Abstract
BACKGROUND:
Goodpasture syndrome was originally described as an association of alveolar haemorrhage and glomerulonephritis. It occurs when the immune system attacks and destroys healthy body tissue.
AIM:
We are presenting a patient with a clinical picture of pulmonary haemorrhage and glomerulonephritis, which is diagnosed by renal biopsy.
CASE PRESENTATION:
His illness began a year and a half before being diagnosed. In that period he had occasional exacerbations. He was received at our Clinic in extremely serious condition, and after stabilisation of his medical condition, there was made a biopsy of the kidney. The p-ANCA was 8.93 U/ml (neg < 3, poz > 5 U/ml). Histopathological diagnosis of biopsy of the kidney was: Glomerulonephritis extra capillaries focalis, segmentalis et globalis. Based on this he was diagnosed with Goodpasture syndrome. He received corticosteroid therapy and cyclophosphamide, with good response to treatment, and he is currently in a stable condition, receiving only corticosteroid therapy.
CONCLUSION:
Goodpasture syndrome is a severe illness caused by the formation of antibodies to the glomerular basement membrane and alveolus with consequential damage to renal and pulmonary function. With current therapy, long-term survival is more than 50%.
Keywords: alveolar haemorrhage, glomerulonephritis, immune system, anti-glomerular basement membrane (anti-GBM) antibodies, Goodpasture syndrome
Introduction
Goodpasture syndrome was originally described as an association of alveolar haemorrhage and glomerulonephritis. It occurs when the immune system attacks and destroys healthy body tissue, so it is defined as an autoimmune disease. Gender distribution is reported differently in different studies, according to some dates it is twice more in men than in women, and the age at presentation can range from the first to the ninth decade. Goodpasture syndrome is manufactured by rapidly progressive glomerulonephritis and alveolar haemorrhage in association with the presence of anti-glomerular basement membrane (anti-GBM) antibodies and another capillary basement membrane. Although it is rare, it is very severe immunological disease [1, 2].
Like other autoimmune conditions, the anti-GBM disease is thought to result from an environmental insult in a person with genetic susceptibility. The human leukocyte antigen (HLA) serotype HLA-DR15 has been strongly associated with the anti-GBM disease. An initial insult to the pulmonary vasculature is required for exposure of the alveolar capillaries to the anti-GBM antibodies. Environmental factors that may lead to such exposure include the following: exposure to organic solvents or hydrocarbons, smoking, infection (eg, influenza A2), cocaine inhalation, exposure to metal dust, lymphocyte depletion therapy, such as alemtuzumab, extracorporeal shock wave lithotripsy [3-5].
Symptoms: constitutional symptoms (eg, malaise, chills and fever, arthralgias) may precede or develop concurrently with pulmonary or renal manifestations; hemoptysis is the presenting symptom when the disease affects the lungs. The level of hemoptysis may vary and, in a small percentage of patients, may be absent. Other pulmonary symptoms include a cough, dyspnea, and shortness of breath. Massive pulmonary haemorrhage leading to respiratory failure may occur. Chest pain is present in less than half of the patients. Renal manifestations include hematuria, oedema, high blood pressure and eventually uremia. Significant anaemia may result from persistent intrapulmonary bleeding [6].
Physical examination findings in patients with the anti-GBM disease include the following: tachypnea, inspiratory crackles over lung bases, cyanosis, hepatosplenomegaly (may be present), hypertension (present in 20% of cases), rash, oedema [6].
Diagnosis can be established the bay presence of pulmonary haemorrhage, pulmonary radiography, kidney biopsy and positive resultants of anti-GBM antibodies. The treatment of this syndrome should be initiated as soon as possible using a combination of corticosteroid therapy, cytostatics and plasmapheresis [7].
Case Report
The patient DS, male, born in 1967, has been admitted to the Clinic of Pulmonology and Allergology on May 15th, 2016. On inspection severely ill, presenting with a productive cough, expectoration mixed with blood, chills, and fever, which has been present for three days and measured increased body temperature up to 39°C. The patient has complained of nausea, fatigue, and chest tightness, as well. Formerly he had worked as a driver; presently he works with automobile lacquers and has a 10 pack-year smoking history.
He has sought medical advice at his primary care health practitioner since 2014 when he was referred to the Pulmonology Outpatient’s Clinic, whereas he has been prescribed antibiotics. He has been treated at the outpatient’s clinic on several occasions because of fever, nausea, and cough with bloody expectoration, malaise and sideropenic anaemia. These symptoms and signs have been repetitive the last year. He has been investigated at the Clinic of Rheumatology, suspected of the diagnosis of Gilbert syndrome, as well. Later he has been referred to a nephrologist because of the occurrence of proteinuria, hematuria and increased values of serum urea and serum creatinine. Therefore, a kidney biopsy has been indicated, for which the patient has not given his consent to be performed (April, the 14th, 2016). A computer tomography of the chest has been performed using intravenous contrast, with a series of scans during the arterial phase, with the following findings: interstitial fibrous changes bilaterally “ground-glass” opacities of the lung parenchyma, bilaterally with centrilobular emphysema in the proximal parts (April, the 28th, 2016).
The last admission has been at the department of infectious diseases of the clinical hospital in Kumanovo, and because of the severity of the general condition he has been referred to the University Clinic of Pulmonology and Allergy in May, the 15th, 2016. He has been admitted in a severely ill condition, presenting with fever (39°C) the last three days, headaches, vertigo, cough accompanied by hemoptysis, tachypneic, tachycardia, severe dyspnoea and malaise. The patient has shown decreased oxygen saturation (SatO2 = 50%) and hypoxemia (PaO2 = 6.35 kPa), anemia (Hgb = 7.9 g/L; Hct = 23.2%, RBC = 2.7 x 10^12/L; WBC = 16.9 x 10^9/L), ESR = 60 mm/h, urea = 13.9 mmol/L, creatinine = 206 mmol/L, total proteins = 56 g/L, albumins = 33 g/L, Fe in serum = 8.1 mmol/L, CRP = 134 U/L, troponins = 777 U/L, CK = 144 U/L, CK-MB = 29 U/L, LDH = 686 U/L, proteinuria, hematuria. Chest X-ray found a massive, diffuse lung consolidation bilaterally, with reactive hila (Fig. 1, 2).
Figure 1.
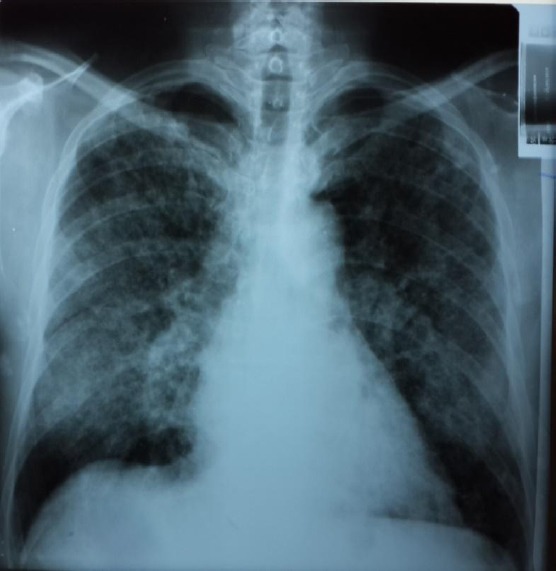
RTG Posteranterior: Chest X-ray found a massive, diffuse lung consolidation bilaterally, with reactive hila
Figure 2.
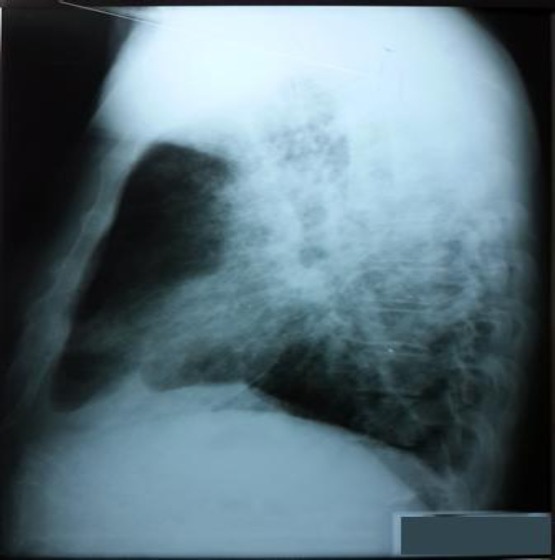
RTG lateral view: Chest X-ray found a massive, diffuse lung consolidation, with reactive hila
Because of suspected Wegener’s disease, parenteral corticosteroids, antibiotics and oxygen supplementation has been administered, with consequent improvement of the patient’s condition, when the patient has been referred to a kidney biopsy at a private clinic in Skopje. Anti-dsDNA = 2.32 U/ml (neg < 40, pos > 60 U/ml), Anti-MPO p-ANCA = 8.93 U/ml (neg < 3, pos > 5 U/ml).
The histopathological findings of the kidney biopsy, performed in May, the 15th, 2016, where as follows: Microscopically the sections of the samples have revealed 11 glomeruli with surrounding tubular interstitium. Ten out of eleven glomeruli have had relatively preserved architectonics, without increased cellularity, endocapillary and extra capillary, whereas in one-to-two glomeruli the GBM (glomerular basement membrane) is slightly is chemically wrinkled.
In one of the glomeruli, there has been found a segmental lesion of GBM collapse with synechiae in between the visceral and parietal epithelium. In the surrounding tubular interstitium, there are focuses of tubular atrophy and interstitial fibrosis (< 25%). The immunofluorescence technique carried out has shown linear IgG deposits along the GBM in eight out of eight glomeruli, whereas in two of the glomeruli there is globally collapsed GBM, and in another two there can be seen segmental epithelial proliferations with a slight impression of GBM.
The diagnosis made is Extracapillary Glomerulonephritis, focal and segmental (2/19) and global (2/19). According to the above, the diagnosis of Goodpasture´s syndrome has been made.
The patient has been admitted for the second time at the University Clinic of Nephrology, on June, the 7th, 20116, with the following values of the blood and biochemistry analyses: WBC = 15.5 × 10^9/L; serum urea = 11.6 mmol/L; creatinine = 137 mmol/L; urine: specific weight = 1014; pH = 6, proteins (+), glucose (-), blood (+), RBC = 4-5 in the sediment, WBC = 3-4 in the sediment. Anti GBM antibodies were negative during this hospitalization.
Urinary tract ultrasound (June, the 9th, 2016): the kidneys, bilaterally are with proper size and shape, right: 110 x 52 mm, on the left 113 x 54 mm, with a granular parenchyma – 19 mm diameter, with slightly increased echogenicity from where the hypoechogenic medulla is noticeable. There is no stasis. The urinary bladder was without any changes. The prostate is oval, homogenous, weighing approximately 21 gramme.
During the hospital admission the patient has been treated with immunosuppressive therapy, initially with high-dose methylprednisolone, 500 mg per day, three days in a row, with tapering the dosage, and using cyclophosphamide in one occasion. During the hospital admission, he had not had any respiratory symptoms, and the other parameters have been improving. Hence, plasmapheresis has not been indicated. Blood pressure measurements, as follows: 115/80 – 126/89 mmHg, with no need of anti-hypertensive therapy. There were noted slight decreases in the serum creatinine and the proteinuria measurements. The diuresis noted 2000 ml. The patient has been discharged with an improved general condition and prescribed the following drugs: Decortin 60 mg, one tablet per day; Ranitidine 150 mg, twice per day. The follow-up examination at the University Clinic of Pulmonology and Allergy, performed in August, the 1st, 2016 has revealed a patient in good general condition. The blood gas analyses, as follows: SaO2 = 96.4%; PaO2 = 9.46 kPa; PaCO2 = 3.67 kPa, normal spirometry measurements. The chest X-ray has shown bilaterally pleura-diaphragmatic adhesions, other findings were normal (Fig. 3, 4). At the moment the patient is stable and he is being treated with oral corticosteroids.
Figure 3.
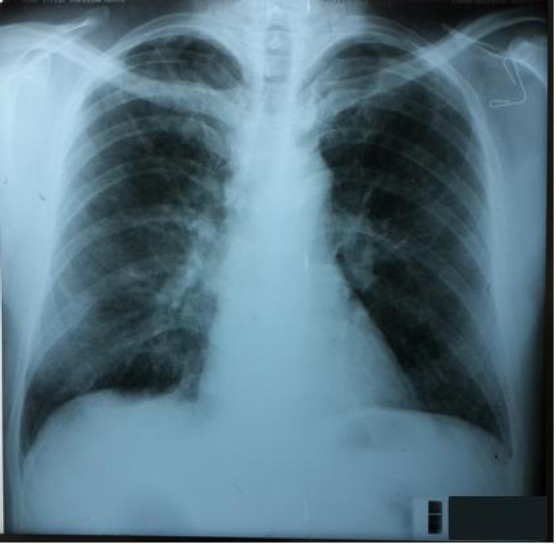
RTG Posteranterior: Chest X-ray has shown bilaterally pleura-diaphragmatic adhesions, other findings are normal
Figure 4.
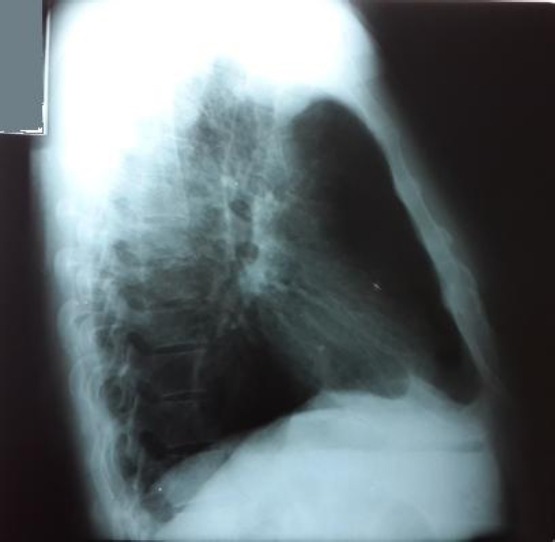
RTG lateral view: Chest X-ray has shown pleura-diaphragmatic adhesions, other findings are normal
Discussion
Ernest Goodpasture first described the disorder in 1919 [8]. He reported a case of pulmonary haemorrhage and glomerulonephritis during an influenza epidemic. In the 1950s, Krakower and Greenspon identified GBM as the antigen [9]. In 1967, Lerner, Glassock, and Dixon confirmed that the antibodies taken from the diseased kidneys produced nephritis in experimental animals [10]. The discovery of anti-GBM antibodies led to the understanding of the pathogenesis of Goodpasture syndrome. Anti-GBM disease is an uncommon disorder [11].
Careful attention to the medical history, physical examination, and targeted laboratory evaluation often suggests the underlying cause [12].
Our patient has been diagnosed almost after one year and half, after his symptoms appeared for the first time, when his condition became very severe, with breathlessness, massive haemopthises, renal failure, anemia, hematuria. Formerly he had worked as a driver; presently he works with automobile lacquers, and has a 10 pack-year smoking history. Anti-MPO p-ANCA was 8.93 U/ml (neg < 3, pos > 5 U/ml). The diagnosis was made as an extracapillary glomerulonephritis, focal and segmental (2/19) and global (2/19). The patient on his admission on Clinic of pulmology and allergology has shown decreased oxygen saturation (SatO2 = 50%) and hypoxemia (PaO2 = 6.35 kPa), anemia (Hgb = 7.9 g/L; Hct = 23.2%, RBC = 2.7 x 10^12/L; WBC = 16.9 x 10^9/L), ESR = 60mm/h, urea = 13.9 mmol/L, creatinine = 206 mmol/L, total proteins = 56 g/L, albumins = 33 g/L, Fe in cepym 8.1mmol/L, CRP = 134 U/L, troponins = 777 U/L, CK = 144 U/L, CK-MB = 29 U/L, LDH = 686 U/L, proteinuria, hematuria. Chest X-ray found a massive, diffuse lung consolidation bilaterally, with reactive hila.
The incidence of the anti-GBM disease is estimated to be 0.5-1.8 cases per million per year in both European white and Asian populations. It is responsible for 1-5% of all types of glomerulonephritis and for 10-20% of crescentic glomerulonephritis [13].
From 60-80% of patients have clinically apparent manifestations of pulmonary and renal disease, 20-40% has renal disease alone, and less than 10% have a disease that is limited to the lungs. Anti-GBM disease occurs more commonly in white people than in black people, but it also may be more common in certain ethnic groups, such as the Maoris of New Zealand. The age distribution is bimodal, 20-30 years and 60-70 years. The prevalence of the disease is higher in men in the younger age group and women in the older age subgroup [13, 14].
A subgroup of patients is double-positive for anti-GBM antibodies and antineutrophilic cytoplasmic antibodies. The peak age incidence for this subgroup is 60-70 years, with a male predominance [15].
Patients presenting with serum creatinine levels greater than 4 mg/dL, oliguria, and more than 50% crescents on renal biopsy rarely recover. They usually progress to end-stage renal failure that requires long-term dialysis. In a retrospective analysis of patients with the anti-GBM disease who started renal replacement therapy for end-stage renal disease (ESRD) in Australia and New Zealand (ANZDATA Registry), the median survival rate was 5.93 years with death predicted by older age and history of pulmonary haemorrhage. Conditions that affect the lung and kidney (pulmonary-renal syndromes) are important in the differential diagnosis. These include granulomatosis with polyangiitis (Wegener granulomatosis), systemic lupus erythematosus, microscopic polyangiitis, and other forms of systemic vasculitides. Distinguishing granulomatosis with polyangiitis from Goodpasture syndrome is particularly important. Interestingly, some patients with Goodpasture syndrome may present with antineutrophilic cytoplasmic antibodies (ANCAs), which are predominantly observed in patients with granulomatosis with polyangiitis. Pulmonary-renal syndromes are less commonly a manifestation of IgA-mediated disorders (eg, IgA nephropathy or Henoch-Schönlein purpura) and of immune complex–mediated renal disease (eg, essential mixed cryoglobulinemia). Rarely, rapidly progressive glomerulonephritis alone can cause pulmonary-renal syndromes through a mechanism involving renal failure, volume overload, and pulmonary oedema with hemoptysis [16-18].
In the past, Goodpasture syndrome was usually fatal. Aggressive therapy with plasmapheresis, corticosteroids, and immunosuppressive agents has dramatically improved prognosis. With this approach, the 5-year survival rate exceeds 80% and fewer than 30% of patients require long-term dialysis. In a study of patients admitted to intensive care units for the acute manifestation of small-vessel vasculitis, including anti-GBM disease, delayed administration of cyclophosphamide was associated with a higher mortality rate [19, 20].
On the Clinic of nephrology during the hospital admission our patient has been treated with immunosuppressive therapy, initially with high-dose methylprednisolone, 500 mg per day, three days in a row, with tapering the dosage, and using cyclophosphamide in one occasion. In this moment the patient is in remission with only “in one occasion use” of cyclophosphamide and plasmapheresis. He is being treated only with oral corticosteroids. The prognosis of this case, for now, is not so bad although delayed diagnose.
In conclusion, Goodpasture syndrome is a severe illness caused by the formation of antibodies to the glomerular basement membrane and alveolus with consequential damage to renal and pulmonary function. With current therapy, long-term survival is more than 50%. Before, the mortality was higher than 90%. The treatment involved corticosteroid therapy, cytostatic therapy and plasmapheresis. Very rarely it appears only with pulmonary involvement, so it can be concluded all cases of repeated pulmonary haemorrhage should raise suspicion of this syndrome.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Castro C, Gourley M. Diagnostic Testing and Interpretation of Tests for Autoimmunity. J Allergy Clin Immunol. 2010;125(2):238–S247. doi: 10.1016/j.jaci.2009.09.041. PMid: 20061009. PMCid: PMC2832720. https://doi.org/10.1016/j.jaci.2009.09.041 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan P, Leung M. Sequential occurrence of anti-glomerular basement membrane disease 9 years after anti-neutrophil cytoplasmic antibody-associated vasculitis. Oxf Med Case Reports. 2016;4:91–93. doi: 10.1093/omcr/omw026. https://doi.org/10.1093/omcr/omw026 . PMid: 27123311. PMCid: PMC4845091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava G, Rao P, Segal M, Geetha Sh. Characteristics and outcome of crescentic glomerulonephritis in patients with both antineutrophil cytoplasmic antibody and anti-glomerular basement membrane antibody. Clinical Rheumatology. 2013;2(9):1317–1322. doi: 10.1007/s10067-013-2268-5. https://doi.org/10.1007/s10067-013-2268-5 . PMid: 23624587. [DOI] [PubMed] [Google Scholar]
- 4.Olson SW, Arbogast CB, Baker TP, Owshalimpur D, Oliver DK, Abbott KC, Yuan CM. Asymptomatic autoantibodies associate with future anti-glomerular basement membrane disease. J Am Soc Nephrol. 2011;22:1946–1952. doi: 10.1681/ASN.2010090928. https://doi.org/10.1681/ASN.2010090928 . PMid: 21868497. PMCid: PMC3279953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parekh N, Epstein E, El-Sayegh S. Necrotizing RPGN with linear anti IgG deposits in a patient with history of granulomatosis with polyangiitis: a case report. International Journal of Nephrology and Renovascular Disease. 2014;7:441–446. doi: 10.2147/IJNRD.S61621. https://doi.org/10.2147/IJNRD.S61621 . PMid: 25473306. PMCid: PMC4251529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spyros A, Manali ED, Kalomenidis I, Kapotsis GE, Karakatsani A, Roussos C. Bench-to-bedside review: Pulmonary–renal syndromes –an update for the intensivist. Critical Care. 2007;11:213. doi: 10.1186/cc5778. https://doi.org/10.1186/cc5778 . PMid: 17493292. PMCid: PMC2206392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swainson C, Robson J, Urbaniak S, Keller A, Kay A. Treatment of Goodpasture’s disease by plasma exchange and immunosuppression. Clin Exp Immunol. 1978;32(2):233–242. PMid: 668199. PMCid: PMC1541272. [PMC free article] [PubMed] [Google Scholar]
- 8.Collins RD. Dr Goodpasture: “I was not aware of such a connection between lung and kidney disease”. Ann Diagn Pathol. 2010;14(3):194–8. doi: 10.1016/j.anndiagpath.2010.02.003. https://doi.org/10.1016/j.anndiagpath.2010.02.003 . PMid: 20471565. [DOI] [PubMed] [Google Scholar]
- 9.Greenspon SA, Krakower CA. Direct evidence for the antigenicity of the glomeruli in the production of nephrotoxic serums. AMA Arch Pathol. 1950;49(3):291–7. PMid: 15406262. [PubMed] [Google Scholar]
- 10.Lerner RA, Glassock RJ, Dixon FJ. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967;126(6):989–1004. doi: 10.1084/jem.126.6.989. https://doi.org/10.1084/jem.126.6.989 . PMid: 4964566. PMCid: PMC2138413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazidi P, Bajestani S, Khan M, Khair T, Laos L. Goodpasture’s Syndrome: A Case Report And Review Of Literature. The Internet Journal of Internal Medicine. 2001;7:2. [Google Scholar]
- 12.Kalluri R, Wilson CB, Weber M, Gunwar S, Chonko AM, Neilson EG, Hudson BG. Identification of the alpha 3 chain of type IV collagen as the common autoantigen in antibasement membrane disease and Goodpasture syndrome. J Am Soc Nephrol. 1995;(4):1178–85. doi: 10.1681/ASN.V641178. PMid: 8589284. [DOI] [PubMed] [Google Scholar]
- 13.Shiferaw B, et al. Goodpasture’s Disease: An Uncommon Disease With an Atypical Clinical Course. J Clin Med Res. 2016;8(1):52–55. doi: 10.14740/jocmr2379w. https://doi.org/10.14740/jocmr2379w . PMid: 26668684. PMCid: PMC4676347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molecular characteristics of the Goodpasture autoantigen. Hudson BG, Kalluri R, Gunwar S, Noelken ME, Mariyama M, Reeders ST. Kidney Int. 1993;43(1):135–9. doi: 10.1038/ki.1993.22. PMid: 7679455. [DOI] [PubMed] [Google Scholar]
- 15.Vucković B, Ilić T, Mitić I, Knezević V, Vodopivec S, Curić S. Med Pregl.[Goodpasture’s syndrome--case report] Med Pregl. 2004;57(7-8):391–5. doi: 10.2298/mpns0408391v. PMid: 15626299. [DOI] [PubMed] [Google Scholar]
- 16.Sinha V, Hibbert C. Near-lethal acute kidney injury due to Goodpasture’s syndrome: A case report. Journal of the Intensive Care Society. 2015;0(0):1–5. doi: 10.1177/1751143715593560. https://doi.org/10.1177/1751143715593560 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salam N, Rezki H, Fadili W, Hachim K, Ramdani B. Goodpasture’s syndrome - Four Case Reports. Saudi J Kidney Dis Transpl (serial online) 2007;18:235–8. PMid: 17496401. [PubMed] [Google Scholar]
- 18.Rossert J. Goodpasture’s disease. Orphanet encyclopedia. 2002. http://www.orpha.net/data/patho/GB/uk-goodpasture.pdf .
- 19.Figurek A, Vlatkovic V, Vojvodic D, Grujic M. Anti-GBM rapidly progressive glomerulonephritis (Syndroma Goodpasture): A case report. Serbian Journal of Experimental and Clinical Research. 2014;15(3):157–159. https://doi.org/10.5937/sjecr1403157F . [Google Scholar]
- 20.Levy JB, Turner AN, Rees AJ, et al. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033–42. doi: 10.7326/0003-4819-134-11-200106050-00009. https://doi.org/10.7326/0003-4819-134-11-200106050-00009 . PMid: 11388816. [DOI] [PubMed] [Google Scholar]


