for pairwise comparison. Independent t-test was used to compare between experimental toothpaste and mouthwash against selected bacterial and fungal strains. The significance level was set at P ≤ 0.05.
Results
The different extraction methods (ethanol, acetone and ethyl acetate) of different parts of M Oleifera plant were assessed against S aureus, S mutans and C. albicans. The means and standard deviations (SD) values for the Inhibition Zones (mm) were displayed in Tables 1 & 2 and Figures 4 & 5. For experimental mouthwash and toothpaste dental remedies; means and SDs of the Inhibition Zones (mm) against the same microbial strains are represented in Table 3 and Fig. 6.
Table 1.
Means and Standard deviations (SDs) for the Inhibition Zones (mm) for different parts of Moringa Oleifera plant using different extraction methods
| Methods of extractions | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Ethanolic | Acetone | Ethyl acetate | ||||||
| Mean | SD | Mean | SD | Mean | SD | |||
| Staphylococcus aureus | Leaves Extract | 19.25aA | 0.96 | 11.25aB | 0.96 | 9.00aC | 0.82 | ≤0.001* |
| Roots Extract | 9.25aA | 0.50 | 5.50cB | 0.58 | 4.50cC | 0.58 | ≤0.001* | |
| Seeds Extract | 3.25c | 0.96 | 2.50d | 0.58 | 2.75d | 0.50 | 0.354 | |
| Mix Extract (1:1:1) | 10.00A | 0.82 | 6.75bB | 0.50 | 5.75bB | 0.96 | ≤0.001* | |
| p-value | ≤0.001* | ≤0.001* | ≤0.001* | |||||
Means with the same lower Case letter within each row are not significantly different at ≥0.05. Means with the same Upper Case letter within each Column are not significantly different at p≤0.05.
= Significant, NS= Non-significant.
Table 2.
Means and Standard deviations (SDs) for the Inhibition Zones (mm) for different parts of Moringa Oleifera plant using different extraction methods
| Methods of extraction | p-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Ethanolic | Acetone | Ethyl acetate | ||||||
| Mean | SD | Mean | SD | Mean | SD | |||
| Streptococcus mutans | Leaves Extract | 13.00aA | 1.15 | 8.25aB | 0.50 | 7.25aB | 0.96 | ≤0.001* |
| Roots Extract | 10.50bA | 0.58 | 5.25bB | 0.50 | 4.00cC | 0.82 | ≤0.001* | |
| Seeds Extract | 4.75dA | 0.96 | 3.25cB | 0.96 | 2.25dB | 0.50 | 0.007* | |
| Mix Extract (1:1:1) | 8.00cA | 0.82 | 5.75bB | 0.50 | 5.25bB | 0.50 | ≤0.001* | |
| p-value | ≤0.001* | ≤0.001* | ≤0.001* | |||||
Means with the same lower Case letter within each row are not significantly different at p≥0.05. Means with the same Upper Case letter within each Column are not significantly different at p≤0.05.
= Significant, NS= Non-significant.
Figure 4.
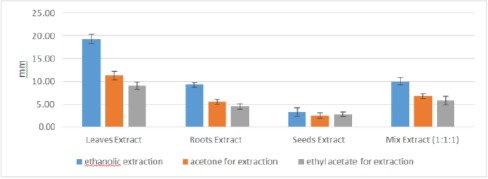
Mean Inhibition Zones (mm) for different parts of Moringa Oleifera plant against S. aureus growth using different extraction methods
Figure 5.
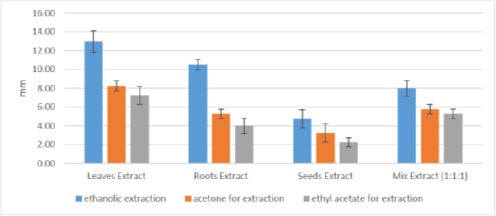
Mean Inhibition Zones (mm) for different parts of Moringa Oleifera plant against S. mutans growth using different extraction methods
Table 3.
Means and Standard deviations (SD) of the Inhibition Zones (mm) for mouth wash and tooth paste
| Dental remedies | p-value | ||||
|---|---|---|---|---|---|
| Mouth Wash | Tooth Paste | ||||
| Mean | SD | Mean | SD | ||
| Staphylococcus aureus | 11.75 | 1.26 | 17.75 | 0.96 | ≤0.001* |
| Streptococcus mutans | 9.25 | 0.96 | 13.75 | 0.96 | 0.001* |
| Candidia albicans | 0.00 | 0.00 | 13.25 | 1.50 | ≤0.001* |
Means with the same lower Case letter within each row are not significantly different at p≥0.05. Means with the same Upper Case letter within each Column are not significantly different at p≤0.05.
= Significant, NS= Non-significant.
Figure 6.
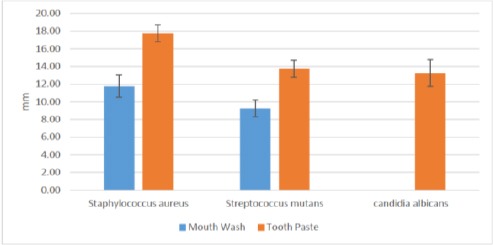
Mean Inhibition Zones (mm) for experimental mouthwash and tooth paste
Comparison of the mean inhibition zones against S. aureus growth; revealed a statistical significance difference regarding the extraction methods (p ≤ 0.05). Where all ethanol extracts demonstrated highest mean inhibition values followed by acetone except for seeds extract and lowest values were for acetyl acetate extracts. The later seeds extract showed slightly higher mean value than acetone extract, however, it was statistically insignificant (P ≥ 0.05) Table 1 and Figure 4.
Regarding plant parts, leaves extracts had highest mean inhibition values against S. aureus growth, followed by roots, plant parts mix and finally seeds extracts. Ethanol leaves extract recorded inhibition zone diameter (19.25 ± 0.96) followed by acetone (11.25 ± 0.96) and the lowest value was (9 ± 0.82) for acetyl acetate, respectively. Roots extracts mean values were: (9.25 ± 0.50), (5.50 ± 0.58) and (4.50 ± 0.58) for ethanol, acetone and ethyl acetate extracts respectively. While the mix extracts values were: (10 ± 0.82), (6.75 ± 0.50) and (5.75 ± 0.96) for ethanol, acetone and acetyl acetate respectively. All extracts showed a significant difference (p ≤ 0.05) except seeds extracts; where all seeds extracts showed insignificant difference despite the differences in extraction method (P ≥ 0.05) Table 1 and Figure 4.
Regarding the mean inhibition zones against S. mutans growth; statistical analysis revealed significance differences concerning the extraction methods and the plant parts evaluated (p ≤ 0.05). These results are comparable to that obtained against S. aureus. Where ethanol extracts demonstrated highest significant mean inhibition values followed by acetone extracts and the lowest values were for acetyl acetate extracts (P ≤ 0.05) Table 2 and Figure 5.
Again, leaves extract had the highest mean inhibition zone values followed by the roots, mix and at last seeds extracts exhibited the lowest mean value and these results were statistically significant (p ≤ 0.05) Table 2 and Figure 5. On contrary, no inhibition zone was detected against C. albicans fungus for the different plant part extracts using the three extraction methods.
When comparing the mean inhibition zone values for toothpaste and mouth wash remedies, the toothpaste showed the higher mean inhibition zone values than the mouthwash against S. aureus, S. mutans and C. albicans respectively and it statistically significant (P ≤ 0.05) Table 3 and Figure 6.
The toothpaste exhibited mean inhibition values (17.75 ± 0.96), (13.75 ± 0.96) and (13.25 ± 1.5) against S aureus, S. mutans and C. albicans respectively. While mouth values were: (11.25 ±1.26), (9.25 ± 0.96) against S. aureus and S. mutans respectively. The absence of inhibition zones against C albicans. Hence, only the experimental toothpaste had inhibition effect against C. albicans growth.
Discussion
In the present study, the antimicrobial and antifungal activities of Moringa oleifera plant parts (leaves, roots, seeds and their mixture) were tested against some oral pathogens as S. aureus, S. mutans and C. albicans. These microorganisms are associated with respiratory infection [22], dental caries [23] and oral candidiasis [24].
As it was reported in the literature that aqueous extracts of plants were generally exhibited little or no antimicrobial activities [25-27]. Therefore, in this study the three organic solvents; ethanol, acetone and acetyl acetate were used to prepare the M. oleifera plant extracts to explore the one with best antimicrobial activities. The results of this study revealed that all extracts of Moringa oleifera parts (leaves, roots, seeds and their mixture) have antimicrobial potentials (inhibition zones) against S. aureus and S. mutans with varying effectiveness. Differences in antimicrobial potency were found to vary with the type of organic solvent used to extract the active components and to plant part being evaluated (Leaves, seeds, roots, and their mixture).
On contrary, none of the extracts showed any antifungal activity against C. albicans, this is in agreement with two previous studies conducted by (Masika et al, 2012, Patel et al, 2014) [27, 28] who demonstrated that ethanolic leaf extract has no antifungal effect against C. albicans. The findings of this study, however, are in contrast to (Mahdi et al, 2014) [29] who reported that ethanol and acetone leaf extracts showed antifungal activity against C. albicans. The difference could be attributed to variation in the environment where the plant was collected, the season and the physiological stage of the plant when leaves were harvested [30].
While ethanolic extract, demonstrated the most effective antimicrobial activity against the S. aureus and S. mutans compared to acetone and acetyl acetate the extracts. Leaves extract also, showed greatest antimicrobial potential compared to other plant parts (seeds, roots, and the mixture). These latter results are in agreement with (Rahman et al, 2013 and Devedra et al, 2011) [31, 32] who mentioned that Moringa oleifera leaves contain bio-active substances with antibacterial potentials against a wide range of microorganisms. The leaves of M. oleifera plant are known to contain phytochemicals compounds as flavonoids, saponins, tannins and other phenolic compounds that have antimicrobial activities [33-35]. This would explain the antimicrobial activities observed in this study could be attributed to such compounds.
According to the results of this study, the plant leaf extract can be the added as a natural antimicrobial constituent in dental products to control oral pathogens. Such products will be supposed to have a preventive/therapeutic effect thus helping to remove dental plaque, the most important factor in oral infectious diseases such as dental caries and periodontal disease. Ethanol leaf extract of Moringa plant was used as an active constituent to formulate an experimental toothpaste and mouthwash. Other natural additives were used as a negative additive to configure dental products. The antimicrobial characteristics of dental products were examined against selected bacterial and fungal strains. The results showed that experimental toothpaste exhibited effective antimicrobial and antifungal activities against the all tested microorganisms. While, the experimental mouthwash revealed lesser antimicrobial potentials compared to toothpaste.
The previous observations could be attributed to several reasons as the difference in antibacterial activities is due to dissimilarities in the composition of both toothpaste and mouthwash base ingredients; variation in the proportion of added leaves extract for each remedy. Another explanation is that the ingredients of experimental toothpaste base formula do not suppress the antimicrobial activity of leaves extract [36]. Unexpectedly, the antifungal activity of the experimental toothpaste was found which could be attributed to the possible synergistic effect between leaf extract and the ingredients of toothpaste base formula. The results of the current study supported the antibacterial potential M. oleifera plant and could be the basis for promising applications of medicinal plant in the pharmaceutical industry to control oral pathogens.
In conclusions, within the limitations of present in vitro study: (1) the present study confirmed the antibacterial characteristics of Moringa oleifera extracts; (2) the plant extracts can be used to formulate new dental products to control oral pathogens owing to their antimicrobial potentials. However, further studies are required to clarify the optimal concentration, cell toxicity and physical stability before its clinical application
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Albuquerque AP, Monteiro JM, Ramos MA, Amorim ELC. Medicinal and magic plants from a public market in Northeastern Brazil. J Ethnopharmacol. 2007;110:76–91. doi: 10.1016/j.jep.2006.09.010. https://doi.org/10.1016/j.jep.2006.09.010 . PMid: 17056216. [DOI] [PubMed] [Google Scholar]
- 2.Costa RA, Vieira GHF, Silva GC, Vieira RHSF, Sampaio SS. Susceptibility “in vitro” antimicrobial Vibrio spp strains isolated from shrimp (Litopenaeus vannamei) and water creating these animals from a shrimp farm in CearáForeword. Braz J Vet Res Anim Sci. 2008;45:458–62. [Google Scholar]
- 3.Hofer E, Quintaes BR, Reis EMF, Rodrigues DP, Seki LM, Feitosa IS. Emergence of multiple drug resistance in Vibrio cholerae isolated from patients with gastroenteritis in Ceará, Brazil. Rev Soc Bras Med Trop. 1999;32:151–6. doi: 10.1590/s0037-86821999000200006. PMid: 10228365. [DOI] [PubMed] [Google Scholar]
- 4.Astal ZY, Ashour AERA, Kerrit AAM. Antimicrobial activity of some medicinal plant extracts in Palestine. Pak J Med Sci. 2005;21:187–193. [Google Scholar]
- 5.Rojas JJ, Ochoa VJ, Ocampo SA, Munoz JF. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complement Altern Med. 2006;6(1):2. doi: 10.1186/1472-6882-6-2. https://doi.org/10.1186/1472-6882-6-2 . PMid: 16483385. PMCid: PMC1395329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chea A, Jonville MC, Bun SS, Laget M, Elias R, Duménil G. In vitro antimicrobial activity of plants used in Cambodian traditional medicine. Am J Chin Med. 2007;35:867–73. doi: 10.1142/S0192415X07005338. https://doi.org/10.1142/S0192415X07005338 . PMid: 17963325. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira DF, Pereira AC, Figueiredo HC, Carvalho DA, Silva G, Nunes AS. Antibacterial activity of plant extracts from Brazilian southeast region. Fitoterapia. 2007;78:142–5. doi: 10.1016/j.fitote.2006.09.027. https://doi.org/10.1016/j.fitote.2006.09.027 . PMid: 17169500. [DOI] [PubMed] [Google Scholar]
- 8.Soberón JR, Sgariglia MA, Sampietro DA, Quiroga EN, Vattuone MA. Antibacterial activity of plant extracts from northwestern Argentina. J App Microbiol. 2007;102:1450–61. doi: 10.1111/j.1365-2672.2006.03229.x. https://doi.org/10.1111/j.1365-2672.2006.03229.x . PMid: 17578409. [DOI] [PubMed] [Google Scholar]
- 9.Zuo GY, Wang GC, Zhao YB, Xu GL, Hao XY, Han J. Screening of Chinese medicinal plants for inhibition against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) J Ethnopharmacol. 2008;120:287–90. doi: 10.1016/j.jep.2008.08.021. https://doi.org/10.1016/j.jep.2008.08.021 . PMid: 18804522. [DOI] [PubMed] [Google Scholar]
- 10.Masika PJ, Afolayan AJ. Antimicrobial activity of some plants used for treatment. International Journal of Antimicrobial Agents. 2002;26(5):129–134. doi: 10.1016/s0378-8741(02)00242-8. [DOI] [PubMed] [Google Scholar]
- 11.Sharif MD, Banik GR. Status and utilization of medicinal plants in Rangamati of Bangladesh. Res J Agric Biol Sci. 2006;2(6):268–273. [Google Scholar]
- 12.Doughari JH, El-mahmood AM, Manzara S. Studies on the anti-Carica papaya bacterial activity of root extracts of L. Afri. J Microbiol Res. 2007:037–041. [Google Scholar]
- 13.Abalaka ME, Olonitola OS, Onaolapo JA. Evaluation of acute toxicity of Momordica charantia extract, using wistar rats to determine safety level and usefulness of the plant ethnochemotheraphy. Int J Appl Sci. 2009;3:1–6. [Google Scholar]
- 14.Khawaja TM, Tahira M, Ikram UK. Moringa oleifera: a natural gift - A review. J Pharm Sci Res. 2010;2:775–81. [Google Scholar]
- 15.Mehta LK, Balaraman R, Amin AH, Bafna PA, Gulati OD. Effects of fruits of Moringa oleifera on the lipid profile of normal and hypo-cholesterolemic rabbits. Journal of Ethno pharmacology. 2003;86:191–195. doi: 10.1016/s0378-8741(03)00075-8. https://doi.org/10.1016/S0378-8741(03)00075-8 . [DOI] [PubMed] [Google Scholar]
- 16.Razis AFA, Ibrahim MD, Kntayya SB. Health benefits of Moringa oleifera. Asian Pac J Cancer Prev. 2014;15:15–20. doi: 10.7314/apjcp.2014.15.20.8571. [DOI] [PubMed] [Google Scholar]
- 17.Caceres A, Cabrera O, Morales O, Mollinedo P, Mendia P. Pharmacological properties of Moringa oleifera. 1: Preliminary screening for antimicrobial activity. J Ethnopharmacol. 1991;33:213–216. doi: 10.1016/0378-8741(91)90078-r. https://doi.org/10.1016/0378-8741(91)90078-R . [DOI] [PubMed] [Google Scholar]
- 18.Suarez M, Entenza JM, Doerries C. Expression of a plant-derived peptide harbouring water-cleaning and antimicrobial activities. Biotechnol Bioeng. 2003;81:13–20. doi: 10.1002/bit.10550. https://doi.org/10.1002/bit.10550 . PMid: 12432576. [DOI] [PubMed] [Google Scholar]
- 19.Ghebremichael K A, Gunaratna K R, Henriksson H, Brumer H, Dalhammar G. A simple purification and activity assay of the coagulant protein from Moringa Oleifera seed. Water Research. 2005;39(11):2338–2344. doi: 10.1016/j.watres.2005.04.012. https://doi.org/10.1016/j.watres.2005.04.012 . PMid: 15921719. [DOI] [PubMed] [Google Scholar]
- 20.Suarez M, Haenni M, Canarelli S, Fisch F, Chodanowski P, Servis C, Michielin O, Moreillon P, Mermod N. Structure–function characterization and optimization of a plant- derived antibacterial peptide. Antimicrob Agents Chemother. 2005;49(9):3847–57. doi: 10.1128/AAC.49.9.3847-3857.2005. https://doi.org/10.1128/AAC.49.9.3847-3857.2005 . PMid: 16127062. PMCid: PMC1195432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez PL, Acosta AE, Mendez RL. A cluster analysis model for caries risk assessment. Arch Oral Biol. 2004;49:719–725. doi: 10.1016/j.archoralbio.2004.02.012. https://doi.org/10.1016/j.archoralbio.2004.02.012 . PMid: 15275859. [DOI] [PubMed] [Google Scholar]
- 22.Sumi Y, Miura H, Sunakawa M, Michiwaki Y, Sakagami N. Colonization of denture plaque by respiratory pathogens in dependent elderly. Gerodontology. 2002;19:25–9. doi: 10.1111/j.1741-2358.2002.00025.x. https://doi.org/10.1111/j.1741-2358.2002.00025.x . PMid: 12164235. [DOI] [PubMed] [Google Scholar]
- 23.Hahnel S, Rosentritt M, Handel G, Bürgers R. Influence of saliva substitute films on initial Streptococcus mutans adhesion to enamel and dental substrata. J Dent. 2008;36:977–983. doi: 10.1016/j.jdent.2008.08.004. https://doi.org/10.1016/j.jdent.2008.08.004 . PMid: 18789569. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg D, Eyal S. Early formation of Streptococcus sobrinus biofilm on various dental restorative materials. Journal of Dentistry. 2002;30(1):47–51. doi: 10.1016/s0300-5712(01)00058-6. https://doi.org/10.1016/S0300-5712(01)00058-6 . [DOI] [PubMed] [Google Scholar]
- 25.Aiyegoro OA, Akinpelu DA, Afolayan AJ, Okoh AI. Antibacterial activities of crude stem bark extracts of Distemonathus benthamianus Baill. J Bio Sci. 2008;8(2):356–361. https://doi.org/10.3923/jbs.2008.356.361 . [Google Scholar]
- 26.Ashafa AOT, Grieson DS, Afolayan AJ. Antimicrobial activity of extract from Felicia muricata Thunb. J Bio Sci. 2008;6:1062–1066. [Google Scholar]
- 27.Masika M, Julius P Voster. Antimicrobial activities of Moringa oleifera Lam leaf extracts. African Journal of Biotechnology. 2012;11(11):2797–2802. [Google Scholar]
- 28.Patel P, Patel N, Patel D, Desai S, Meshram D. Phytochemical analysis and antifungal activity of Moringa oliefera. International Journal of Pharmacy and Pharmaceutical Sciences. 2014;6(5):144–147. [Google Scholar]
- 29.Mahdi L H, Shafiq S A, Galoob A K, Muslem S N. Effect of plant extracted Moringa Oeifera Lam on some isolated pathogens from mouth and teeth. World Journal of Pharmaceutical Research. 2014;3(10):17–22. [Google Scholar]
- 30.Taylor JLS, Staden J. The effect of age season and growth conditions on anti- inflammatory activity in Eucomis autumnalis (Mill.). Chitt. Plant extracts. Plant Growth Regul. 2001;34(1):39–47. https://doi.org/10.1023/A:1013366926113 . [Google Scholar]
- 31.Rahman MS, Zerin LMN, Anwar MN. Antibacterial and antifungal activity of Moringa Oleifera stem bark. The Chittagong Univ. J B Sci. 2008;3(1 & 2):109–117. [Google Scholar]
- 32.Devedra BN, Sriniva N, Prasad V SS L, Latha PS. Antimicrobial activity of Moringa Oleifera Lam Leaf extract against selected bacterial and fungal strains. International Journal of Pharma and Bio Sciences. 2011;2(3):13–18. [Google Scholar]
- 33.Sato Y, Shibata H, Arai T, Yamamoto A, Okimura Y, Arakaki N, Higuti T. Variation in synergistic activity by flavones and its related compounds on the increased susceptibility of various strains of methicillin-resistant Staphylococcus aureus to β-lactam antibiotics. Int J Antimicrob Agents. 2004;24(3):226–233. doi: 10.1016/j.ijantimicag.2004.02.028. https://doi.org/10.1016/j.ijantimicag.2004.02.028 . PMid: 15325425. [DOI] [PubMed] [Google Scholar]
- 34.Cushine TPT, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrobial Agents. 2005;26(5):343–356. doi: 10.1016/j.ijantimicag.2005.09.002. https://doi.org/10.1016/j.ijantimicag.2005.09.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mboto CI, Eja ME, Adegoke AA, Iwatt GD, Asikong BE, Takon I, Udo SM, Akeh M. Phytochemical properties and antimicrobial activities of combined effect of extracts of the leaves of Garcinia Kola, Vernonia amygdalina and honey on some medically important microorganisms. Afr J Microbiol Res. 2009;3(9):557–559. [Google Scholar]
- 36.Vander F, Cummins D. Anti-plaque dentifrices: current status and prospects. Int Dent J. 1991;41(2):117–23. [PubMed] [Google Scholar]


