Abstract
Exposure to neurotoxic, chiral PCBs has been associated with neurodevelopmental disorders, but their metabolism in humans remains unexplored. We investigated the enantioselective metabolism of PCB 95 by human liver microsomes (HLMs) to potentially neurotoxic, hydroxylated metabolites (OH-PCBs). OH-PCB profiles formed in experiments with HLMs differed from metabolite profiles reported for rodent species. The second eluting atropisomer of 2,2′,3,5′,6-pentachlorobiphenyl-4′-ol, the major metabolite, was preferentially formed by all HLM preparations investigated. Differences in metabolite formation rates were observed with single donor HLMs. The metabolism of PCBs and its role in PCB-mediated neurodevelopmental disorders need to be further characterized.
PCBs represent a human health concern because they are detected in environmental samples and human tissues.1,2 Epidemiological studies suggest negative associations between developmental exposures to PCBs and neurophysiological function in infancy and childhood.5 Animal studies demonstrate that developmental exposures to PCBs, such as PCB 95, cause behavioral deficits in rodents due to the disruption of calcium homeostasis.5 Higher levels of PCB 95 have been observed in the brain from individuals diagnosed with a neurodevelopmental disorder compared to that in neurotypical individuals.2 PCB 95 and its hydroxylated metabolites (OH-PCBs) are potent sensitizers of ryanodine receptors,6 calcium release channels implicated in the developmental neurotoxicity of PCBs.5 PCB 95 and its metabolites exist as two stable rotational isomers or atropisomers that are nonsuperimposable mirror images of each other. PCB 95 is enantioselectively metabolized by P450 enzymes, thus resulting in atropisomerically enriched PCB 95 in brain tissue.7,8 Although PCBs atropselectively alter end points implicated in PCBs’ developmental neurotoxicity,1,5 the oxidation of PCB 95 by HLMs has not been investigated previously.
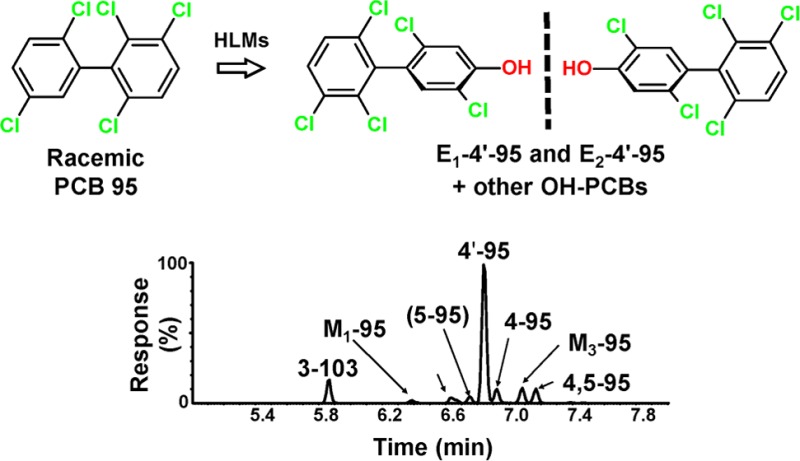
Initial microsomal metabolism studies with pooled HLMs (pHLM; pool of 50, mixed gender; Xenotech, Lenexa KS, USA) were carried out to identify hydroxylated PCB 95 metabolites potentially formed in humans (Figure 1 and Figures S1 to S14).9 Analysis by GC/time-of-flight MS after extraction and derivatization with diazomethane revealed the formation of six mono- and two dihydroxylated metabolites (as methylated derivatives). The identification of the metabolites was based on accurate mass determination and the isotope patterns of the [M]+ ion (expected abundance ratio: 1:1.6:1.0:0.3). The fragmentation patterns of the derivatized mono- or dihydroxylated metabolites were consistent with meta- or para-methoxylated pentachlorobiphenyl derivatives, with characteristic fragments such as [M-CH3]+ and [M-CH3-CO-Cl2]+; see Supporting Information.10 The proposed chemical structures and abbreviations of the metabolites are shown in Figure 1A. The formation of 3-103 (a 1,2-shift product), 5-95, 4′-95, 4-95, and 4,5-95 was further confirmed by comparing relative retention times and MS data of the metabolites to those of authentic standards.
Figure 1.
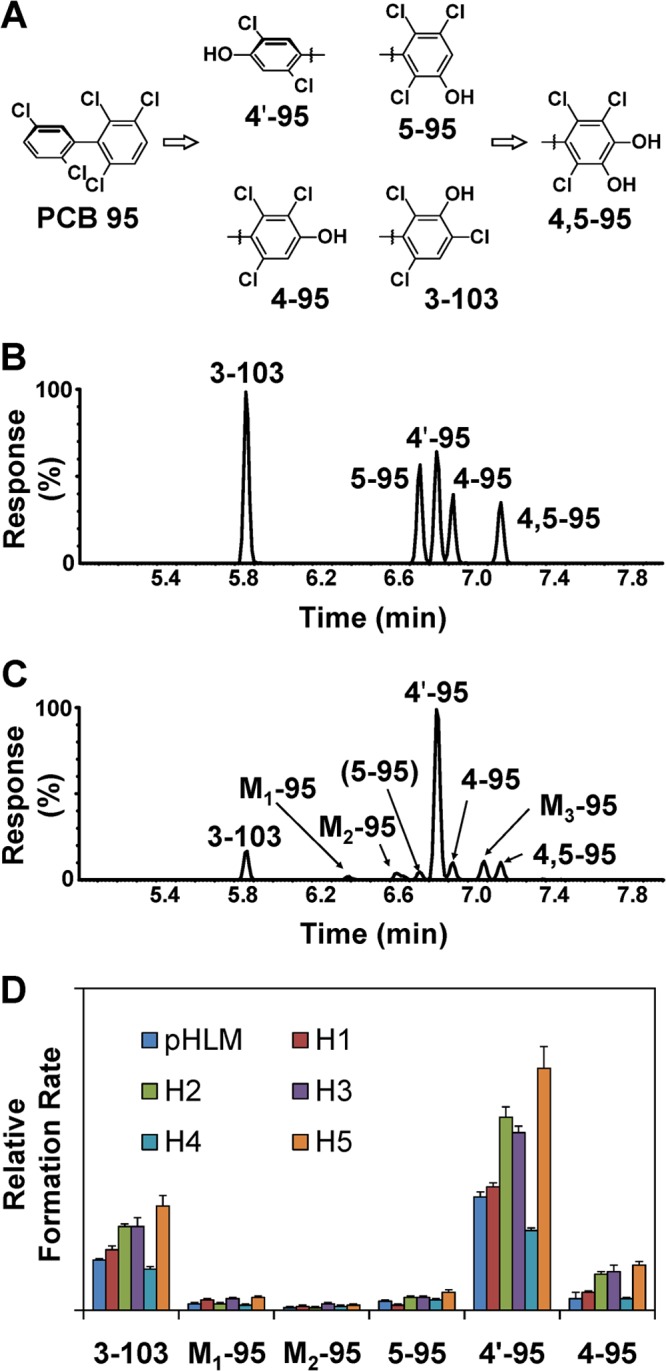
Metabolism of PCB 95 by pHLMs results in the formation of hydroxylated metabolites (analyzed as methylated derivatives), with para-hydroxylated metabolites being the major metabolites. (A) Metabolism scheme showing the chemical structure and abbreviations of PCB 95 metabolites (only one atropisomer is shown for clarity reasons). Gas chromatograms of (B) PCB 95 metabolite standards and (C) metabolites formed in an incubation with pHLMs (50 μM PCB 95, 90 min, 37 °C, 0.3 mg/mL protein, and 1 mM NADPH). (D) Relative formation rates of OH-PCB metabolites (relative peak area/nmol P450/min ×102) in incubations with pHLMs and single donor HLMs (H1 to H5). Data are the mean ± standard deviation, n = 3.
GC with electron capture detection (ECD; see Supporting Information) was used to determine levels and relative formation rates9 of the OH-PCB metabolites in incubations with HLMs (Figure 1D; Tables S1 to S5). Incubation conditions for the determination of formation rates were optimized for time, protein, NADPH, and PCB 95 concentrations (i.e., 50 μM PCB 95; 5 min incubation at 37 °C; 0.1 mg/mL microsomal protein; and 1 mM NADPH).11 The para position of the 2,5-dichloro substituted phenyl ring was the major site of PCB 95 metabolism, with 4′-95 accounting for 73% of total OH-PCBs formed. Metabolite formation rates in experiments with pHLMs folowed the rank order 4′-95 ≫ 4-95∼3-103 > 5-95 ≫ 4,5-95. M1-95, M2-95, and M3-95 were minor metabolites.
Similar to our findings with PCB 95 reported herein, PCB 52 (2,2′,5,5′-tetrachlorobiphenyl) and PCB 101 (2,2′,4,5,5′-pentachlorobiphenyl) are preferentially metabolized in the para position by human P450 enzymes.12,13 These earlier studies identified P450 2A6 as the primary P450 isoform involved in the para-oxidation of a 2,5-dichloro substituted phenyl ring. In contrast, PCB 136 (2,2′,3,3′,6,6′-hexachlorobiphenyl), a symmetrical congener with two 2,3,6-trichloro substituted phenyl rings, is oxidized by pHLMs to both para- and meta-hydroxylated metabolites.9,14 Metabolism studies with recombinant enzymes suggest a role of P450 2B6 in the metabolism of chiral PCBs with a 2,3,6-trichloro substitution.15
Studies using rodent models reveal different OH-PCB profiles in vitro and in vivo, without the predominant formation of 4′-95 observed with HLMs. Briefly, PCB 95 is preferentially metabolized to 5-95 and an unidentified OH-PCB by rat P450 2B116 and rat liver microsomes from rats pretreated with phenobarbital.17 The unidentified metabolite does not correspond to 4′-95 and likely is 2,2′,3,5′,6-pentachlorobiphenyl-3′-ol (3′-95).17 However, 4′-95 is present in the blood and liver from rats orally exposed to PCB 95.7 Metabolism studies with PCB 95 revealed the formation of 5-95 as a major metabolite in liver tissue slices from phenobarbital-treated mice.18 Studies in pregnant mice exposed to PCB 95 during gestation and lactation showed no detectable levels of 4′-95 in the blood and liver of the dams.8
To explore interindividual differences in the metabolism of PCB 95, incubations with HLMs from five randomly selected individual donors were performed (Table S1). All microsomal preparations yielded similar metabolite profiles after a 5 min incubation time, with an approximate ratio of 3-103:5-95:4′-95:4-95 = 2:1:20:4. Irrespective of the incubation conditions, these four OH-PCBs and 4,5-95 accounted for <5% of the total PCB added to each incubation (Figure 1D; Table S1). Depending on the OH-PCB congener, the OH-PCB formation rates differed 2- to 4-fold between individual donor HLMs. In general, the metabolism rates followed the rank order H5 > H1 > H2 ∼ H3 > pHLM > H4 (Tables S2–S5).
The atropisomeric enrichment, expressed as an enantiomeric fraction (EF), of PCB 95 and 4′-95 was assessed as described previously (Figure 2, Table S6, and Figures S15 and S16).11 Long-term incubations were performed with several HLM preparations to obtain nonracemic chiral signatures of the parent PCB. A significant enrichment (EF = 0.55) of the PCB 95 atropisomer eluting first on the GC column (E1-PCB 95) was observed in incubations with two HLM preparations. No enrichment was observed with most HLM experiments because the excess of racemic PCB 95 masked the atropisomeric enrichment due to a low conversion of the parent PCBs. The 4′-95 atropisomer eluting second on the GC column (E2-4′-95) was significantly enriched under these conditions in all HLM preparations investigated. Apparent EF values of 4′-95 ranged from 0.10 to 0.26. HLM incubations with a 15 min incubation time also revealed an enrichment of E2-4′-95 (Table S6). These observations are consistent with a preferential metabolism of E2-PCB 95 to E2-4′-95 by human P450 enzymes.
Figure 2.
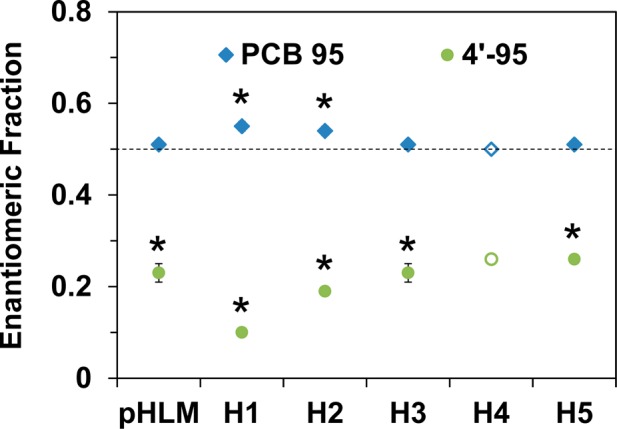
Metabolism of racemic PCB 95 in incubations with different HLMs results in the atropselective formation of E2-4′-95 and for HLMs from donors H1 and H2, a depletion of E2-PCB 95. Incubations were performed for 120 min; Table S6). The dotted line indicates the EF value of the racemic standards. Data are the mean ± standard deviation. Open symbols indicate n = 1. *Significantly different from the racemic standard (p < 0.05).
Our results suggest that interindividual differences in the metabolism of PCB 95 are one factor that modulates chiral signatures of PCB and their OH-PCB metabolites in humans. Further studies are needed to identify the P450 isoforms involved in the metabolism of PCB 95 in humans, determine if PCB 95 metabolites are present in human target tissues and, ultimately, assess how their atropisomers affect end points implicated in the neurodevelopmental toxicity of PCBs.
Acknowledgments
We thank Drs. S. Joshi, S. Vyas, and H. Wu from the University of Iowa for synthesizing the PCB standards and Mr. V. Parcel from the University of Iowa HRMS Facility for help with the GC-MS analysis.
Glossary
Abbreviations
- 3-103
2,2′,4,5′,6-pentachlorobiphenyl-3-ol
- 4-95
2,2′,3,5′,6-pentachlorobiphenyl-4-ol
- 4′-95
2,2′,3,5′,6-pentachlorobiphenyl-4′-ol
- 5-95
2,2′,3,5′,6-pentachlorobiphenyl-5-ol
- 4,5-95
4,5-dihydroxy-2,2′,3,5′,6-pentachlorobiphenyl
- E1
atropisomer eluting first on the enantioselective GC column
- E2
atropisomer eluting second on the enantioselective GC column
- EF
enantiomeric fraction
- HLM
human liver microsomes
- OH-PCBs
hydroxylated polychlorinated biphenyls
- PCB 52
2,2′,5,5′-pentachlorobiphenyl
- PCB 95
2,2′,3,5′,6-pentachlorobiphenyl
- PCB 101
2,2′,4,5,5′-pentachlorobiphenyl
- PCB 136
2,2′,3,3′,6,6′-hexachlorobiphenyl
- pHLM
pooled human liver microsomes
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemrestox.6b00371.
Experimental details of microsomal incubations and chemical analysis; levels and formation rates of OH-PCB metabolites; enantiomeric fractions; and mass spectra of OH-PCB derivatives (PDF)
This study was supported by funding from NIH (ES013661 and ES005605). E.U. gratefully acknowledges support from the Iowa Superfund Research Program Training Core.
The authors declare no competing financial interest.
Supplementary Material
References
- Lehmler H.-J.; Harrad S. J.; Hühnerfuss H.; Kania-Korwel I.; Lee C. M.; Lu Z.; Wong C. S. (2010) Chiral polychlorinated biphenyl transport, metabolism, and distribution: A review. Environ. Sci. Technol. 44, 2757–2766. 10.1021/es902208u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. M.; Woods R.; Chi L. H.; Schmidt R. J.; Pessah I. N.; Kostyniak P. J.; LaSalle J. M. (2012) Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environ. Mol. Mutagen. 53, 589–598. 10.1002/em.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah I. N.; Cherednichenko G.; Lein P. J. (2010) Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol. Ther. 125, 260–285. 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niknam Y.; Feng W.; Cherednichenko G.; Dong Y.; Joshi S. N.; Vyas S. M.; Lehmler H.-J.; Pessah I. N. (2013) Structure-activity relationship of select meta- and para-hydroxylated non-dioxin-like polychlorinated biphenyls: from single RyR1 channels to muscle dysfunction. Toxicol. Sci. 136, 500–513. 10.1093/toxsci/kft202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamou M.; Uwimana E.; Flannery B. M.; Kania-Korwel I.; Lehmler H. J.; Lein P. J. (2015) Subacute nicotine co-exposure has no effect on 2,2′,3,5′,6-pentachlorobiphenyl disposition but alters hepatic cytochrome P450 expression in the male rat. Toxicology 338, 59–68. 10.1016/j.tox.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I.; Barnhart C. D.; Lein P. J.; Lehmler H.-J. (2015) Effect of pregnancy on the disposition of 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) atropisomers and their hydroxylated metabolites in female mice. Chem. Res. Toxicol. 28, 1774–1783. 10.1021/acs.chemrestox.5b00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Kammerer A.; Lehmler H. J. (2014) Microsomal oxidation of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) results in species-dependent chiral signatures of the hydroxylated metabolites. Environ. Sci. Technol. 48, 2436–2444. 10.1021/es405433t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman Å.; Klasson Wehler E.; Kuroki H.; Nilsson A. (1995) Synthesis and mass spectrometry of some methoxylated PCB. Chemosphere 30, 1921–1938. 10.1016/0045-6535(95)00073-H. [DOI] [Google Scholar]
- Wu X.; Pramanik A.; Duffel M. W.; Hrycay E. G.; Bandiera S. M.; Lehmler H.-J.; Kania-Korwel I. (2011) 2,2′,3,3′,6,6′-Hexachlorobiphenyl (PCB 136) is enantioselectively oxidized to hydroxylated metabolites by rat liver microsomes. Chem. Res. Toxicol. 24, 2249–2257. 10.1021/tx200360m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T.; Kakimoto K.; Takenaka S.; Koga N.; Uehara S.; Murayama N.; Yamazaki H.; Kim D.; Guengerich F. P.; Komori M. (2016) Roles of human CYP2A6 and monkey CYP2A24 and 2A26 cytochrome P450 enzymes in the oxidation of 2,5,2′,5′-tetrachlorobiphenyl. Drug Metab. Dispos. 44, 1899–1909. 10.1124/dmd.116.072991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw J. E.; Waller D. P. (2006) Specific human CYP 450 isoform metabolism of a pentachlorobiphenyl (PCB-IUPAC# 101). Biochem. Biophys. Res. Commun. 344, 129–133. 10.1016/j.bbrc.2006.03.122. [DOI] [PubMed] [Google Scholar]
- Schnellmann R. G.; Putnam C. W.; Sipes I. G. (1983) Metabolism of 2,2′,3,3′,6,6′-hexachlorobiphenyl and 2,2′,4,4′,5,5′-hexachlorobiphenyl by human hepatic microsomes. Biochem. Pharmacol. 32, 3233–3239. 10.1016/0006-2952(83)90209-5. [DOI] [PubMed] [Google Scholar]
- Warner N. A.; Martin J. W.; Wong C. S. (2009) Chiral polychlorinated biphenyls are biotransformed enantioselectively by mammalian cytochrome P-450 isozymes to form hydroxylated metabolites. Environ. Sci. Technol. 43, 114–121. 10.1021/es802237u. [DOI] [PubMed] [Google Scholar]
- Lu Z.; Kania-Korwel I.; Lehmler H. J.; Wong C. S. (2013) Stereoselective formation of mono- and dihydroxylated polychlorinated biphenyls by rat cytochrome P450 2B1. Environ. Sci. Technol. 47, 12184–12192. 10.1021/es402838f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I.; Duffel M. W.; Lehmler H.-J. (2011) Gas chromatographic analysis with chiral cyclodextrin phases reveals the enantioselective formation of hydroxylated polychlorinated biphenyls by rat liver microsomes. Environ. Sci. Technol. 45, 9590–9596. 10.1021/es2014727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Duffel M.; Lehmler H.-J. (2013) Oxidation of polychlorinated biphenyls by liver tissue slices from phenobarbital-pretreated mice is congener-specific and atropselective. Chem. Res. Toxicol. 26, 1642–1651. 10.1021/tx400229e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


