Abstract
Immunization with merozoite surface protein 4/5 (MSP4/5), the murine malaria homologue of Plasmodium falciparum MSP4 and MSP5, has been shown to protect mice against challenge by parasites expressing the homologous form of the protein. The gene encoding MSP4/5 was sequenced from a number of Plasmodium yoelii isolates in order to assess the level of polymorphism in the protein. The gene was found to be highly conserved among the 13 P. yoelii isolates sequenced, even though many of the same isolates showed pronounced variability in their MSP119 sequences. Nonsynonymous mutations were detected only for the isolates Plasmodium yoelii nigeriensis N67 and Plasmodium yoelii killicki 193L and 194ZZ. Immunization and challenge of BALB/c mice showed that the heterologous MSP4/5 proteins were able to confer a level of protection against lethal Plasmodium yoelii yoelii YM challenge infection similar to that induced by immunization with the homologous MSP4/5 protein. To explore the limits of heterologous protection, mice were immunized with recombinant MSP4/5 protein from Plasmodium berghei ANKA and Plasmodium chabaudi adami DS and challenged with P. y. yoelii YM. Interestingly, significant protection was afforded by P. berghei ANKA MSP4/5, which shows 81% sequence identity with P. y. yoelii YM MSP4/5, but it was abolished upon reduction and alkylation. Significant protection was not observed for mice immunized with recombinant P. c. adami DS MSP4/5, which shows 55.7% sequence identity with P. y. yoelii YM MSP4/5. This study demonstrates the robustness of MSP4/5 in conferring protection against variant forms of the protein in a murine challenge system, in contrast to the situation found for other asexual-stage proteins, such as MSP119 and AMA1.
Malaria is the most important parasitic disease infecting humans, resulting in ∼300 to 500 million clinical cases and 1 to 3 million deaths per year (30). Plasmodium falciparum is responsible for the most severe and fatal form of malaria. Due to the emergence of drug-resistant malaria parasites and insecticide-resistant mosquito vectors, there is an urgent need to develop a vaccine capable of reducing both morbidity and mortality from infection, as well as the transmission of drug-resistant malaria parasites.
Twenty years of antigen identification and characterization has yielded many potential vaccine candidates (26), but there is still no vaccine against human malaria. A recognized problem is the high level of polymorphism of antigens that are targets of an antimalarial immune response, especially those exposed on the surface of the merozoite and the infected red blood cell (3, 7, 13). Indeed, in rodent malaria challenge studies, two leading vaccine candidates, MSP119 (28, 29) and AMA1 (10), were found to protect from homologous but not heterologous challenge. The ability to confer protection against infection by parasites expressing variant forms of an antigen would be the hallmark of a robust vaccine that would have an extended life span.
Two recently characterized P. falciparum merozoite surface proteins, MSP4 (22) and MSP5 (35), are candidates for inclusion in a blood stage malaria vaccine. We have identified the rodent malaria homologue of these genes (MSP4/5) (4), and all the predicted proteins show structural similarities, including an N-terminal signal sequence, a C-terminal glycosylphosphatidylinositol (GPI) anchor, and a single epidermal growth factor (EGF)-like domain (4, 16). A membrane-bound, surface-exposed location on the merozoite has been demonstrated by Triton X-114 partitioning and immunofluorescence (4, 16).
In contrast to many blood stage antigen genes, msp4 and msp5 show limited sequence diversity. The mature protein of msp5 has no reported diversity among the P. falciparum isolates examined (35). Only limited antigenic diversity for MSP4 has been detected, with nine residues exhibiting polymorphism (34). It is not known whether this conservation is due to functional constraints or a lack of immune (or other) selection pressure.
Recent studies utilizing recombinant MSP4/5 expressed in Escherichia coli have demonstrated its efficacy in protecting BALB/c mice against a lethal Plasmodium yoelii yoelii YM blood stage challenge (17, 19). The present study aimed to extend these findings by determining the ability of MSP4/5 to confer protection against heterologous challenge. We first determined the level of polymorphism present in the sequence of MSP4/5 from various rodent malaria isolates and expressed variant sequences as recombinant proteins in E. coli. Mice were immunized, and antibodies were used to determine cross-reactivity by Western blotting and enzyme-linked immunosorbent assay (ELISA) against the heterologous proteins. The protective efficacies of the variant proteins compared to that of P. y. yoelii YM MSP4/5 were determined by challenging immunized mice with the lethal parasite line P. y. yoelii YM. To explore the limits of heterologous protection, mice were immunized with MSP4/5 from the rodent malaria parasite species Plasmodium berghei ANKA and Plasmodium chabaudi adami DS, which show 81 and 55.7% sequence identity with MSP4/5 of the P. y. yoelii YM challenge strain, respectively.
MATERIALS AND METHODS
Experimental animals, parasites,and genomic DNA.
Female BALB/c mice aged 6 to 8 weeks were purchased from Central Animal Services (Monash University, Victoria, Australia). Michael Good (Queensland Institute of Medical Research, Brisbane, Australia) kindly provided the P. y. yoelii YM and 17XNL parasites. P. y. yoelii 1AR, P. y. yoelii 2BG, and Plasmodium yoelii killicki 193L parasite stabilates were kindly provided by David Walliker (University of Edinburgh, Edinburgh, Scotland). P. y. yoelii 265BY genomic DNA was kindly provided by Laurent Renia (INSERM, CHU Pitie-Salpetriere, Paris, France). Genomic DNAs from the P. yoelii isolates 193L, 194ZZ, N67, 1AR, 2BR, 2CL, 33X, 3AE, 17XB, and 17XS were prepared as described previously (2). The primary isolates were obtained from thicket rats (Thamnomys rutilans) at different locations and times (2), with the exception of P. y. yoelii 17XB and 17XS, which are avirulent lines derived from cloning of 17XL.
Sequencing of MSP4/5 from various P. yoelii isolates and cloning into an E. coli expression vector.
Sequencing was performed using the PRISM BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, Calif.) according to the manufacturer's instructions. Sequencing reaction products were separated on a model 373 automated DNA sequencer (Applied Biosystems, Branchburg, N.J.). PCRs were performed using Ampli-Taq Gold (Applied Biosystems, Branchburg, N.J.), and the PCR products were directly sequenced on both strands. When polymorphisms were identified, direct sequencing of PCR products was repeated to confirm the sequence. The sequences were analyzed by Sequencher software version 3.1.1 (Gene Codes Corp., Ann Arbor, Mich.), and alignments were compiled using Eclustalw (WebANGIS; The Australian Genomic Information Centre, The University of Sydney, Sydney, New South Wales, Australia).
For recombinant-protein expression, all genes lacking the predicted signal and GPI anchor sequence were cloned into the pTrcHisA vector (Invitrogen Corp., Carlsbad, Calif.) as previously described (16). Primers p648 and p623 (Table 1) were used to amplify msp4/5 from genomic DNAs of the various P. yoelii isolates. These primers were designed from the sequence encoding the N-terminal signal and C-terminal GPI anchor sequences of P. y. yoelii YM MSP4/5. PCRs were performed under the following conditions: one cycle of 94°C for 3 min; 35 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min; and one cycle of 72°C for 5 min. Splice overlap extension PCR (12) was performed on P. y. killicki 193L and Plasmodium yoelii nigeriensis N67 with primers p623, p624, p625, and p648 (Table 1) to remove the intron. PCR with primers p626 and p1106 (Table 1) was then used to remove the predicted signal and anchor sequences from the original PCR product before cloning it into pUC18 and sequencing it. A clone with the correct sequence was selected, digested with NcoI/BglII, and cloned into NcoI/BglII-digested pTrcHisA. Error-free recombinant plasmids were then transformed into E. coli BL21(DE3) (Novagen, Milwaukee, Wis.) for protein expression.
TABLE 1.
Primers used in this study for PCR amplification of msp4/5 from various P. yoelii isolates
| Primer | Sequencea |
|---|---|
| p623 | 5′ GTTAATATATCATGGAAACTAAAA 3′ |
| p624 | 5′ AATCGCCATTTGTATTACCTGGTTGTACTGAAGGTT 3′ |
| p625 | 5′ AACCTTCAGTACAACCAGGTAATACAAATGGCGATT 3′ |
| p626 | 5′ GGAAGATCTTTAATGATGATGATGATGATGTGAATGCGCACTGAGTAATTCG 3′ |
| p648 | 5′ AAAAAAATGAAAATCACAAATTAC 3′ |
| p1106 | 5′ GGCATGCCATGGTTCATGAAACTTCTTTAA 3′ |
Restriction endonuclease recognition sites are underlined.
Recombinant six-His-tagged fusion protein expression and purification.
Purification of six-His-tagged proteins was conducted using TALON Metal Affinity Resin (Clontech, Palo Alto, Calif.) as described previously (16). Protein purity and integrity were assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the gels were stained with Coomassie brilliant blue. The protein concentration was determined using the Bio-Rad (Hercules, Calif.) protein assay following the manufacturer's instructions. When required, proteins were concentrated to the required volume using an Ultrafree-15 centrifugal filter device (Millipore Corp., Bedford, Mass.) with a 10,000-Da molecular mass cutoff and stored at −80°C.
Vaccination and challenge infection.
Groups of female BALB/c mice were immunized with 25 μg of recombinant MSP4/5 protein emulsified in complete Freund's adjuvant (Difco Laboratories, Detroit, Mich.) administered intraperitoneally (i.p.). Two subsequent boosters of antigen emulsified in incomplete Freund's adjuvant (Difco Laboratories) were delivered i.p. at monthly intervals. Control mice were injected with phosphate-buffered saline (PBS) emulsified in the appropriate Freund's adjuvant. Sera were collected before the initial injection (preimmunization sera) and 2 days before challenge (prechallenge sera). Twelve to 14 days after the final injection, the mice were challenged i.p. with 105 P. y. yoelii YM-parasitized red blood cells. Parasitemia was monitored microscopically by Giemsa-stained thin blood smears, and the percent parasitemia was determined by counting 500 cells per slide.
Immunochemical assays.
Antibody responses were determined by ELISA as previously described (33) using E. coli-derived P. y. yoelii YM MSP4/5 as the coating antigen. Western blotting was performed as previously described (16) using the various E. coli-derived MSP4/5 proteins.
Statistics.
All statistical analyses were carried out using GraphPad (San Diego, Calif.) Prism software. To determine significant differences in survivor outcome between groups, Fisher's exact test was performed. For determining significant differences in peak parasitemia or prechallenge antibody levels, the Mann-Whitney U test was performed. The Spearman rank correlation test was used to check the association between optical density values of prechallenge sera and peak parasitemia and between percent amino acid identity and peak parasitemia, as well as between percent amino acid identity and percent survival.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper are available in the GenBank database under the accession numbers AY563008 to AY563020.
RESULTS
Sequence analysis of msp4/5 proteins from various P. yoelii isolates.
The gene encoding MSP4/5 was sequenced in a number of P. yoelii isolates in order to assess the level of polymorphism in the gene. Deduced amino acid sequences (lacking the N-terminal signal and C-terminal GPI anchor sequences) were compared by an Eclustalw alignment (Fig. 1). Nonsynonymous mutations relative to P. y. yoelii YM were observed only for the isolates P. y. nigeriensis N67, P. y. killicki 193L, and P. y. killicki 194ZZ. P. y. killicki 193L and 194ZZ have the same MSP119 sequence, and the MSP4/5 sequence was found to be identical. The most variant forms were P. y. killicki 193L and 194ZZ, with eight nonsynonymous mutations and two synonymous mutations, and P. y. nigeriensis N67, with nine nonsynonymous mutations and four synonymous mutations. There was a single synonymous point mutation detected in the EGF-like domains of P. y. yoelii 17XB, 3AE, and 2BR.
FIG. 1.
Alignment of predicted MSP4/5 protein sequences from various P. yoelii isolates and clones. The full sequence comprising the extracellular domain from residues 23 to 193 is shown for P. y. yoelii YM, the parasite line that was used for all challenge experiments. The EGF-like domain is indicated in boldface. For the other parasite proteins, identical residues are indicated by dots and amino acids differing from those of P. y. yoelii YM are indicated.
All amino acid sequence variations observed in MSP4/5 were due to point mutations, except for one case in which an aspartic acid residue was inserted into the aspartic acid-glutamic acid repeat region of P. y. nigeriensis N67. Amino acid substitutions were clustered in the N terminus of MSP4/5, whereas the EGF-like domain was in general conserved, except for P. y. nigeriensis N67, in which there is a residue substituted in the EGF-like domain. In all cases, the six cysteine residues in the EGF-like domain were completely conserved.
Sequence variation was also observed in the intron with five single-point mutations, a 1-bp deletion, and a 16-bp deletion observed in P. y. killicki 193L and 194ZZ. There were seven point mutations and a 1-bp deletion detected for P. y. nigeriensis N67. P. y. yoelii 1AR and 2BG have identical 29-bp insertions in the intron but no other sequence variation. The introns of the remainder of the strains are completely conserved.
Analysis of recombinant MSP4/5 proteins from various rodent malaria isolates expressed in E. coli.
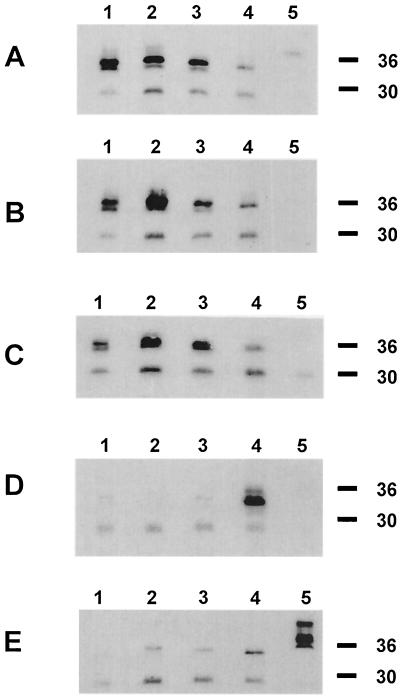
The MSP4/5 proteins from P. y. yoelii YM, P. y. killicki 193L, P. y. nigeriensis N67, P. berghei ANKA, and P. c. adami DS were expressed as recombinant His-tagged proteins in E. coli. The purified proteins were analyzed by Western blotting with an anti-His antibody and sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing and nonreducing conditions, and each generally had a unique pattern of bands (data not shown). P. y. yoelii YM and P. y. killicki 193L MSP4/5 were exceptions, as they had identical banding patterns. Under reducing conditions, P. y. yoelii YM and P. y. killicki 193L MSP4/5 both migrate at ∼36 kDa. P. y. nigeriensis N67 and P. berghei ANKA MSP4/5 migrate at just below 36 kDa. P. c. adami DS MSP4/5 migrates at ∼40 kDa.
In order to ascertain the comparative immunoreactivities of the variant MSP4/5 proteins, immunoblotting was performed using polyclonal sera raised to the recombinant proteins on reducing (Fig. 2) and nonreducing (data not shown) gels. Antibodies raised to recombinant P. y. yoelii YM, P. y. killicki 193L, and P. y. nigeriensis N67 MSP4/5 cross-reacted strongly with each other. The reactivity of each of the sera with recombinant P. berghei ANKA MSP4/5 was reduced, while reactivity was absent for recombinant P. c. adami DS MSP4/5, except for the P. y. yoelii YM antisera, which showed low levels of reactivity. Antibodies raised to recombinant P. berghei ANKA MSP4/5 weakly recognized P. y. yoelii YM and P. y. nigeriensis N67 MSP4/5, but not P. y. killicki 193L or P. c. adami DS MSP4/5. Antibodies raised to recombinant P. c. adami DS MSP4/5 faintly recognized P. berghei ANKA, P. y. nigeriensis N67, and P. y. killicki 193L MSP4/5, but not P. y. yoelii YM MSP4/5. The band migrating at 30 kDa has been determined to be a contaminating E. coli protein present in all purifications (16).
FIG. 2.
Immunoblot analysis of purified E. coli-derived MSP4/5-His proteins from P. y. yoelii YM (lane 1), P. y. killicki 193L (lane 2), P. y. nigeriensis N67 (lane 3), P. berghei ANKA (lane 4) and P. c. adami DS (lane 5). The proteins were reduced prior to being loaded. The membrane was probed with mouse antisera diluted 1:2,500 raised against P. y. yoelii YM (A), P. y. killicki 193L (B), P. y. nigeriensis N67 (C), P. berghei ANKA (D), and P. c. adami DS (E) MSP4/5. Molecular mass standards (in kilodaltons) are on the right.
Under nonreducing conditions, broad cross-reactivity was observed among all recombinant proteins (data not shown). This broad cross-reactivity was also observed by ELISA when prechallenge sera were tested against yeast-derived P. y. yoelii YM MSP4/5 (19) and when serum raised against P. y. yoelii YM parasites was reacted with the various recombinant MSP4/5 proteins (data not shown).
Immunization of mice with variant MSP4/5 proteins and challenge with P. y. yoelii YM.
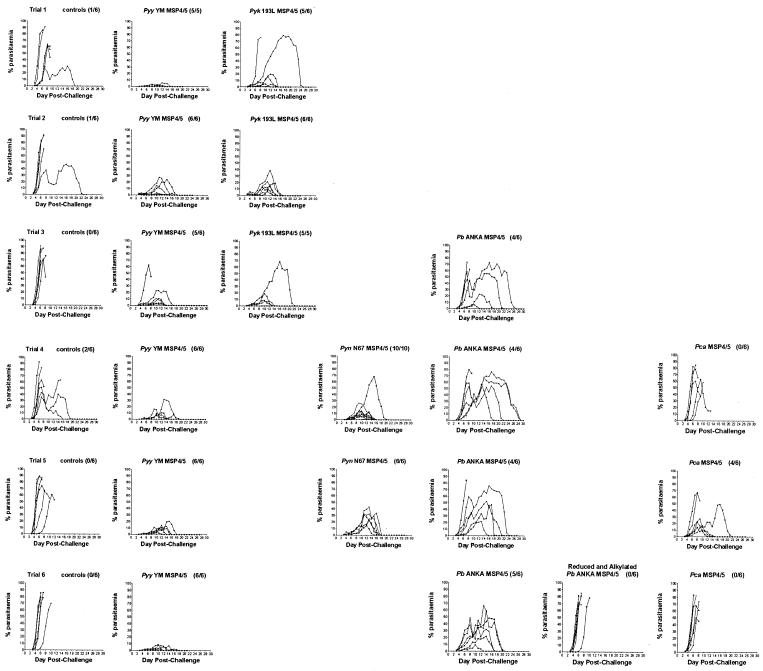
Groups of BALB/c mice were immunized with three doses of PBS (control) or 25 μg of recombinant MSP4/5-His protein emulsified in adjuvant at monthly intervals. Two weeks after the final immunization, the mice were challenged with 105 P. y. yoelii YM-parasitized red blood cells, and the percent parasitemia was determined daily starting at day 3. Six separate vaccination trials were performed (Fig. 3), and they are summarized in Tables 2 and 3. Statistical comparisons of survival outcome using Fisher's exact test and peak parasitemias using the Mann-Whitney test are summarized in Table 4.
FIG. 3.
Blood-stage parasitemia in groups of mice in trials 1 to 6. Each trial is displayed horizontally, and groups immunized with identical preparations are aligned vertically. The number of survivors out of the total number of mice is shown in brackets next to the title of each graph. Pyy, P. y. yoelii; Pyk, P. y. killicki; Pyn, P. y. nigeriensis; Pb, P. berghei ANKA; Pca, P. c. adami.
TABLE 2.
Summary of vaccination trials
| Trial no. | Immunizing antigena | Peak parasitemiab
|
Survival (no. of survivors/total no.) | ||
|---|---|---|---|---|---|
| Range (%) | Mean (%) | Days | |||
| 1 | Controls | 30-91 | 66 | 6-16 | 1/6 |
| P. y. yoelii YM MSP4/5 | 0-6 | 2 | 8-12 | 5/5 | |
| P. y. killicki 193L MSP4/5 | 4-79 | 33 | 8-19 | 5/6 | |
| 2 | Controls | 47-92 | 78 | 6-16 | 1/6 |
| P. y. yoelii YM MSP4/5 | 2-27 | 12 | 10-16 | 6/6 | |
| P. y. killicki 193L MSP4/5 | 6-39 | 19 | 9-14 | 6/6 | |
| 3 | Controls | 68-92 | 80 | 6-8 | 0/6 |
| P. y. yoelii YM MSP4/5 | 2-23 | 19 | 7-12 | 5/6 | |
| P. y. killicki 193L MSP4/5 | 0.2-69 | 21 | 10-16 | 5/5 | |
| P. berghei ANKA MSP4/5 | 6-73 | 49 | 7-16 | 4/6 | |
| 4 | Controls | 42-92 | 66 | 6-15 | 2/6 |
| P. y. yoelii YM MSP4/5 | 0.6-32 | 12 | 9-17 | 6/6 | |
| P. y. nigeriensis N67 MSP4/5 | 1-68 | 17 | 9-15 | 10/10 | |
| P. berghei ANKA MSP4/5 | 54-80 | 65 | 8-17 | 4/6 | |
| P. c. adami DS MSP4/5 | 58-85 | 71 | 6-10 | 0/6 | |
| 5 | Controls | 61-89 | 78 | 6-11 | 0/6 |
| P. y. yoelii YM MSP4/5 | 6-20 | 12 | 10-15 | 6/6 | |
| P. y. nigeriensis N67 MSP4/5 | 14-43 | 29 | 13-18 | 6/6 | |
| P. berghei ANKA MSP4/5 | 22-84 | 56 | 8-16 | 4/6 | |
| P. c. adami DS MSP4/5 | 14-67 | 39 | 8-18 | 4/6 | |
| 6 | Controls | 70-86 | 80 | 6-10 | 0/6 |
| P. y. yoelii YM MSP4/5 | 0-9 | 5 | 10-16 | 6/6 | |
| P. berghei ANKA MSP4/5 | 9-67 | 40 | 10-15 | 5/6 | |
| RA P. berghei MSP4/5 | 71-85 | 80 | 6-10 | 0/6 | |
| P. c. adami DS MSP4/5 | 50-83 | 71 | 6-8 | 0/6 | |
The amount of immunizing antigen was 25 μg. RA, reduced and alkylated.
Number of parasites per 500 red blood cells counted; all mice were challenged with P. y. yoelii YM.
TABLE 3.
Summary of relationships among percent amino acid identity, survival, and average peak parasitemia for mice immunized with various recombinant MSP4/5 proteins and challenged with P. y. yoelii YM
| Parameter | Valuea
|
||||||
|---|---|---|---|---|---|---|---|
| Control mice | P. y. yoelii YM MSP4/5 immunized | P. y. killicki 193L MSP4/5 immunized | P. y. nigeriensis N67 MSP4/5 immunized | P. berghei ANKA MSP4/5 immunized | Reduced and alkylated P. berghei ANKA MSP4/5 immunized | P. c. adami MSP4/5 immunized | |
| Amino acid identity with P. y. yoelii YM MSP4/5 (%) | N/A | 100 | 95.3 | 94.7 | 81 | 81 | 55.7 |
| No. survived/total | 4/36 | 34/35 | 16/17 | 16/16 | 17/24 | 0/6 | 4/18 |
| % Survival | 11 | 97 | 94 | 100 | 71 | 0 | 22 |
| Avg % peak parasitemia ± SEM | 74 ± 2.6 | 16 ± 2.9 | 24 ± 6.2 | 22 ± 4.3 | 53 ± 4.6 | 80 ± 2.0 | 60 ± 5.3 |
N/A, not applicable.
TABLE 4.
Statistical significance of differences in peak parasitemia and survival outcome of mice immunized with various recombinant MSP4/5 proteins and challenged with P. y. yoelii YM
| Immunizing MSP4/5 antigen | Parameter | Significancec
|
||||
|---|---|---|---|---|---|---|
| Control mice | P. y. yoelii YM MSP4/5 | P. y. killicki 193L MSP4/5 | P. y. nigeriensis N67 MSP4/5 | P. berghei ANKA MSP4/5 | ||
| P. y. yoelii YM | Peak parasitemiaa | P < 0.0001 (SD) | ||||
| Survivalb | P < 0.0001 (SD) | |||||
| P. y. killicki 193L | Peak parasitemia | P < 0.0001 (SD) | P = 0.0328 (SD) | |||
| Survival | P < 0.0001 (SD) | P = 1.5152 (NSD) | ||||
| P. y. nigeriensis N67 | Peak parasitemia | P < 0.0001 (SD) | P = 0.1093 (NSD) | P = 0.8854 (NSD) | ||
| Survival | P < 0.0001 (SD) | (NSD) | ||||
| P. berghei ANKA | Peak parasitemia | P = 0.0001 (SD) | P < 0.0001 (SD) | P = 0.0013 (SD) | P = 0.0004 (SD) | |
| Survival | P < 0.0001 (SD) | P < 0.0479 (SD) | ||||
| Reduced and alkylated P. berghei ANKA | Peak parasitemia | P = 0.9372 (NSD) | P = 0.0022 (SD) | |||
| Survival | (NSD) | P = 0.0022 (SD) | ||||
| P. c. adami DS | Peak parasitemia | P = 0.4522 (NSD) | P = 0.0003 (SD) | P = 0.0046 (SD) | P = 0.0017 (SD) | P = 0.1547 (NSD) |
| Survival | P = 0.6581 (NSD) | P < 0.0001 (SD) | P = 0.0044 (SD) | |||
Statistical analysis of peak parasitemias was performed with the Mann-Whitney U test.
Statistical analysis of survival outcome was performed with Fisher's exact test.
NSD, no significant difference; SD, significant difference (P < 0.05).
There was a statistically significant inverse correlation between percent amino acid identity and peak parasitemia (Spearman rank correlation coefficient; r = −0.9747; P = 0.0167). When survival for each challenge was considered, there was a statistically significant correlation between percent amino acid identity and percent survival (Spearman rank correlation test; r = 0.8053; P < 0.0001).
Analysis of antibody responses of immunized mice.
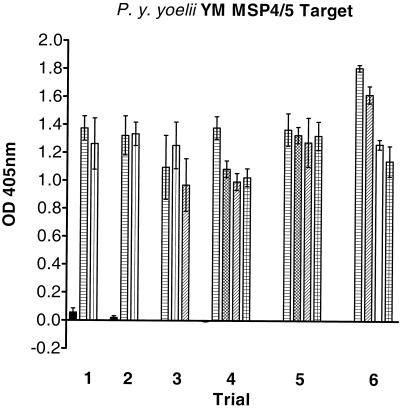
The prechallenge antibody responses of the control and recombinant-protein-immunized mice were determined by ELISA using recombinant P. y. yoelii YM MSP4/5 protein as the target (Fig. 4). The Mann-Whitney test was used to determine statistical significance between antibody responses. There was a statistically significant difference in antibody response between the control mice and the P. y. yoelii YM, P. y. killicki 193L, P. y. nigeriensis N67, P. berghei ANKA, and P. c. adami DS MSP4/5-immunized mice. For the first three trials (analyzed separately), there was no statistically significant difference among antibody responses for the P. y. yoelii YM, P. y. killicki 193L, and P. berghei ANKA MSP4/5-immunized mice. Statistically significant differences were evident for trial 4. P. y. yoelii YM MSP4/5-immunized mice had a statistically significantly higher antibody response than mice immunized with P. y. nigeriensis N67, P. berghei ANKA, and P. c. adami DS MSP4/5. For trial 5, there were no statistically significant differences in antibody levels. For trial 6, the antibody levels of mice immunized with P. berghei ANKA, reduced and alkylated P. berghei ANKA, and P. c. adami MSP4/5 were significantly lower than those of the P. y. yoelii YM MSP4/5-immunized mice.
FIG. 4.
Prechallenge antibody levels of immunized mice. Mice were immunized with PBS (adjuvant control; solid bars), recombinant E. coli-derived MSP4/5-His from P. y. yoelii YM (horizontally hatched bars), P. y. killicki 193L (vertically hatched bars), P. y. nigeriensis N67 (cross-hatched bars), P. berghei ANKA (diagonally hatched bars), reduced and alkylated P. berghei ANKA (open bars), and P. c. adami DS (checkered bars). The sera were diluted 1:5,000 in duplicate, and antibody levels were determined by ELISA on E. coli-derived P. y. yoelii YM MSP4/5. The mean optical density (OD) values at 405 nm are indicated, and the error bars represent standard errors of the mean. There were three, four, and five groups of mice in trials 1 and 2, trial 3, and trials 4, 5, and 6, respectively.
There was a statistically significantly higher prechallenge antibody response in the surviving mice immunized with P. berghei ANKA (P = 0.0422) and P. c. adami (P = 0.0203) MSP4/5.
There was a statistically significant inverse correlation between the antibody level and peak parasitemia for P. y. yoelii YM (r = −0.3442; P = 0.0429), P. berghei ANKA (r = −0.5558; P = 0.0048), and P. c. adami DS (r = −0.6670; P = 0.0025) MSP4/5-immunized mice, but not for P. y. killicki 193L, or P. y. nigeriensis N67 MSP4/5-immunized mice.
DISCUSSION
Limited sequence diversity was identified in the msp4/5 genes from 13 P. yoelii isolates, even though many of the same isolates differ markedly in their MSP119 sequences (ranging from 2 to 22% in a 98-amino-acid stretch) (2). Nonsynonymous mutations were detected only for the isolates P. y. killicki 193L and 194ZZ and P. y. nigeriensis N67. Compared to P. y. yoelii YM MSP4/5, the greatest variations in sequence were detected for P. y. killicki 193L and 194ZZ and P. y. nigeriensis N67. P. y. killicki 193L and 194ZZ MSP4/5 differs from that of P. y. yoelii YM at 8 amino acids out of 171 (4.7%), whereas P. y. nigeriensis N67 MSP4/5 differs from that of the challenge strain at 9 amino acid positions out of 171 (5.3%). The MSP119 sequences of these strains differ from that of the challenge strain by 14 out of 98 (14.3%) and 22 out of 98 (22.4%) amino acids, respectively. As determined for several other antigens of Plasmodium spp., there were more nonsynonymous than synonymous mutations observed (3, 13).
While the mature MSP5 protein was found to be completely conserved across a number of isolates (35), the sequence variations identified in MSP4/5 and MSP4 were found to be similar. In both cases, they is located in the N-terminal region of the proteins, are due to point mutations, and occur at similar rates (overall, 3.3% for MSP4) (34). Only one amino acid change was identified in the EGF-like domain of MSP4/5 in a single P. yoelii isolate (P. y. nigeriensis N67). Similarly, only one amino acid change was identified in the EGF-like domain of MSP4 of P. falciparum (34).
Cross-reactivity in the antibody response to the recombinant proteins was explored by immunoblotting and ELISA. Under reducing conditions, immunoblots probed with polyclonal antisera raised to the recombinant MSP4/5 proteins showed strong cross-reactivity between P. y. yoelii YM, P. y. killicki 193L, and P. y. nigeriensis N67 MSP4/5 and marked differences in cross-reactivity for recombinant P. berghei ANKA and P. c. adami DS MSP4/5, which show 81 and 55.7% identity with that of the P. y. yoelii YM challenge strain, respectively (Fig. 2). The cross-reactivity observed among P. y. yoelii YM, P. berghei ANKA, and P. c. adami DS MSP4/5 is similar to that observed previously for parasite lysates (16) and appears to be related to the percent amino acid identity.
In contrast, broad cross-reactivity was observed by immunoblotting of the nonreduced proteins (data not shown). Similarly, broad cross-reactivity was apparent by ELISA, where prechallenge sera were tested against recombinant P. y. yoelii YM MSP4/5 protein (Fig. 4) or titrated on yeast-derived P. y. yoelii YM MSP4/5 (data not shown) and where convalescent phase sera raised in mice to a Triton X-114 detergent phase extract of P. y. yoelii YM parasites was titrated out on the recombinant MSP4/5 proteins (data not shown). Overall, the clear hierarchy of reactivity related to the primary amino acid sequence was not evident when nonreduced recombinant proteins were analyzed. The levels of cross-reactivity were very similar and did not appear to be consistently related to percent amino acid identity.
There was a statistically significant difference between survival outcomes in the control mice and mice immunized with the recombinant MSP4/5 proteins of P. y. yoelii YM, P. y. killicki 193L, P. y. nigeriensis N67, and P. berghei ANKA. This implies that immunization with any of the variant recombinant proteins, except that of P. c. adami DS, provides greater protection in terms of survival outcome than adjuvant alone. There was no statistically significant difference between survival outcomes in the P. y. yoelii YM, P. y. killicki 193L,and P. y. nigeriensis N67 MSP4/5-immunized mice, but survival was significantly reduced for P. berghei ANKA MSP4/5-immunized mice.
The effects of antigenic differences on protective efficacy were most pronounced with respect to peak parasitemia. P. y. yoelii YM MSP4/5-immunized mice had a peak parasite burden significantly lower than those of the P. y. killicki 193L and P. berghei ANKA MSP4/5-immunized mice, while there was no significant difference from that of P. y. nigeriensis N67 MSP4/5-immunized mice. P. berghei ANKA MSP4/5-immunized mice had a statistically significant higher peak parasite burden compared to P. y. killicki 193L and P. y. nigeriensis N67 MSP4/5-immunized mice, but perhaps surprisingly, it was not statistically significantly different from that of P. c. adami DS MSP4/5-immunized mice. The peak parasite burdens of P. y. killicki 193L and P. y. nigeriensis N67 MSP4/5-immunized mice were not significantly different.
Overall, there was a statistically significant inverse correlation between the percent amino acid identity and the peak parasitemia (Spearman rank correlation coefficient; r = −0.9747; P = 0.0167). This suggests that with respect to the peak parasite burden, the heterologous recombinant MSP4/5 proteins are not as efficacious in controlling peak parasitemia as the homologous protein. There was also a statistically significant correlation between percent amino acid identity and percent survival (Spearman rank correlation coefficient; r = 0.8053; P < 0.0001). This suggests that with respect to survival outcome, the heterologous recombinant MSP4/5 proteins are not as efficacious in conferring protection as the homologous protein. Furthermore, as the recombinant proteins become more divergent in their primary amino acid sequences, the protective efficacy they afford decreases accordingly. Interestingly, there was a statistically significant inverse correlation between peak parasitemia and antibody level for P. y. yoelii YM, P. berghei ANKA, and P. c. adami DS MSP4/5-immunized mice but not for P. y. killicki 193L or P. y. nigeriensis N67 MSP4/5-immunized mice. There were also statistically significantly higher prechallenge antibody responses in the surviving mice immunized with P. berghei ANKA and P. c. adami MSP4/5, suggestive of antibody-mediated protective efficacy.
The near-complete conservation of the EGF-like domain of MSP4/5, MSP4, and MSP5 contrasts with findings reported for MSP119 and AMA1, where polymorphism exists in disulfide-bonded regions (11, 15, 23, 25, 31). This may be related to these conformational structures being critical for protection, as demonstrated by reduction and alkylation experiments (1, 20, 21). In contrast, in homologous challenge studies, reduced and alkylated MSP4/5 is still able to afford protection (17). Furthermore, in rodent malaria challenge studies, MSP119 (28) and AMA1 (10) did not protect from heterologous challenge in their recombinant forms. There were differences in 17 out of 96 (17.7%) and 36 out of 567 (6.4%) amino acids, respectively, between the immunizing antigen and the challenge strain. For MSP119, this difference is much greater than the 8 amino acids out of 120 (6.7%) known to be polymorphic to a limited extent for P. falciparum (14, 15, 27, 32). However for AMA1, this difference is only slightly greater than the largest difference between the products of two alleles for P. falciparum, which is 32 out of 622 amino acids (5.1%) (23).
Antibody cross-reactivity for P. y. yoelii YM, P. berghei ANKA, and P. c. adami DS MSP4/5 is reduction sensitive, and for P. berghei ANKA MSP4/5, so too is protection. The EGF-like domain is the only structure predicted to be reduction sensitive from sequence analysis, and when compared across the three species, this domain differs at only 6 amino acids out of 37 (16%) (16). Given this level of conservation, and the reduction-sensitive nature of cross-reactivity, it is perhaps surprising that P. berghei ANKA MSP4/5 confers protection against P. y. yoelii YM challenge whereas P. c. adami DS MSP4/5 does not. Previous reduction and alkylation experiments using P. y. yoelii MSP4/5 have found that homologous protection is not dependent on the conformation of the EGF-like domain of MSP4/5, although peak parasitemias were elevated (17). It could be argued that the difference between the parasite burdens in mice in the reduced and nonreduced recombinant protein preparations equates to the protection afforded by reduction-sensitive conformation; however, there is no difference in survival outcome. Perhaps the antibody responses can be divided into two groups, those directed against reduction-insensitive linear epitopes and those directed against reduction-sensitive epitopes, and both types of response alone are capable of affording some degree of homologous protection. However the EGF-like domain alone, expressed cytoplasmically in E. coli as a glutathione-S-transferase fusion or expressed periplasmically as a maltose binding protein fusion, conferred almost no protection (data not shown). Indeed, it appears that the full-length molecule is required to confer protection.
For MSP4, reduction and alkylation appear to transmit a conformational change through the molecule that alters antibody binding at epitopes remote from the EGF-like domain (33). We hypothesize that the tertiary structure of MSP4/5 may depend upon a partially or completely folded EGF-like domain. As recombinant P. y. yoelii YM and P. berghei ANKA MSP4/5 provide significant protection but P. c. adami DS MSP4/5 does not, comparing the primary amino acid sequences may highlight important protective N-terminal epitopes whose conformations, and perhaps functions, depend on an intact EGF-like domain. This type of analysis identifies blocks of similarity throughout the N terminus of MSP4/5 which would be useful to compare with structural data. Other considerations are that P. c. adami MSP4/5 may be too divergent to provide significant protection or may provide protection only if high antibody levels are achieved. Indeed, the four mice immunized with P. c. adami MSP4/5 that survived challenge had statistically significantly higher prechallenge antibody responses.
It was anticipated that differences in the primary amino acid sequence would result in decreased antibody binding and affinity, and consequently decreased protective immunity. Although no clear hierarchy in cross-reactivity related to the primary amino acid sequence was identified for nonreduced protein, the protective efficacy of the recombinant proteins is consistent with the hierarchy of polymorphism. The nature of the reduction-sensitive conformation, as well as the affinities and fine specificities of antibodies raised to these regions, has not been investigated and may provide an explanation for the different outcomes in P. berghei ANKA and P. c. adami DS MSP4/5-immunized mice, as may exploring the role of T-cell immunity in conferring protection.
Previous studies have found that mice immunized with whole-parasite preparations were afforded various degrees of heterologous protection within the rodent malaria parasite pairs Plasmodium vinckei-P. chabaudi and P. berghei-P. yoelii, and to a lesser extent between these two groups (8, 9, 24). Although different mechanisms of immunity (for example, cellular versus antibody) are implicated as being important in clearing P. yoelii and P. chabaudi infections and may in some cases have a component that is nonspecific in nature (6), the degree of protection loosely follows the phylogenic relatedness of the rodent malaria parasites (8, 9, 24). It is interesting that this study supports this finding.
This study demonstrates the robustness of a single recombinant antigen, MSP4/5, in conferring protection against a heterologous malaria challenge. There remains the question of its limits in terms of longevity of protection and how much the formulation can be improved, for example, by immunizing with antigen combinations (18), refolding (5), or using different adjuvants. The nature of the reduction-sensitive epitopes shared by P. y. yoelii YM, P. berghei ANKA, and P. c. adami DS MSP4/5 and how they relate to protection also need to be addressed. If such immunization regimes are to be adopted for P. falciparum MSP4 and MSP5, it must be in conjunction with adjuvants suitable for human use and capable of inducing an enduring immune response of sufficient magnitude and suitable type.
Acknowledgments
We thank Michael Good and David Walliker for kindly supplying parasite stabilates, Pearline Benjamin and Sola Ogun for parasite growth and DNA preparation, Irene Ling for parasite growth and DNA preparation and critical reading of the manuscript, and Laurent Renia for supplying P. y. yoelii 265BY genomic DNA.
This work was supported by research project grants from the National Health and Medical Research Council and the UNDP-World Bank-WHO Special Program for Research Training in Tropical Diseases (TDR). M.W.G. is the recipient of an Australian Postgraduate Award scholarship.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Anders, R. F., P. E. Crewther, S. Edwards, M. Margetts, M. L. Matthew, B. Pollock, and D. Pye. 1998. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 16:240-247. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin, P. A., I. T. Ling, G. Clottey, L. M. Valero, S. A. Ogun, S. L. Fleck, D. Walliker, W. D. Morgan, B. Birdsall, J. Feeney, and A. A. Holder. 1999. Antigenic and sequence diversity at the C terminus of the merozoite surface protein-1 from rodent malaria isolates, and the binding of protective monoclonal antibodies. Mol. Biochem. Parasitol. 104:147-156. [DOI] [PubMed] [Google Scholar]
- 3.Black, C. G., and R. L. Coppel. 2000. Synonymous and non-synonymous mutations in a region of the Plasmodium chabaudi genome and evidence for selection acting on a malaria vaccine candidate. Mol. Biochem. Parasitol. 111:447-451. [DOI] [PubMed] [Google Scholar]
- 4.Black, C. G., L. Wang, A. R. Hibbs, E. Werner, and R. L. Coppel. 1999. Identification of the Plasmodium chabaudi homologue of merozoite surface proteins 4 and 5 of Plasmodium falciparum. Infect. Immun. 67:2075-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns, J. M., C. C. Belk, and P. D. Dunn. 2000. A protective glycosylphosphatidylinositol-anchored membrane protein of Plasmodium yoelii trophozoites and merozoites contains two epidermal growth factor-like domains. Infect. Immun. 68:6189-6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, I. A., A. C. Allison, and F. E. G. Cox. 1976. Protection of mice against Babesia and Plasmodium with BCG. Nature 259:309-311. [DOI] [PubMed] [Google Scholar]
- 7.Coppel, R. L., K. M. Davern, and M. J. McConville. 1994. Immunochemistry of parasite antigens, p. 475-532. In C. J. van Oss and M. H. V. van Regenmortel (ed.), Immunochemistry. Marcel Dekker Inc., New York, N.Y.
- 8.Cox, F. E. G. 1970. Protective immunity between malarial parasites and piroplasms in mice. Bull. W. H. O. 43:325-336. [PMC free article] [PubMed] [Google Scholar]
- 9.Cox, F. E. G., and A. Voller. 1966. Cross-immunity between the malaria parasites of rodents. Ann. Trop. Med. Parasitol. 60:297-303. [DOI] [PubMed] [Google Scholar]
- 10.Crewther, P. E., M. L. Matthew, R. H. Flegg, and R. F. Anders. 1996. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect. Immun. 64:3310-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly, T. M., J. M. Burns, and C. A. Long. 1992. Comparison of the carboxy terminal, cysteine-rich domain of the merozoite surface protein 1 from several strains of Plasmodium yoelii. Mol. Biochem. Parasitol. 52:279-282. [DOI] [PubMed] [Google Scholar]
- 12.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 13.Hughes, M. K., and A. L. Hughes. 1995. Natural selection on Plasmodium surface proteins. Mol. Biochem. Parasitol. 71:99-113. [DOI] [PubMed] [Google Scholar]
- 14.Jongwutiwes, S., K. Tanabe, and H. Kanbara. 1993. Sequence conservation in the C-terminal part of the precursor to the major merozoite surface proteins (MSP1) of Plasmodium falciparum from field isolates. Mol. Biochem. Parasitol. 59:95-100. [DOI] [PubMed] [Google Scholar]
- 15.Kang, Y., and C. A. Long. 1995. Sequence heterogeneity of the C-terminal, Cys-rich region of the merozoite surface protein-1 (MSP-1) in field samples of Plasmodium falciparum. Mol. Biochem. Parasitol. 73:103-110. [DOI] [PubMed] [Google Scholar]
- 16.Kedzierski, L., C. G. Black, and R. L. Coppel. 2000. Characterization of the merozoite surface protein 4/5 gene of Plasmodium berghei and Plasmodium yoelii. Mol. Biochem. Parasitol. 105:137-147. [DOI] [PubMed] [Google Scholar]
- 17.Kedzierski, L., C. G. Black, and R. L. Coppel. 2000. Immunization with recombinant Plasmodium yoelii merozoite surface protein 4/5 protects mice against lethal challenge. Infect. Immun. 68:6034-6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kedzierski, L., C. G. Black, M. W. Goschnick, A. W. Stowers, and R. L. Coppel. 2002. Immunization with a combination of merozoite surface proteins 4/5 and 1 enhances protection against lethal challenge with Plasmodium yoelii. Infect. Immun. 70:6606-6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kedzierski, L., C. G. Black, A. W. Stowers, M. W. Goschnick, D. C. Kaslow, and R. L. Coppel. 2001. Comparison of the protective efficacy of yeast-derived and E. coli-derived recombinant merozoite surface protein 4/5 against lethal challenge by Plasmodium yoelii. Vaccine 19:4661-4668. [DOI] [PubMed] [Google Scholar]
- 20.Ling, I. T., S. A. Ogun, and A. A. Holder. 1994. Immunization against malaria with a recombinant protein. Parasitol. Immunol. 16:63-67. [DOI] [PubMed] [Google Scholar]
- 21.Majarian, L. H., T. M. Daly, W. P. Weindanz, and C. A. Long. 1984. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J. Immunol. 132:3131-3137. [PubMed] [Google Scholar]
- 22.Marshall, V. M., A. Silva, M. Foley, S. Cranmer, L. Wang, D. J. McColl, D. J. Kemp, and R. L. Coppel. 1997. A second merozoite surface protein (MSP4) of Plasmodium falciparum that contains an epidermal growth factor-like domain. Infect. Immun. 65:4460-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall, V. M., L. X. Zhang, R. F. Anders, and R. L. Coppel. 1996. Diversity of the vaccine candidate AMA-1 of Plasmodium falciparum. Mol. Biochem. Parasitol. 77:109-113. [DOI] [PubMed] [Google Scholar]
- 24.McColm, A. A., and L. Dalton. 1983. Heterologous immunity in rodent malaria: comparison of the degree of cross-immunity generated by vaccination with that produced by exposure to live infection. Ann. Trop. Med. Parasitol. 77:355-377. [DOI] [PubMed] [Google Scholar]
- 25.Miller, L. H., T. Roberts, M. Shahabuddin, and T. F. McCutchan. 1993. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein (MSP-1). Mol. Biochem. Parasitol. 59:1-14. [DOI] [PubMed] [Google Scholar]
- 26.Nussenzweig, R. S., and C. A. Long. 1994. Malaria vaccines: multiple targets. Science 265:1381-1383. [DOI] [PubMed] [Google Scholar]
- 27.Qari, S. H., Y. P. Shi, I. F. Goldman, B. L. Nahlen, M. Tibayrene, and A. A. Lal. 1998. Predicted and observed alleles of Plasmodium falciparum merozoite surface protein 1 (MSP-1), a potential malaria vaccine antigen. Mol. Biochem. Parasitol. 92:241-252. [DOI] [PubMed] [Google Scholar]
- 28.Renia, L., I. T. Ling, M. Marussig, F. Miltgen, A. A. Holder, and D. Mazier. 1997. Immunization with a recombinant C-terminal fragment of Plasmodium yoelii merozoite surface protein 1 protects mice against homologous but not heterologous P. yoelii sporozoite challenge. Infect. Immun. 65:4419-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotman, H. L., T. M. Daly, and C. A. Long. 1999. Plasmodium: immunization with carboxyl-terminal regions of MSP-1 protects against homologous but not heterologous blood-stage parasite challenge. Exp. Parasitol. 91:78-85. [DOI] [PubMed] [Google Scholar]
- 30.Sachs, J., and P. Malaney. 2002. The economic and social burden of malaria. Nature 415:680-685. [DOI] [PubMed] [Google Scholar]
- 31.Thomas, A. W., A. P. Waters, and D. Carr. 1990. Analysis of variation in PF83, an erythrocytic merozoite vaccine candidate antigen of Plasmodium falciparum. Mol. Biochem. Parasitol. 42:285-288. [DOI] [PubMed] [Google Scholar]
- 32.Tolle, R., H. Bujard, and J. A. Cooper. 1995. Plasmodium falciparum: variations within the C-terminal region of merozoite surface antigen-1. Exp. Parasitol. 81:47-54. [DOI] [PubMed] [Google Scholar]
- 33.Wang, L., C. G. Black, V. M. Marshall, and R. L. Coppel. 1999. Structural and antigenic properties of merozoite surface protein 4 of Plasmodium falciparum. Infect. Immun. 67:2193-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, L., V. M. Marshall, and R. L. Coppel. 2002. Limited polymorphism of the vaccine candidate merozoite surface protein 4 of Plasmodium falciparum. Mol. Biochem. Parasitol. 120:301-303. [DOI] [PubMed] [Google Scholar]
- 35.Wu, T., C. G. Black, L. Wang, A. R. Hibbs, and R. L. Coppel. 1999. Lack of sequence diversity in the gene encoding merozoite surface protein 5 of Plasmodium falciparum. Mol. Biochem. Parasitol. 103:243-250. [DOI] [PubMed] [Google Scholar]