Abstract
AIM:
There are no reports regarding the influence of vitamin D on thymosin ß4 and the cluster of differentiation CD4 levels which are important for maintaining a healthy immune system. Consequently, we aimed to explore this relationship through a study.
MATERIAL AND METHODS:
The study was carried out on 35 subjects, screened for 25-hydroxy vitamin D[25 (OH) D] using ELISA method and they were divided into two groups: Group 1 consists of 10 healthy subjects with sufficient vit. D level > 24.8 ng/ml. Group 2 consists of 25 subjects suffering, severely, from vitamin D deficiency at level < 11.325 ng/ml. Also, Thymosin ß4, CD4 and zinc levels were performed.
RESULTS:
There were significant differences between the two groups in the concentration levels of thymosin β4, as the group 1 has shown higher levels (P = 0.005). Whereas, CD4 and zinc levels didn’t show any significant difference between the two groups. At the same time, a significant positive correlation has been observed between vitamin D, thymosin β4, and CD4 at (r = 0.719; P = 0.001), and (r = 0.559, P = 0.001) respectively.
CONCLUSION:
We concluded that vitamin D may be an essential factor that influence or determine the level of thymosin β4. This study is the first that focused on demonstrating that sufficient level of vitamin D may have the ability to influence the thymic hormone thymosin β4 levels. Further studies on large scale of subjects are needed to explore the positive correlation we had found between vitamin D and thymosin β4 and CD4.
Keywords: Vitamin D, thymosin beta-4, immunity
Introduction
The impact of Vitamin D has been observed in many aspects [1]. Regarding immune system and human health lower levels of vitamin D may cause of immune dysregulation [2]. In addition, Wei and Christakos in 2015 revealed that vitamin D may have immunomodulatory properties [3]. Vitamin D influences human health because vitamin D receptor and its activating enzyme 1-α-hydroxylase (CYP27B1) are expressed in activated lymphocytes, macrophages and dendritic cells [1]. This suggests an important impact of vitamin D especially in the field of human immunology [4-6]. Vitamin D intake is highly dependent on nutritional habits where 47% of vitamin D source come from food supplements [7].
Thymosin ß-4 is one of the thymic hormones [8], it is abundant in human cells and tissues, representing 70–80% of the total thymosin content [9] [10] it is an active peptide with 43 amino acids [8] it is omnipresent as intracellular protein, bind to and sequester G-actin to modulate cell migration [11]. Several physiological properties of Tβ4 have been reported; [12] repairing and remodeling of skin, neural system and heart tissues following injury [13], assisting in the development of B cells to plasma cells to produce antibodies [14] implicated in lymphocyte maturation and differentiation [9, 15], controlling cell morphogenesis and motility [16] preventing fibrosis [17], acting as a modulator of wound healing and inflammation [18] and regulating immunity [19]. Tβ4 is the major actin-sequestering molecule in all eukaryotic cells [20]. (Tβ4) is considered to play a significant role in the cellular metabolism due to its actin-sequestering properties [12].
A cluster of differentiation cells- often referred to as CD4 cells- are glycoprotein located on the surface of various types of immune cells restricted to T helper lymphocytes. It has an important function such as signal amplification and T- cell activation [21]. CD4 is a co-receptor that assists the T cell receptor (TCR) in communicating with an antigen-presenting cell [22].
Zinc was found to be necessary for a normal functioning of the immune system [23], altered zinc levels disturb the functions of innate immunity [24], and mild zinc deficiency depresses immunity [25].
This is the first investigation to describe the relationship between vitamin D deficiency, thymosin ß4, and CD4 levels.
Subjects and Methods
Throughout the period from January to March 2015 subjects were recruited from the outpatient clinic of the Centre of medical excellence at the NRC, where 40 subjects were screened by full medical history, thorough clinical examination, nutritional questionnaire, and anthropometric measurement. Screened subjects were enrolled into the study according to the following inclusion /exclusion criteria:
Inclusion: Age ranging from 18-40 years, both sexes, suffering from easy fatigability and lethargy and marked Vit D deficiency < 12 ng/ml.
Exclusion: Systemic diseases (cardiac, hepatic, renal, pulmonary, etc) malignancy of whatever nature, any autoimmune disease.
Thus, 25 subjects (males, females) suffering from easy fatiguability and lethargy, of average age of 27.28 ± 1.5 were enrolled into the study as group), in addition to 10 age and sex matched healthy subjects having normal levels of Vit D > 30 ng/ml as a control group (group 2).
Methods
Blood samples were drawn from all subjects to estimate complete blood picture. Plasma was separated for determination of vitamin D, thymosin β4, CD4 and trace element zinc and stored at -80°C until used.
Plasma 25(OH)D, thymosin β4, CD4 levels were determined using a commercial enzyme immunoassay according to the manufacturer instructions (Glory Science Co., Ltd, 2400 veterans Blvd. Suite 16-101, Del Rio, TX78840, USA), performed at National Research Centre medical physiology department serum vitamin D sufficiency was defined as > 20 ng/ml and severely deficient < 12 ng/ml according to the committee to review dietary reference intakes for vitamin D and calcium [26]. Zinc levels were estimated by spectrophotometric method (Salucea, Haansberg 19, 4874 NJ EttenLeur, Netherlands).
Statistical analysis
Independent sample student’s t-test (two tails)was used to determine the significant difference between the two groups and expressed as mean±SE Pearson correlations were used to analyse the association between 25(OH)D with CD4 and thymosin ß4, chi-square test for non-parametric data was performed to examine the relation between the number of subjects with energy and those lacking energy as a symptom of vitamin D deficiency.
Results
The total number of Group 1 was 10 (8 females and 2 males) and the total number of Group 2 was 25 (21 female and 4 males) as shown in Table 1.
Table 1.
Gender distribution
| Gender | Group 1 (n = 10) | Group 2 (n = 25) |
|---|---|---|
| Females | 8 (80%) | 21 (84%) |
| Males | 2 (20%) | 4 (16%) |
Age and BMI are shown in Table 2.
Table 2.
Age and BMI of subjects
| Parameters | Mean ± SD Group 1 N = 10 | Mean ± SD Group 2 N = 25 | Significance p |
|---|---|---|---|
| Age years | 28.5 ± 4.64 | 28 ± 7.51 | P = 0.636 NS |
| BMI Kg/m2 | 26.81 ± 3.89 | 27.41 ± 5.52 | P = 0.717 NS |
BMI:(body mass index); NS : Non-significant.
As shown in Table 3, group 1 had significantly higher 25 (OH) D levels in comparison to group 2 at (p < 0.001) the majority were deficient with 25 (OH) D levels. In addition there was a significant difference between thymosin beta 4 in group 1 where it has shown remarkable increase in comparison to group 2, in addition, an increased level of CD4 has been observed in group 1 in comparison to group 2 but this increase wasn’t statistically significant, zinc levels didn’t show any significant difference between the two groups.
Table 3.
Comparison between Group I and II regarding vitamin D, CD4, Thymosin ß4, and Zinc
| Parameters | Mean ± SE Group 1 N = 10 | Mean ± SE Group 2 N = 25 | Significance p |
|---|---|---|---|
| Vitamin D µg/l | 34.77 ± 2.82 | 5.16 ± 0.63 | P = 0.001 |
| CD4 pg/ml | 2.32 ± 0.88 | 0. 737 ± 0.05 | P = 0.108NS |
| Thymosinß4 ng/ml | 9.400E2 ± 202 | 1.844E2 ± 60.65 | P = 0.005 |
| Zinc µg/dl | 1.800E2 ± 9.35 | 2.06E2 ± 17.63 | P = 0.201 NS |
p < 0.001 = very highly significant difference; CD4: Cluster of differentiation 4.
Figure 1 demonstrate the significant positive correlations of this short study where Figure 1A and Figure 1B represent the positive correlation between vitamin D, thymosin ß-4 and CD4 (r = 0.719, p = 0.001, r = 0.559, p = 0.001 respectively). Where Figure 1C has shown a significant positive correlation between thymosinß-4 and CD4 at r = 0.755, p = 0.001.
Figure 1.
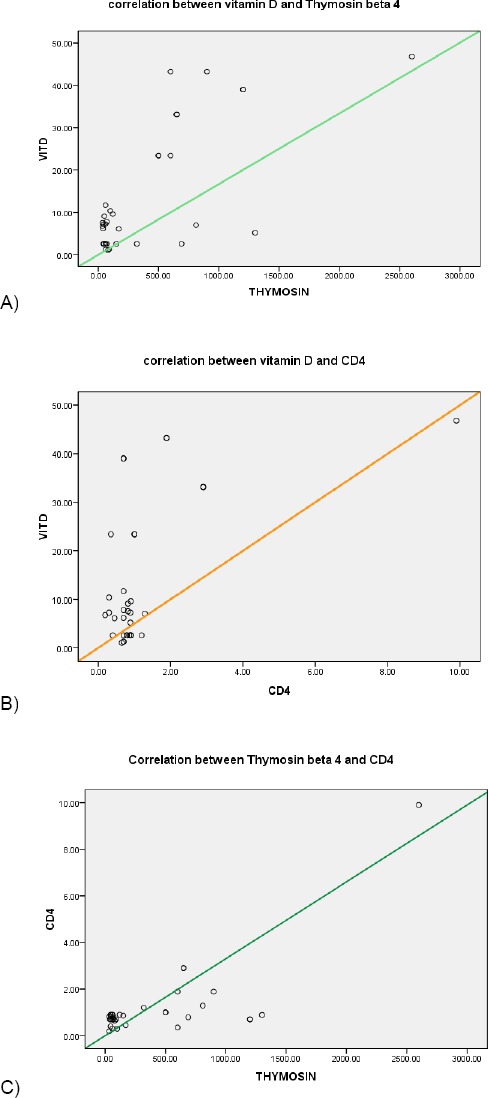
A) positive correlation between vitamin D and thymosin ß-4 at r=0.719, p= 0.001; B) positive correlation between vitamin D and CD4 at r=0. 559, p= 0.001; C) A positive correlation between Thymosin beta 4 and CD4 at r=0.755, p= 0.001
The results of a chi-square test of independence: A chi-square test of independence was performed to examine the relation between the number of subjects with energy and those lacking energy (lethargy) as a symptom of vitamin D deficiency, is shown in Figure 2. Subjects with vitamin D deficiency suffer from lethargy than sufficient subjects, the difference was significant, χ2 (1, N = 35) = 6.632, p < 0.001.
Figure 2.
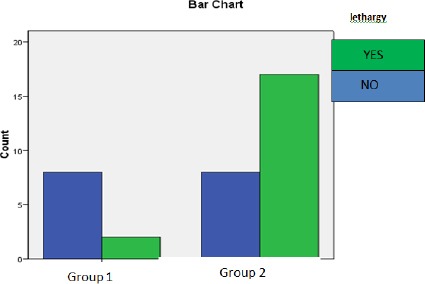
A bar chart showing the difference between Group 1 and 2 regarding lethargy
Discussion
In this study we identified that the majority of subjects were severely deficient in vitamin D where the ratio was 2:5 in which (28.58%) were sufficient and (71.42%) were severely deficient in vitamin D which could be attributed to blood sampling at the end of winter; an observation noted in the studies carried by both Anderson, and Pittawaw as they found vitamin D levels to depend on season [27] [28], because Vitamin D levels are in their lowest levels after winter and their higher at summer [29]. Our current study found a strongly significant correlation between vitamin D, Thymosin beta 4 and CD4. Thymosin beta 4 is the most abundant thymosin in human cells and tissues, it represents 70–80% of the total thymosin content [9] and implicated in lymphocyte maturation and differentiation [15] while vitamin D receptor VDR is found nearly in every tissue and cell type in the body [30] and resides in the cytoplasm in the absence of VDR ligands [31]. When stimulated with 1alpha,25-(OH)(2)D(2) or 1,25(OH)(2)D(3), VDR moves from the cytoplasm into the nucleus [31].
Thus, the strongly significant correlation between vitamin D, Thymosin beta 4 and CD4 found in this study may raise a speculation about a release of thymosin beta 4 secondary to vitamin D stimulated VDR in the thymus. The correlation found between vitamin D and CD4 could be explained that vitamin D has effects on adaptive immune cells because of the expression of the nuclear (vitamin D receptor) as well as vitamin D-activating enzymes in both T- and B-cells [32]. The VDR expression by these cells is very low in resting conditions but when activated, T- and B cells up-regulate VDR expression significantly, allowing regulation of up to 500 vitamin D responsive genes which influence their differentiation and proliferation [33] [34] therefore leading to a shift from a proinflammatory to a more tolerogenic immune status [35].
A recent study by Hewison who proposed that vitamin D influence on T cells function by the direct conversion of 25(OH)D to calcitriol by T-cells, and the effects of calcitriol on T-cells in which calcitriol have indirect effects on antigen presentation to T cells [36]. This study also revealed a strong positive correlation between thymosin β4 and CD4in agreement with Knutsen and colleagues in 1999 [37] which could be attributed to the fact that thymosin β4 is the predominant form of thymic hormones [38], and that its primary function is to stimulate the production of T- cells which are targets of thymosin activity [39]. In our study vitamin D was in positive correlation with CD4 -that represent helper cells - which has found to contain the significant amount of VDR [40]. Our study was in agreement with (Ritterhouse et al) [41] vitamin D regulates T-helper 1 (Th1) and dendritic cell function [42], which suggest that vitamin D support the innate and the adaptive immune system.
We didn’t find any significant difference regarding zinc levels between group 1 and 2 or any correlation between zinc and vitamin D, Thymosin beta-4 and CD4 because the sources of zinc like whole grains, cereals and legumes, was available for our subjects according to questionnaire; as whole grains are high in zinc [43]. In addition, a study conducted by Hess, 2007 revealed that zinc levels in the serum are not an indicator marker of zinc status because it is detectable in a population with risk and severe deficiency [44]. A recent study by Chiplokara and Kawade 2012 observed that zinc deficiency is very rare but moderate is widespread [45]. Vitamin D dietary intake is highly dependent on nutritional habits. However, a study with a global perspective found that 6 to 47% of vitamin D intake comes from dietary supplements [46] [7]. Thus, without supplementation, vitamin D status strongly will depend on endogenous vitamin D production which is also influenced by latitude, skin pigmentation, season, and lifestyle such as clothing [47] [48].
In conclusion, Vitamin D is obtained from limited dietary sources and the high vitamin D deficiency found in this study emphasises the importance of increased awareness and supplementation. It is apparent that vitamin D influences Tβ4 and CD4 levels so supplementation with vitamin D is essential to support immunity. More experimental trials in laboratories are needed to measure the levels of thymosin beta 4 in the compartments of thymus by its direct stimulation with vitamin D and measuring its concentration in vitro to explore the strong correlation found between vitamin D and thymosinβ4.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Battault S, Whiting SJ, Peltier SL, Sadrin S, Gerber G, Maixent JM. Vitamin D metabolism, functions and needs: From science to health claims. Eur J Nutr. 2013;52:429–441. doi: 10.1007/s00394-012-0430-5. https:/doi.org/10.1007/s00394-012-0430-5. PMid: 22886046. [DOI] [PubMed] [Google Scholar]
- 2.Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev. 2012;12:127–136. doi: 10.1016/j.autrev.2012.07.007. https:/doi.org/10.1016/j.autrev.2012.07.007 . PMid: 22776787. [DOI] [PubMed] [Google Scholar]
- 3.Wei R, Christakos S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients. 2015;7:8251–8260. doi: 10.3390/nu7105392. https:/doi.org/10.3390/nu7105392 . PMid: 26404359. PMCid: PMC4632412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–886. doi: 10.231/JIM.0b013e31821b8755. https:/doi.org/10.2310/JIM.0b013e31821b8755 . PMid: 21527855. PMCid: PMC3166406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Zvi I, Aranow C, Mackay M, Stanevsky A, Kamen DL, et al. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS One. 2010;5:e9193. doi: 10.1371/journal.pone.0009193. https:/doi.org/10.1371/journal.pone.0009193 . PMid: 20169063. PMCid: PMC2821911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerman M, Burnham J, Behrens E. 1,25 dihydroxyvitamin D3 limits monocyte maturation in lupus sera. Lupus. 2011;20:749–753. doi: 10.1177/0961203310394542. https:/doi.org/10.1177/0961203310394542 . PMid: 21447602. [DOI] [PubMed] [Google Scholar]
- 7.Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, Chope G, Hyppönen E, Berry J, Vieth R, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357–1364. doi: 10.3945/ajcn.111.031070. https:/doi.org/10.3945/ajcn.111.031070 . PMid: 22552031. PMCid: PMC3349454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landuyt B, Schoofs L, Luyten W, Arckens L. Thymosin beta 4 mRNA and peptide expression in phagocytic cells of different mouse tissues. Peptides. 2009;30:1822–1832. doi: 10.1016/j.peptides.2009.07.010. https:/doi.org/10.1016/j.peptides.2009.07.010 . PMid: 19631707. [DOI] [PubMed] [Google Scholar]
- 9.Huff T, Müller CS, Otto AM, Netzker R, Hannappel E. beta-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001;33:205–220. doi: 10.1016/s1357-2725(00)00087-x. https:/doi.org/10.1016/S1357-2725(00)00087-X . [DOI] [PubMed] [Google Scholar]
- 10.Hannappel E, Xu GJ, Morgan J, Hempstead J, Horecker BL. Thymosin beta 4: a ubiquitous peptide in rat and mouse tissues. Proc Natl AcadSci U S A. 1982;79:2172–2175. doi: 10.1073/pnas.79.7.2172. https:/doi.org/10.1073/pnas.79.7.2172 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bubb MR. Thymosin beta 4 interactions. Vitamins and hormones. 2003;66:297–316. doi: 10.1016/s0083-6729(03)01008-2. https:/doi.org/10.1016/S0083-6729(03)01008-2 . [DOI] [PubMed] [Google Scholar]
- 12.Piludu M, Piras M, Pichiri G, Coni P, Orrù G, Cabras T, Messana I, Faa G, Castagnola M. Thymosin Beta 4 May Translocate from the Cytoplasm in to the Nucleus in HepG2 Cells following Serum Starvation. An Ultrastructural Study. PLoS One. 2015;10(4):e0119642. doi: 10.1371/journal.pone.0119642. https:/doi.org/10.1371/journal.pone.0119642 . PMid: 25835495. PMCid: PMC4383617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu TJ, Wang Q, Ma XW, Zhang Z, Zhang W, Xue XC, Cun Zhang C, Hao Q, Li WN, Zhang YQ, Li M. A novel dimeric thymosin beta 4 with enhanced activities accelerates the rate of wound healing. Drug Des Devel Ther. 2013;7:1075–1088. doi: 10.2147/DDDT.S50183. PMid: 24109178. PMCid: PMC3792846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Górski A, Korczak-Kowalska G, Nowaczyk M, Gaciong Z, E Skopińska-Rózewska E. Thymosin: an immunomodulator of antibody production in man. Immunology. 1982;47(3):497–501. PMid: 6215339. PMCid: PMC1555552. [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein AL. History of the discovery of the thymosins. Ann N Y Acad Sci. 2007;1112:1–13. doi: 10.1196/annals.1415.045. https:/doi.org/10.1196/annals.1415.045 . PMid: 17600284. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein AL, Hannappel E, Sosne G, Kleinman HK. Thymosin β4: a multi-functional regenerative peptide. Basic properties and clinical applications. Expert Opin Biol Ther. 2012;12(1):37–51. doi: 10.1517/14712598.2012.634793. https:/doi.org/10.1517/14712598.2012.634793 . PMid: 22074294. [DOI] [PubMed] [Google Scholar]
- 17.Bock-Marquette I, Saxena A, White MD, DiMaio JM, Srivasta D. Thymosin beta 4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. https:/doi.org/10.1038/nature03000 . PMid: 15565145. [DOI] [PubMed] [Google Scholar]
- 18.Freeman KW, Bowman BR, Zetter BR. Regenerative protein thymosin-4 is a novel regulator of purinergic signaling. FASEB J. 2011;25:907–915. doi: 10.1096/fj.10-169417. https:/doi.org/10.1096/fj.10-169417. PMid: 21106936. [DOI] [PubMed] [Google Scholar]
- 19.Rath NC, Kannan L, Liyanage R, Lay JrJO. Thymosin beta in macrophage J Endocrinol Reprod. 2007;2:55–61. [Google Scholar]
- 20.Ballweber E, Hannappel E, Huff T, Stephan H, Haener M, Taschner N, et al. Polymerisation of chemically cross-linked actin: thymosin beta(4) complex to filamentous actin: alteration in helical parameters and visualisation of thymosin beta(4) binding on F-actin. J Mol Biol. 2002;315:613–625. doi: 10.1006/jmbi.2001.5281. https:/doi.org/10.1006/jmbi.2001.5281 . PMid: 11812134. [DOI] [PubMed] [Google Scholar]
- 21.Wang M, He HJ, Turko IV, Phinney KW, Wang L. Quantifying the cluster of differentiation 4 receptor density on human T lymphocytes using multiple reaction monitoring mass spectrometry. Anal Chem. 2013;85(3):1773–7. doi: 10.1021/ac3031306. https:/doi.org/10.1021/ac3031306 . PMid: 23286534. [DOI] [PubMed] [Google Scholar]
- 22.Ansari-Lari MA, Muzny DM, Lu J, Lu F, Lilley CE, Spanos S, Malley T, Gibbs RA. A gene-rich cluster between the CD4 and triosephosphate isomerase genes at human chromosome 12p13. Genome Res. 1996;6(4):314–26. doi: 10.1101/gr.6.4.314. https:/doi.org/10.1101/gr.6.4.314 . PMid: 8723724. [DOI] [PubMed] [Google Scholar]
- 23.Rink L, Gabriel P. Zinc and the immune system. Proc Nutr Soc. 2000;59(4):541–52. doi: 10.1017/s0029665100000781. https:/doi.org/10.1017/S0029665100000781 . PMid: 11115789. [DOI] [PubMed] [Google Scholar]
- 24.Maares M, Haase H. Zinc and immunity: An essential interrelation. Arch Biochem Biophys. 2016 doi: 10.1016/j.abb.2016.03.022. pii: S0003-9861(16)30074-1. [DOI] [PubMed] [Google Scholar]
- 25.Barnett JB, Dao MC, Hamer DH, Kandel R, Brandeis G, Wu D, Dallal GE, Jacques PF, Schreiber R, Kong E, Meydani SN. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2016;103(3):942–51. doi: 10.3945/ajcn.115.115188. https:/doi.org/10.3945/ajcn.115.115188 . PMid: 26817502. [DOI] [PubMed] [Google Scholar]
- 26.Committee to Review Dietary Reference Intakes for Vitam D and Calcium IoM Dietary Reference Intakes for Calcium and Vitamin D. Washington, D.C: The National Academies Press; 2011. pp. 1–1116. [Google Scholar]
- 27.Andersen R, Brot C, Jakobsen J, Mejborn H, Mølgaard C, Skovgaard LT, Trolle E, Tetens I, Ovesen L. Seasonal changes in vitamin D status among Danish adolescent girls and elderly women: The influence of sun exposure and vitamin D intake. Eur J Clin Nutr. 2013;67:270–274. doi: 10.1038/ejcn.2013.3. https:/doi.org/10.1038/ejcn.2013.3 . PMid: 23388663. [DOI] [PubMed] [Google Scholar]
- 28.Pittaway JK, Ahuja KDK, Beckett JM, Bird M-L, Robertson IK, Ball MJ. Make vitamin D while the sun shines, take supplements when it doesn’t: A longitudinal, observational study of older adults in Tasmania, Australia. PLoS One. 2013;8:e59063. doi: 10.1371/journal.pone.0059063. https:/doi.org/10.1371/journal.pone.0059063 . PMid: 23527088. PMCid: PMC3601102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross AC, Taylor CL, Yaktine AL, Valle HBD. Washington (DC): National Academies Press (US); 2011. Dietary Reference Intakes for Calcium and Vitamin D Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. [PubMed] [Google Scholar]
- 30.Bikle DD. Vitamin D Metabolism, Mechanism of Action, and Clinical applications. Chemistry & Biology. 2014;21(3):319–329. doi: 10.1016/j.chembiol.2013.12.016. https:/doi.org/10.1016/j.chembiol.2013.12.016 . PMid: 24529992. PMCid: PMC3968073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu-Wong JR, Nakane M, Ma J, Dixon D, Gagne G, Vitamin D. receptor (VDR) localization in human promyelocytic leukemia cells. Leuk Lymphoma. 2006;47(4):727–32. doi: 10.1080/10428190500398898. https:/doi.org/10.1080/10428190500398898 . PMid: 16690532. [DOI] [PubMed] [Google Scholar]
- 32.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. https:/doi.org/10.1126/science.6310748 . PMid: 6310748. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. https:/doi.org/10.4049/jimmunol.179.3.1634 . PMid: 17641030. [DOI] [PubMed] [Google Scholar]
- 34.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. https:/doi.org/10.1002/jcb.10580 . PMid: 12874827. [DOI] [PubMed] [Google Scholar]
- 35.Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and Immune Function. Nutrients. 2013;5(7):2502–2521. doi: 10.3390/nu5072502. https:/doi.org/10.3390/nu5072502 . PMid: 23857223. PMCid: PMC3738984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hewison M. An update on vitamin D and human immunity. Clin Endocrinol. 2012;76:315–325. doi: 10.1111/j.1365-2265.2011.04261.x. https:/doi.org/10.1111/j.1365-2265.2011.04261.x . PMid: 21995874. [DOI] [PubMed] [Google Scholar]
- 37.Knutsen AP, Freeman JJ, Mueller KR, Roodman ST, Bouhasin JD. Thymosin-α1 stimulates maturation of CD34+stem cells into CD3+4+cells in an in vitro thymic epithelia organ coculture model. Int J Immunopharmacol. 1999;21:15–26. doi: 10.1016/s0192-0561(98)00060-5. https:/doi.org/10.1016/S0192-0561(98)00060-5 . [DOI] [PubMed] [Google Scholar]
- 38.Galy AH, Hadden EM, Touraine JL, Hadden JW. Effects of cytokines on human thymic epithelial cells in culture: IL1 induces thymic epithelial cell proliferation and change in morphology. Cell Immunol. 1989;124(1):13–27. doi: 10.1016/0008-8749(89)90108-1. https:/doi.org/10.1016/0008-8749(89)90108-1 . [DOI] [PubMed] [Google Scholar]
- 39.Kouttab NM, Goldstein A, Lu M, Lu L, Campbell B, Maizel AL. Production of human B and T cell growth factors is enhanced by thymic hormones. Immunopharmacology. 1988;16:97–105. doi: 10.1016/0162-3109(88)90018-5. https:/doi.org/10.1016/0162-3109(88)90018-5 . [DOI] [PubMed] [Google Scholar]
- 40.Deluca HF, Cantorna MT. Vitamin D its role and uses in immunology. The FASEB Journal. 2001;15(14):2579–2585. doi: 10.1096/fj.01-0433rev. https:/doi.org/10.1096/fj.01-0433rev . PMid: 11726533. [DOI] [PubMed] [Google Scholar]
- 41.Ritterhouse LL, Lu R, Shah HB, Robertson JM, Fife DA, Maecker HT, Du H, et al. Vitamin D Deficiency in a Multiethnic Healthy Control Cohort and Altered Immune Response in Vitamin D Deficient European-American Healthy Controls. PloS one. 2014;9(4):e94500. doi: 10.1371/journal.pone.0094500. https:/doi.org/10.1371/journal.pone.0094500 . PMid: 24727903. PMCid: PMC3984168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyakh LA, Sanford M, Chekol S, et al. TGF- beta and vitamin D3 utilize distinct pathways to suppress IL-12 production and modulate rapid differentiation of human monocytes into CD83+dendritic cells. J Immunol. 2005;174:2061–2070. doi: 10.4049/jimmunol.174.4.2061. https:/doi.org/10.4049/jimmunol.174.4.2061 . PMid: 15699136. [DOI] [PubMed] [Google Scholar]
- 43.Yamada T, Alpers DH, et al. Textbook of gastroenterology. 5th ed. Chichester, West Sussex: Blackwell Pub; 2009. p. 495. 498, 499, 1274, 2526. [Google Scholar]
- 44.Hess SY, Peerson JM, King JC, Brown KH. Use of serum zinc concentration as an indicator of population zinc status. Food and nutrition bulletin. 2007;28(3 Suppl):S403–29. doi: 10.1177/15648265070283S303. https:/doi.org/10.1177/15648265070283S303 . PMid: 17988005. [DOI] [PubMed] [Google Scholar]
- 45.Chiplonkar SA, Kawade R. Effect of zinc- and micronutrient-rich food supplements on zinc and vitamin A status of adolescent girls. Nutrition. 2012;28(5):551–8. doi: 10.1016/j.nut.2011.08.019. https:/doi.org/10.1016/j.nut.2011.08.019 . PMid: 22129855. [DOI] [PubMed] [Google Scholar]
- 46.Calvo MS, Whiting SJ. Overview of the proceedings from Experimental Biology 2004 symposium: Vitamin D insufficiency: A significant risk factor in chronic diseases and potential disease-specific biomarkers of vitamin D sufficiency. J Nutr. 2005;135:301–303. doi: 10.1093/jn/135.2.301. PMid: 15671231. [DOI] [PubMed] [Google Scholar]
- 47.Malinda KM, Sidhu GS, Mani H, Banaudha K, Maheshwari RK, et al. Thymosin beta 4 accelerates wound healing. J Invest Dermatol. 1999;113:364–368. doi: 10.1046/j.1523-1747.1999.00708.x. https:/doi.org/10.1046/j.1523-1747.1999.00708.x . PMid: 10469335. [DOI] [PubMed] [Google Scholar]
- 48.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. https:/doi.org/10.1016/S0140-6736(10)60588-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]


