Abstract
In a cross-sectional survey of 187 Gambian children and adults, we have analyzed prevalence, fine specificity, and 19-kilodalton merozoite surface protein 1 (MSP-119)-specific erythrocyte invasion inhibitory activity of antibodies to MSP-119 but find no significant association between any of these parameters and prevalence or density of malarial parasitemia, except that, after correcting for total anti-MSP-119 antibody levels, individuals with anti-MSP-119 antibodies that compete with an invasion inhibitory monoclonal antibody (12.10) were significantly less likely to have malaria infections with densities of ≥1,000 parasites/μl than were individuals without such antibodies. This association persisted after correction for age and ethnic origin.
Immunogens based on the C-terminal portion of the major Plasmodium falciparum merozoite surface protein, merozoite surface protein-1 (MSP-1), are being developed as potential vaccines against erythrocytic stages of malaria (1, 13). Reproducible antibody-mediated protection against challenge infection has been induced in rodents and primates with vaccines containing the 42-kilodalton MSP-1 (MSP-142) or MSP-119 C-terminal fragments of the protein (4, 5, 12, 15, 16, 22-24). By contrast, immunoepidemiological surveys of human populations naturally exposed to malaria have produced conflicting evidence regarding a protective role for antibodies to MSP-119, with some studies showing a positive association between anti-MSP-119-specific antibody levels and protection (3, 10) and others showing no association (7) or even a negative association (25). We have suggested (20) that this is due to variation between individuals in the fine specificity of the antibody response to MSP-119, with only some antibody specificities being able to mediate antiparasitic functions, such as inhibition of merozoite invasion of erythrocytes (inhibitory antibodies), while other specificities may have no effect (neutral antibodies) or may even block the activity of invasion inhibitory antibodies (blocking antibodies) (2, 8, 11, 13, 17). In support of this hypothesis, recent results have demonstrated marked interindividual and interpopulation variation in the fine specificity of anti-MSP-119 antibodies (measured by their ability to compete with MSP-119-specific monoclonal antibodies [MAbs] for binding to recombinant MSP-119 and by patterns of binding to modified recombinant proteins in which specific antibody epitopes have been eliminated), which is associated with variation in protective immunity (20). As total anti-MSP-119 antibody levels do not correlate with immunity, we have developed a functional assay to assess the efficacy of anti-MSP-119 antibodies by using a chimeric transgenic line of P. falciparum in which the gene encoding MSP-119 (PfMSP-119) has been replaced by the functionally equivalent but antigenically unrelated sequence from the murine parasite Plasmodium chabaudi (PcMSP-119) (18, 19). By comparing this construct to the native parent under identical conditions within a single assay, it is possible to correct for many of the nonspecific factors which lead to variability in a traditional invasion assay and to isolate just that component of invasion inhibition attributable to antibody to MSP-119. With this assay it has been shown that a major part of the invasion inhibitory activity of malaria immune human sera can be attributed to antibodies to PfMSP-119 (18) and that MSP-119-specific invasion inhibitory activity is associated with resistance to reinfection in a population from an area of moderate malaria endemicity in Kenya (14).
As a further step towards identifying the epitope specificity of MSP-119-specific invasion inhibitory antibodies, and to further test their association with resistance to parasitemia, we have compared gross MSP-119 titers, competition titers against MSP-119-specific MAbs, and binding of serum antibodies to modified MSP-119 recombinant proteins to (i) the MSP119-specific invasion inhibitory activity of the same sera and (ii) to the prevalence and density of malaria parasitemia in plasma samples from a cross-sectional study carried out (at the end of the peak malaria transmission season) with 187 individuals aged 1 to 70 years in The Gambia. The study defined responders as subjects whose serum antibodies bound to MSP-119 with an optical density (OD) greater than the means + 3 standard deviations of a panel of 37 European nonimmune sera. Using this definition, the study had a power of 80% to detect a significant association between parasitemia and antibody-dependent measurements with an odds ratio (OR) of 2.5 or more.
The study population (21) and the MSP-119 invasion inhibition assay (18) have been described in detail elsewhere. Antibody binding assays have also been described elsewhere (9, 20). Briefly, mutant and native protein binding was determined by enzyme-linked immunosorbent assay at a serum dilution of 1:1,000 with recombinant MSP-119-glutathione S transferase fusion proteins corrected for binding to glutathione S transferase alone. Mutant binding for MSP-119-positive sera was analyzed as the ratio of mutant OD to wild-type OD. Competition assays were carried out in enzyme-linked immunosorbent assay format by allowing human serum at dilutions of 1:50 and 1:250 to bind to plates coated with recombinant MSP-119 followed (after washing) by the appropriate mouse MAb at a predetermined dilution, detected with an anti-mouse immunoglobulin G conjugate. For each plate, competition was determined as the percentage reduction of OD in wells treated with serum compared to levels for wells which had not been treated with serum.
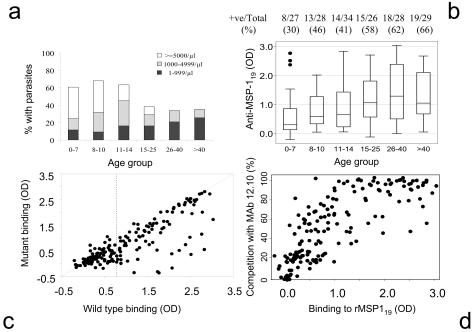
The prevalence of parasitemia and the prevalence of parasite densities above 1,000 infected red blood cells/μl of blood were both negatively associated with age (χ2 trend = 3.8, degree of freedom [df] = 1, P = 0.05, and χ2 trend = 17.7, df = 1, P < 0.001, respectively) (Fig. 1a), while the prevalence of seropositive individuals (OD > 0.77) and median and mean concentrations (measured as OD) of antibodies to MSP-119 (Fig. 1b) increased with age (χ2 trend = 9.5, df = 1, P = 0.002, and t [linear trend of OD] = 4.16, df = 1, P < 0.001, respectively). There was no significant association between anti-MSP-119 seropositivity and the presence or absence of parasitemia (χ2 = 2.76, df = 1, P = 0.10) or between anti-MSP-119 concentration (OD) and the presence or absence of parasitemia (χ2 = 0.94, df = 1, P = 0.33) or parasite density category (χ2 = 2.06, df = 1, P = 0.15). After correction for age, individuals with parasitemia of ≥1,000/μl were significantly more likely to be MSP-119 seropositive than were individuals with parasite density of <1,000/μl (OR = 2.3, χ2 = 4.95, df = 1, P = 0.03). However, when corrected for ethnic group this association was lost (χ2 = 2.02, df = 1, P = 0.15), because the major part of the variance was contributed by 15 individuals from a single ethnic group (Fula/Fulani).
FIG. 1.
Age-related changes in parasite density, prevalence, and anti-MSP-119 antibody responses. (a) Parasite prevalence and density by age (n = 186). (b) Distribution of antibodies to MSP-119 by age. In each plot median (line), the interquartile range (25th to 75th centile) (box) and the adjacent values (highest value within 1.5 multiplied by the interquartile range of the 75th centile and lowest value within 1.5 multiplied by the interquartile range of the 25th centile) (vertical lines) are indicated. Points falling outside this range are drawn individually. Positive sera were defined as sera with an OD of >0.77 (means ± 3 standard deviations of a panel of 37 European nonimmune sera). Number (and percentages) of positive sera/number tested in each age group are indicated at the top of the plot. (c) Correlation between binding to wild-type and modified recombinant MSP-119 antigens for individual plasma samples. Vertical dashed line indicates the OD cutoff for positive sera. The diagonal dashed line is a line of identity between mutant OD and wild-type OD. (d) Correlation between binding of plasma antibodies to wild-type recombinant MSP-119 (OD) and the ability of the same plasma to inhibit binding of MAb 12.10 to recombinant MSP-119 (percent).
Of the 88 sera that contained anti-MSP-119 antibodies, between 18 and 33 (20 to 37.5%) (depending on which modified protein was being tested) bound more strongly to wild-type recombinant MSP-119 than to three modified recombinant proteins in which epitopes for blocking MAbs had been eliminated (ratio of OD of mutant to OD of the wild type, <0.75) (20, 26), suggesting that these sera contained antibodies that were more similar in their fine specificity to blocking MAbs than to invasion inhibitory MAbs (Fig. 1c) and that these children might thus be more likely to develop malaria infection. In fact, although the study had 80% power to detect such associations if they actually exist in this population, there was no significant association between ability to discriminate between modified and wild-type proteins and parasite prevalence or parasite density (P > 0.6 in all cases).
The ability of individual sera to compete with MAbs of defined specificity is one indication of the fine specificity of anti-MSP-119 antibodies (20, 26). The abilities of sera to compete with a panel of six different MAbs were significantly correlated (r2 values ranged from 0.43 to 0.82 for all possible pairs, P < 0.0001 in all cases), and competition with MAbs was significantly correlated with OD for binding to wild-type MSP-119 (r2 values ranging from 0.33 to 0.61, P < 0.0001 in all cases). However, in simple logistic regression analysis there was no significant association between competition with any MAb and parasite prevalence (P > 0.4 for all associations) or density (P > 0.1 for all associations).
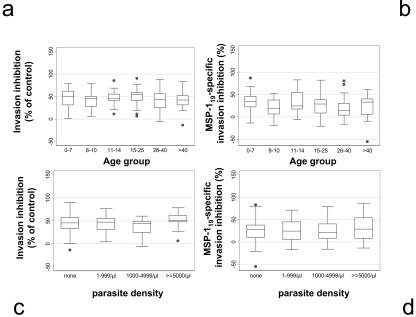
MSP-119-specific invasion inhibition activity for each serum can be measured as the difference between inhibition of invasion of wild-type parasites (PfMSP-119) and inhibition of invasion of transfected parasites (PcMSP-119) (18). Consistent with results of previous studies (6, 14, 18), the majority of the total invasion inhibitory activity (63.5% ± 67.1%) was attributable to anti-MSP-119 antibodies. However, neither total invasion inhibition (χ2 trend = 8.28, df = 5, P = 0.14) nor MSP-119-specific invasion inhibition (χ2 trend = 10.36, df = 5, P = 0.066) was associated with age (Fig. 2a and b), and no association was seen between total or MSP-119-specific invasion inhibition and the presence or absence of parasitemia (χ2 = 0.005, df =1, P = 0.95, and χ2<0.001, df = 1, P = 0.99, respectively) or between MSP-119-specific invasion inhibition and parasite density category (χ2 = 0.78, df = 3, P = 0.85) (Fig. 2c and d). There was a significant, if counterintuitive, positive association between total (non-MSP-119-specific) inhibition of invasion and the presence of high-density parasitemia (≥5,000/μl) (χ2 trend = 5.23, df = 1, P = 0.023). MSP-119-specific invasion inhibition activity (classified as sera that specifically inhibit invasion by ≥50%) was not associated with the presence or absence of anti-MSP-119 antibodies (χ2 = 0.71, P = 0.39), with MSP-119 OD (r2 = 0.0002, P = 0.87), with differential binding to wild-type and modified MSP-119 proteins (χ2 = 0.03, df = 1, P = 0.87), or with the ability of the sera to compete with any of the six MAbs (c2 ranged from 0.015 to 1.17, df =1, P = 0.28 to 0.90).
FIG. 2.
Lack of association between age, parasite density, and invasion inhibitory activity of immune plasma. (a) Total invasion inhibitory activity of plasma by age, with the percent reduction in invasion of PfMSP-119-transformed parasites (containing native MSP-119) compared to invasion levels of control nonimmune serum (100%). (b) MSP-119-specific invasion inhibition by age, with the percent invasion of PcMSP-119 parasites compared to invasion levels of the control nonimmune serum subtracted from the percent invasion of PfMSP-119 parasites. (c) Relationship between total invasion inhibition and ranked parasite density. (d) Relationship between MSP-119-specific invasion inhibition and ranked parasite density. Box limits are the same as those described in the legend to Fig. 1b.
Analysis of the entire antibody response data set by principle component analysis failed to reveal any minor component which correlated with either invasion inhibition or parasitemia. However, as we envisaged that antibodies of different specificities might have opposing effects on parasitemia or invasion inhibition activity (i.e., the activity of invasion inhibitory antibodies being opposed by the activity of invasion-enhancing antibodies), we looked for possible interactions between the ability of antibodies to compete with the different classes of MAbs and other explanatory variables on outcome (parasite prevalence or density) by multiple logistic regression. Statistically significant interactions are shown in Table 1. Not surprisingly, in this analysis, increasing age was significantly associated with decreased risk of having high-density parasitemia (≥1,000/μl). By contrast, seropositivity for MSP-119 antibodies was significantly associated with increased risk of high-density parasitemia. However, the ability of plasma antibodies to inhibit binding of the invasion inhibitory MAb 12.10 (by >50% at a plasma dilution of 1:50) was significantly associated with reduced risk of high-density parasitemia (≥1,000/μl); this was not seen for competition with other MAbs. The inclusion of ethnic origin did not affect this association. Although this relationship is of marginal significance and results from small differences within a highly correlated data set, the association between competition with MAb 12.10 and resistance to high-density parasitemia does confirm previous observations in an entirely different population (20). In a previous study (20), no association was found between competition with MAb 12.10 and protection from malaria with fever (i.e., mild clinical malaria) in Gambian children aged 7 to 9 years, but a significant association was found between competition with MAb 12.10 and resistance to high-density parasitemia in Ugandan children. Thus, in both studies where the association has been tested, possession of anti-MSP119 antibodies that compete for binding with MAb 12.10 is associated with resistance to high-density parasitemia. This is consistent with the notion that the effects of antibodies of specificity similar to that of 12.10 (which inhibits merozoite invasion into erythrocytes) would be most readily detected by their effect on parasite density rather than by their effect on a less direct measure, such as clinical disease.
TABLE 1.
Logistic regression analysis of selected explanatory variables for outcome variable parasite density of >1,000/μl
| Variable | Result for variable
|
|||||
|---|---|---|---|---|---|---|
| MSP-119-positive
|
MSP-119-positive age group
|
MSP-119-positive age group competition with 12.10
|
||||
| OR | P | OR | P | OR | P | |
| Seropositive for: | ||||||
| Anti-MSP-119a | 1.36 | 0.36 | 2.31 | 0.03 | 4.65 | 0.005 |
| Age groupb | 0.57 | <0.01 | 0.58 | <0.001 | ||
| >50% Competition with MAb 12.10a | 0.35 | 0.045 | ||||
OR for parasite density of >1,000/μl for explanatory variable being positive or negative.
OR for parasite density of >1,000/μl between age categories.
Significant differences in infection risk and antibody responses were noted between the three ethnic groups in the study population. Those from the Mandinka ethnic group were significantly less likely than others to be parasitemic (χ2 = 12.2, df = 2, P = 0.002), while Wolofs were significantly more likely to have high-density parasitemias (χ2 = 13.5, df = 2, P = 0.001). Interestingly, Fulas were twice as likely as other individuals to have MSP-119-specific invasion inhibitory antibodies (29.4% for Fulas versus 14.0% for Wolofs and 14.2% for Mandinkas), but because of the low numbers of Fula individuals in the study (9%), this difference was not statistically significant (χ2 = 2.75, df = 2, P = 0.25).
In summary, the only significant associations we have found between malaria infection (prevalence and density of parasitemia) and four separate measures of anti-MSP-119 antibody prevalence, concentration, specificity, or function are (i) between higher parasite density and higher total anti-MSP-119 OD and (ii) between the ability of antibodies to compete with an invasion inhibitory MAb and resistance to higher density parasitemia. Thus, it seems that total anti-MSP-119 antibody levels are indicative of recent reinfection and boosting of the humoral immune response, while certain specificities within this polyclonal population of anti-MSP-119 antibodies may inhibit parasite replication within an infected individual. While this latter finding is in general agreement with previous observations (20), it hardly amounts to strong evidence for the protective role of anti-MSP-119 antibodies. One potential drawback of this study is that as antibodies and infection were measured contemporaneously, antibody responses may have been modified by the boosting effects of the present infection. Thus, it cannot be ruled out that, in some individuals, anti-MSP-119 antibody levels may have fallen below the concentration required for protection at the time of infection but then had been boosted to higher levels in the following days or weeks. This would tend to lead to underestimation of the protective effect of high titers of protective antibodies. Secondly, half of the subjects of the study were aged 15 years or more and would have acquired a substantial degree of immunity to malaria (which may operate independently of any protective effect of anti-MSP-119 antibodies), further weakening the strength of any association between anti-MSP-119 antibodies and parasite prevalence. Although anti-MSP-119 antibodies appear to make up a significant proportion of the total invasion inhibitory activity of immune sera (18), the relative importance of invasion inhibition in terms of the various effector mechanisms that may be required for immunity to malaria infection and/or disease is not presently known. Prospective surveys of semi-immune children in which immune status is determined prior to infection and correlated with subsequent risk of infection are more powerful in this respect, and in one such study significant associations between antibody specificity and resistance to high-density parasitemia was found (20); this analysis now needs to be extended to assessment of MSP-119-specific invasion inhibitory activity. More worrisome is the fact that none of the antibody-related parameters that we have measured here were associated with each other, confirming the lack of association reported for Nigerian sera between competition titers and inhibition of secondary processing of MSP-142 (17). These results underline the complexity of natural responses to MSP-119 and suggest that repeated exposure to malaria may boost responses which confound protection as well as those which are protective. However, these results leave unanswered the question of whether or not these in vitro parameters will prove useful for monitoring the outcome of the clinical trials of MSP-119 vaccines planned for the near future.
Acknowledgments
This study was carried out by a large team of researchers from the Medical Research Council Laboratories in The Gambia. In particular we acknowledge the roles of Steve Allen, Steve Bennett, and Brian Greenwood in the initiation, implementation, and analysis of these studies. We thank Jana McBride, University of Edinburgh, for providing MAb 7.5 and hybridomas for preparation of MAbs 12.10 and 12.8.
Funding for this study came from the Bill and Melinda Gates Foundation (London School of Hygiene and Tropical Medicine-Gates Malaria Partnership), EU contract number QLK2-CT-1999-01293, the UK Medical Research Council, and the National Health and Medical Research Council (NHMRC) of Australia. C. Uthaipibull was in receipt of a Thai Government scholarship; B. S. Crabb is an International Research Scholar of the Howard Hughes Medical Institute.
Editor: W. A. Petri, Jr.
Footnotes
This work is dedicated to the memory of Steve Bennett, who died in March 2003 at the age of 52 years.
REFERENCES
- 1.Angov, E., B. M. Aufiero, A. M. Turgeon, M. Van Handenhove, C. F. Ockenhouse, K. E. Kester, D. S. Walsh, J. S. McBride, M. C. Dubois, J. Cohen, J. D. Haynes, K. H. Eckels, D. G. Heppner, W. R. Ballou, C. L. Diggs, and J. A. Lyon. 2003. Development and pre-clinical analysis of a Plasmodium falciparum merozoite surface protein-1(42) malaria vaccine. Mol. Biochem. Parasitol. 128:195-204. [DOI] [PubMed] [Google Scholar]
- 2.Blackman, M. J., T. J. Scott-Finnigan, S. Shai, and A. A. Holder. 1994. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 180:389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braga, E. M., R. M. Barros, T. A. Reis, C. J. Fontes, C. G. Morais, M. S. Martins, and A. U. Krettli. 2002. Association of the IgG response to Plasmodium falciparum merozoite protein (C-terminal 19 kD) with clinical immunity to malaria in the Brazilian Amazon region. Am. J. Trop. Med. Hyg. 66:461-466. [DOI] [PubMed] [Google Scholar]
- 4.Chang, S. P., S. E. Case, W. L. Gosnell, A. Hashimoto, K. J. Kramer, L. Q. Tam, C. Q. Hashiro, C. M. Nikaido, H. L. Gibson, C. T. Lee-Ng, P. J. Barr, B. T. Yokota, and G. S. N. Hui. 1996. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect. Immun. 64:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly, T. M., and C. A. Long. 1993. A recombinant 15-kilodalton carboxy-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect. Immun. 61:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Koning-Ward, T. F., R. A. O'Donnell, D. R. Drew, R. Thomson, T. P. Speed, and B. S. Crabb. 2003. A new rodent model to assess blood stage immunity to the Plasmodium falciparum antigen merozoite surface protein 119 reveals a protective role for invasion inhibitory antibodies. J. Exp. Med. 198:869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodoo, D., T. G. Theander, J. A. L. Kurtzhals, K. Koram, E. Riley, B. D. Akanmori, F. K. Nkrumah, and L. Hviid. 1999. Antibody levels to conserved parts of Plasmodium falciparum merozoite surface protein 1 (PfMSP1) in Ghanaian children are not associated with protection from clinical malaria. Infect. Immun. 67:2131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan, A. F., P. Burghaus, P. Druilhe, A. Holder, and E. Riley. 1999. Human antibodies to the 19 kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 21:133-139. [DOI] [PubMed] [Google Scholar]
- 9.Egan, A. F., J. A. Chappel, P. A. Burghaus, J. S. Morris, J. S. McBride, A. A. Holder, D. C. Kaslow, and E. M. Riley. 1995. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP119, the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect. Immun. 63:456-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan, A. F., J. Morris, G. Barnish, S. Allen, B. M. Greenwood, D. C. Kaslow, A. A. Holder, and E. M. Riley. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19 kDa C-terminal fragment of the merozoite surface antigen, PfMSP1. J. Infect. Dis. 173:765-769. [DOI] [PubMed] [Google Scholar]
- 11.Guevara Patino, J. A., A. A. Holder, J. S. McBride, and M. J. Blackman. 1997. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 186:1689-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirunpetcharat, C., J. Tian, D. Kaslow, N. van Rooijen, S. Kumar, J. Berzofsky, L. Miller, and M. Good. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 159:3400-3411. [PubMed] [Google Scholar]
- 13.Holder, A., J. Guevara Patino, C. Uthaipibull, S. Syed, I. Ling, T. Scott-Finnigan, and M. Blackman. 1999. Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parassitologia 41:409-414. [PubMed] [Google Scholar]
- 14.John, C. C., R. A. O'Donnell, P. O. Sumba, A. M. Moormann, T. F. de Koning-Ward, C. L. King, J. W. Kazura, and B. S. Crabb. 2004. Evidence that invasion-inhibitory antibodies specific for the 19-kDa fragment of merozoite surface protein-1 (MSP-119) can play a protective role against blood-stage Plasmodium falciparum infection in individuals in a malaria endemic area of Africa. J. Immunol. 173:666-672. [DOI] [PubMed] [Google Scholar]
- 15.Kumar, S., W. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. Guevara Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in Aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 68:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling, I. T., S. A. Ogun, and A. A. Holder. 1994. Immunization against malaria with a recombinant protein. Parasite Immunol. 16:63-67. [DOI] [PubMed] [Google Scholar]
- 17.Nwuba, R. I., O. Sodeinde, C. I. Anumudu, Y. O. Omosun, A. B. Odaibo, A. A. Holder, and M. Nwagwu. 2002. The human immune response to Plasmodium falciparum includes both antibodies that inhibit merozoite surface protein 1 secondary processing and blocking antibodies. Infect. Immun. 70:5328-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Donnell, R. A., T. F. de Koning-Ward, R. A. Burt, M. Bockarie, J. C. Reeder, A. F. Cowman, and B. S. Crabb. 2001. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193:1403-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Donnell, R. A., A. Saul, A. F. Cowman, and B. Crabb. 2000. Functional conservation of the malaria vaccine antigen MSP-119 across distantly related Plasmodium species. Nat. Med. 6:91-95. [DOI] [PubMed] [Google Scholar]
- 20.Okech, B. A., P. H. Corran, J. Todd, A. Joynson-Hicks, C. Uthaipibull, T. G. Egwang, A. A. Holder, and E. M. Riley. 2004. Fine specificity of serum antibodies to Plasmodium falciparum merozoite surface protein, PfMSP-1(19), predicts protection from malaria infection and high-density parasitemia. Infect. Immun. 72:1557-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley, E. M., S. J. Allen, S. Bennett, P. J. Thomas, G. Andersson, A. O'Donnell, S. W. Linsday, M. F. Good, and B. M. Greenwood. 1990. Recognition of dominant T cell stimulating epitopes from the circumsporozoite protein of Plasmodium falciparum and relationship to malaria morbidity in Gambian children. Trans. R Soc. Trop. Med. Hyg. 84:648-657. [DOI] [PubMed] [Google Scholar]
- 22.Singh, S., M. C. Kennedy, C. A. Long, A. J. Saul, L. H. Miller, and A. W. Stowers. 2003. Biochemical and immunological characterization of bacterially expressed and refolded Plasmodium falciparum 42-kilodalton C-terminal merozoite surface protein 1. Infect. Immun. 71:6766-6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stowers, A., V. Cioce, R. Shimp, M. Lawson, G. Hui, O. Muratova, D. Kaslow, R. Robinson, C. Long, and L. Miller. 2001. Efficacy of two alternate vaccines based on Plasmodium falciparum merozoite surface protein 1 in an Aotus challenge trial. Infect. Immun. 69:1536-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stowers, A. W., L. H. Chen, Y. Zhang, M. C. Kennedy, L. Zou, L. Lambert, T. J. Rice, D. C. Kaslow, A. Saul, C. A. Long, H. Meade, and L. H. Miller. 2002. A recombinant vaccine expressed in the milk of transgenic mice protects Aotus monkeys from a lethal challenge with Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 99:339-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolle, R., K. Fruh, O. Doumbo, O. Koita, M. N′diaye, A. Fischer, K. Dietz, and H. Bujard. 1993. A prospective study of the association between the human humoral immune response to Plasmodium falciparum blood stage antigen gp190 and control of malaria infections. Infect. Immun. 61:40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uthaipibull, C., B. Aufiero, S. Syed, B. Hansen, J. Guevara Patino, E. Angov, I. Ling, K. Fegeding, W. Morgan, C. Ockenhouse, B. Birdsall, J. Feeney, J. Lyon, and A. Holder. 2001. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 307:1381-1394. [DOI] [PubMed] [Google Scholar]