Abstract
Maintenance of paroxysmal atrial fibrillation (AF) by fast rotors in the left atrium (LA) or at the pulmonary veins (PVs) is not fully understood. This review describes the role of the heterogeneous distribution of transmembrane currents in the PVs and LA junction (PV-LAJ) in the localization of rotors in the PVs. Experimentally observed heterogeneities in IK1, IKs, IKr, Ito, and ICaL in the PV-LAJ were incorporated into models of human atrial kinetics to simulate various conditions and investigate rotor drifting mechanisms. Spatial gradients in the currents resulted in shorter action potential duration, less negative minimum diastolic potential, slower upstroke and conduction velocity for rotors in the PV region than in the LA. Rotors under such conditions drifted toward the PV and stabilized at the less excitable region. Our simulations suggest that IK1 heterogeneity is dominant in determining the drift direction through its impact on the excitability gradient. These results provide a novel framework for understanding the complex dynamics of rotors in AF.
Keywords: rotors, drift, atrial fibrillation, ionic currents, computer modeling
Introduction
The mechanisms of atrial fibrillation (AF), the most common cardiac arrhythmia in the clinical practice, are not fully understood. Acute AF in normal isolated sheep hearts has been found to often depend on fast rotors localized mainly to the posterior wall of the left atrium (LA) and the pulmonary veins (PVs) junction (PV-LAJ) with fibrillatory conduction toward the rest of the atria.1 Recent clinical data also point to rotors in various atrial sites as a mechanism driving paroxysmal AF.2–4 Previous simulations have already demonstrated that rotors can occur in the PV-LAJ, provided the PV size is adequate, and nonuniform coupling conditions exist.5 However, how the rotors form or drift in the PV-LAJ, as well as the drift underlying ionic mechanisms, have not been investigated systematically.6,7 Earlier studies using cardiac computer models have shown a causal link between rotor drift and spatial heterogeneity in the action potential (AP) properties.8 Rotors drifted toward regions with prolonged action potential duration (APD) or reduced excitability,9,10 which were inscribed mainly due to heterogeneities in multiple ion channels,11 and particularly K+ channels.10
As shown in Figure 1, IK1 and IKr play a role in rotor dynamics by affecting both the membrane APD and excitability.12,13 Figure 1A presents the average duration and frequency of the tachyarrhythmic episodes in the wild-type (WT) and transgenic (TG) mice, in which IK1 was upregulated by overexpressing the Kir2.1.13 There was statistical difference between duration (WT, 3 ± 9; TG, 350 ± 1181 seconds, P < 0.001) and frequency (WT, 26.2 ± 5.2; TG, 44.6 ± 4.3 Hz, P < 0.001) of the arrhythmias in the two groups. In addition, Figure 1A displays a representative snapshot of the distribution of membrane voltage and the underlying inactivation gating variables (h·j) of the sodium current (INa) during simulated reentry in WT and TG mouse hearts. The product of the h and j gates is representative of the excitability of the resting tissue. The voltage maps show that the wavelength is shorter and the diastolic potential is more hyperpolarized (darker blue) over a wider area in the TG cardiac sheet, compared to the WT (values of diastolic potentials were ~−94 mV in TG versus ~−90 mV in WT). This results in greater recovery of Na+ channel availability (maximum value of h·j is 0.808 versus 0.684 in TG versus WT hearts, respectively). Furthermore, the accelerated final phase of repolarization results in a larger area in which Na+ channels have recovered (orange), and thus greater excitability in front of the depolarization wave front. These factors contribute in part to the higher rotor frequency seen in the TG hearts.
Figure 1.
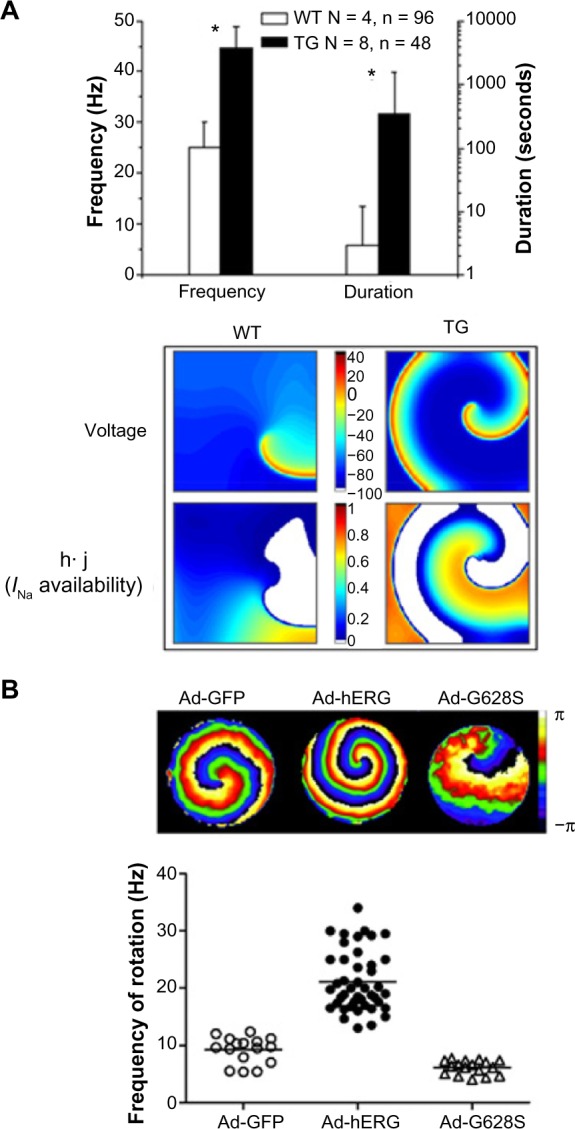
The effect of IK1 and IKr on rotor stability and frequency. (A) Top: average duration and frequency of arrhythmias in four WT and eight TG hearts from mice. Bottom: spatial distribution of transmembrane voltage (top) and inactivation gating variables (h·j gates, bottom) of the INa during stable reentry in a 2 × 2 cm2 cardiac cell sheet. The diastolic potential is more hyperpolarized over a wider area in the TG heart sheet (~−94 mV), compared to the WT case (~−90 mV). The h·j gate map shows that the maximum availability of Na+ channels in TG (0.808) exceeds that of the WT heart (0.684) and occupies a larger area during the excitable gap (from Ref. 13). (B) The effects of hERG infection on reentry frequency in neonatal rat ventricular myocytes monolayers. Top: snapshots from representative optical mapping movies in monolayers infected with Ad-GFP (left), Ad-hERG (middle), or Ad-G628S (right). Bottom: reentry frequencies in individual monolayers infected with Ad-GFP (IKr-overexpression, open circles), Ad-hERG (P < 0.05 versus GFP, closed circles), or Ad-G628S (P = NS versus Ad-GFP; P < 0.05 versus Ad-hERG, open triangles). Horizontal bars represent mean values. One-way analysis of variance (ANOVA) with Tukey’s multiple comparison test (reproduced from Ref. 12).
In addition, sustained functional reentry is seen to accelerate in cardiomyocytes’ monolayers infected by hERG to increase their IKr.12 The top of Figure 1B shows phase maps of single rotors generating spiral waves in control (Ad-GFP, left), IKr-overexpressing (middle), and G628S-overexpressing (IKr blockade, right) monolayers of neonatal rat ventricular myocytes.12 The bottom graph in Figure 1B compares the ranges of individual rotation frequencies of control monolayers with that of IKr-overexpressing and G628S-overexpressing monolayers. The important role of IKr is illustrated by the significantly higher average rotational frequency in IKr-overexpressing monolayers than in GFP controls (21.12 ± 0.81, n = 43 versus 9.21 ± 0.58 Hz, n = 16; P < 0.001), whereas the frequency in the G628S monolayers is slightly lower (6.14 ± 0.3 Hz, n = 17; P = not significant [NS] versus GFP).
While the effect of the individual IK1 and IKr on rotor dynamics and hence on its drift should be discernable, the complex coexisting heterogeneity in the expression of channels at the PV-LAJ, such as in dogs, which show a larger current density of IKr and a smaller density of IK1 in the PV compared to LA,14,15 in conjunction with structural discontinuities (narrow PV sleeves compared to the larger LA), precludes a simple prediction of the rotor dynamics at the PV-LAJ. To address such substrate effects, the data presented in this paper aim to elucidate ionic mechanisms of rotor drifting at the PV-LAJ, a region that is important for paroxysmal AF maintenance in many patients. We review here an investigation in two-dimensional (2D) and pseudo-three-dimensional (3D) models of the PV-LAJ to test the hypothesis that the characteristic heterogeneous dispersion of transmembrane currents during paroxysmal AF, in the absence of remodeling, is a determinant of rotor drift. Our numerical simulations further demonstrate IK1 dominance in conveying a preferential rotor drift direction toward the PVs. We also demonstrate the cycle-by-cycle mechanism by which PV-LAJ regions with longer refractoriness and lower excitability tend to attract rotors. Notwithstanding the reliance on simple models and animal-based ionic kinetics, it is proposed that the specific ionic properties of the human atrial substrate also predispose the PVs, or any other atrial region with reduced IK1 density, to attract rotor activity during AF.16–18
Numerical Methods
Computer simulations were performed on two simple models of the junction between the LA and the PV (Fig. 2)19:
A 50 × 50 mm2 regular 2D square mesh model was implemented and subjected to no-flux boundary conditions.
A pseudo-3D cylindrical surface model of 50 mm length and perimeter constructed by applying no-flux boundary conditions at the LA and PV edges and periodic boundary conditions on the other two edges of a regular 2D mesh.
Figure 2.
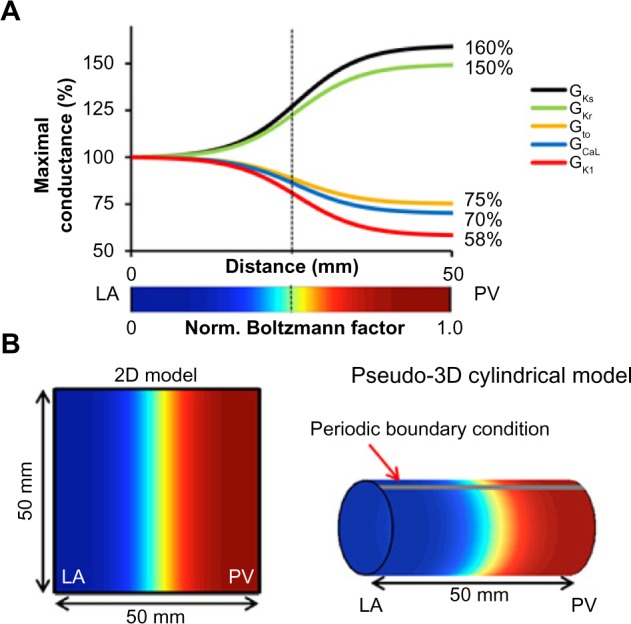
The PV-LAJ models. (A) Conductances for ICaL, IKr, IKs, Ito, and IK1 follow a Boltzmann function (x1/2 = 25 mm, vertical dashed line; Δx = 5 mm) from LA to PV. Color bar: normalized Boltzmann factor across the junction. (B) Simulations were performed on (1) 2D model of 50 × 50 mm2 and (2) cylindrical pseudo-3D model generated by a periodic boundary condition in the 2D model. Cyan-yellow is the steepest gradient region in the PV-LAJ (reproduced from Ref. 19).
The transmembrane potential was based on the Courtemanche-Ramirez-Nattel and Kneller (CRN-K) model of human atrial cell kinetics for AF without remodeling and in the presence of 0.0015 µM acetylcholine.20–22 Electrical activity was computed using a monodomain and numerically stable Euler forward scheme with 100 µm internodal distance, 0.005 ms time step, and isotropic diffusion coefficient of 0.062 mm2/ms adjusted for conduction velocity of 48 cm/s.23,24 Initial conditions were the membrane variables at resting potential following pacing a single cell at 1 Hz for 10 seconds.
Heterogeneous ionic conditions were implemented by assigning a spatial Boltzmann distribution of conductance for IK1, IKs, IKr, Ito, and ICaL between the LA and the PV based on the data from dogs14 and described in Figure 2A (maximum conductance for IKr and IKs was increased by 50% and 60%, respectively, while that for IK1, Ito, and ICaL was diminished by 42%, 25%, and 30%, respectively). Single and paired ionic currents heterogeneities were implemented, as well as three additional conditions of ionic heterogeneity were modeled: (1) all currents varied spatially according to their corresponding Boltzmann function (Condition I); (2) all currents, except IK1, varied (Condition II); and (3) only IK1 varied in space (Condition III). Reentrant excitation patterns were induced by S1–S2 cross-field stimulation protocols,22 and their pivoting points were identified as a singularity point (SP) and tracked in the phase domain from the moment of their appearance.19
Spatial profiles of peak sodium channels availability (h·j)peak were obtained by averaging those parameters in time for every pixel across the model in the last 2 of 5 seconds simulations. Measurements of minimum diastolic potential (MDP) and maximum upstroke velocity (dV/dtmax) followed similar procedure.
Results
Ionic gradients and rotor attraction toward the PV
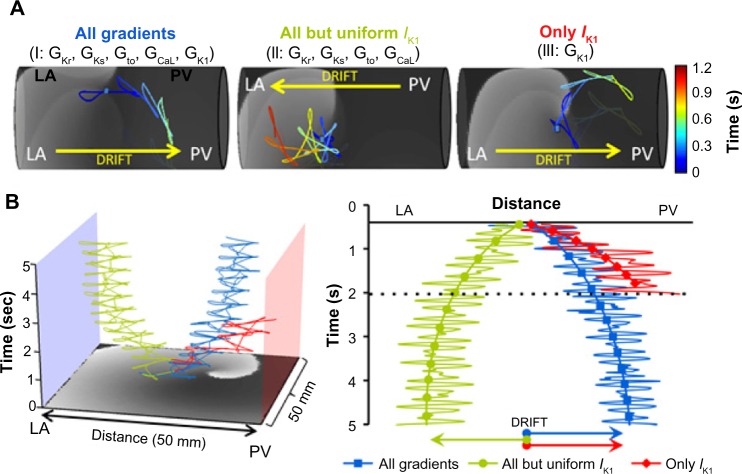
We tested the effect of all individual and pairwise currents on drift by switching them from heterogeneous to uniform (Fig. 3). Figure 3A shows that different ionic current heterogeneity, when applied individually, has a different drift direction. However, when pairs of ionic heterogeneities were combined, Figure 3B shows that the IK1 heterogeneity is the primary ionic factor that determines the direction of the drift toward the PV; whatever IK1 heterogeneity is combined with, the drift direction is always in the direction imposed by the IK1, namely a drift from the LA toward the PV region.
Figure 3.
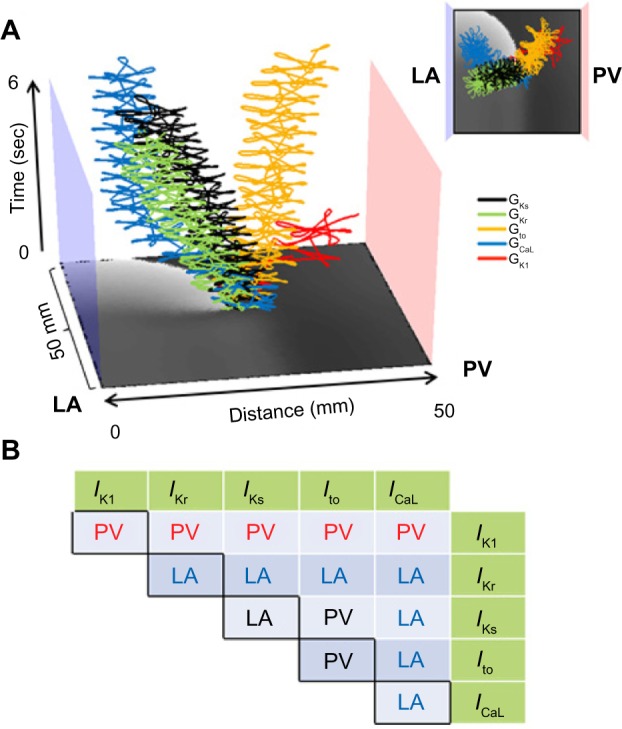
Spatial gradients of individual and paired currents and reentry drift in a flat model. (A) Effect of spatial gradient for each individual current on rotor drift. 2D PV-LAJ model showing spatiotemporal trajectories of SPs of rotors in gradients of IKs (black), IKr (green), Ito (yellow), ICaL (blue), and IK1 (red) as described by the individual Boltzmann distributions in Figure 2A. While Ito and IK1 cause the rotor to drift toward the PV edge, the gradients in the other currents cause the rotor to drift toward the opposite LA edge. Insert: top view of the trajectories. (B) Effect of spatial gradients in paired currents on rotor drift. The table shows the direction of the drift when ionic gradients as indicated in the abscissa and ordinate (green cells) were considered. IK1 is the only current whose gradient leads to PV attraction (in red fonts) of the rotor, regardless of gradient in any other current (reproduced from Ref. 19).
As a demonstration of major dependence of rotor drift in the PV-LAJ on the specific ionic current heterogeneity of IK1 in contrast to all the other combined heterogeneities, we generated rotors in the three ionic conditions implemented in the cylindrical and flat model and tracked the spontaneous trajectory of their SP. Figure 4 demonstrates that the rotor in the heterogeneous PV-LAJ region drifts predictably as a consequence of the particular condition simulated.
Figure 4.
Simulations of rotor drift in the PV-LAJ and IK1 role against all other currents. (A) Rotor dynamics on the cylindrical model using the CRN-K kinetics under three conditions: (1) all currents varied spatially, (2) all currents varied spatially, except IK1, and (3) only IK1 varied spatially. Color-coded traces show the SP trajectory of rotors initiated at the middle of the models (blue dots). Rotors under Conditions I and III drifted toward the PV edge. Drift direction was reversed when IK1 heterogeneity was excluded in Condition II. (B) Time–space plots and arrows show rotor drift for the three conditions in a flat 2D model (reproduced from Ref. 19).
In Condition I, ie, heterogeneity in the currents IK1, IKs, IKr, Ito, and ICaL as characterized at the PV-LAJ in dog,14 the drift is toward the PV edge of the model. However, when all the currents except IK1 are set to be heterogeneous, and IK1 density is maintained homogenously and is equal to the LA region value (Condition II), the drift reverses toward the LA (when the IK1 is homogeneously equal to the PV region – not shown – the drift is also toward the LA). On the other hand, when all the currents are set uniform (either with the LA or the PV values) and only IK1 is set to disperse as in the dog (Condition III), the drift is again toward the PV, but with a faster rate as compared with the drift in Condition I. This set of three scenarios in cylindrical as well as in the flat models, shown in Figure 2, clearly points to the strong effect of IK1 on the direction of the rotor drift in the LA-PV junction area. As controls, we simulated rotors in 2D models with uniform ionic properties of either the LA or the PV that revealed nondrifting rotors. The rotors in the model with uniform LA properties were slightly faster than those in the model with the uniform PV properties (7.7 versus 7 Hz, respectively; other properties of the rotors did not vary by more than about 10%).19
Heterogeneous excitability and rotor drift in the PV-LAJ
Ionic heterogeneity is imposing nonuniform excitability properties and a rotor drift toward a predictable direction.9 In Figure 5, we analyze the role of tissue excitability in rotor drifts by quantifying the spatiotemporal distribution of the product of membrane model parameters h and j (h·j), which determines the INa availability during rotor pivoting.22 Figure 5A illustrates the drift trajectory of a rotor in a 2D model under Condition I and shows a time–space plot (TSP) of h·j along the line of the drift. A closer look at the TSP near the pivoting location during a single cycle shows higher values of h·j at the left side (LA) of the drift than at the right side (PV). Thus, a progressive shift of the pivoting point toward the PV is always toward a region with lower h·j values.
Figure 5.
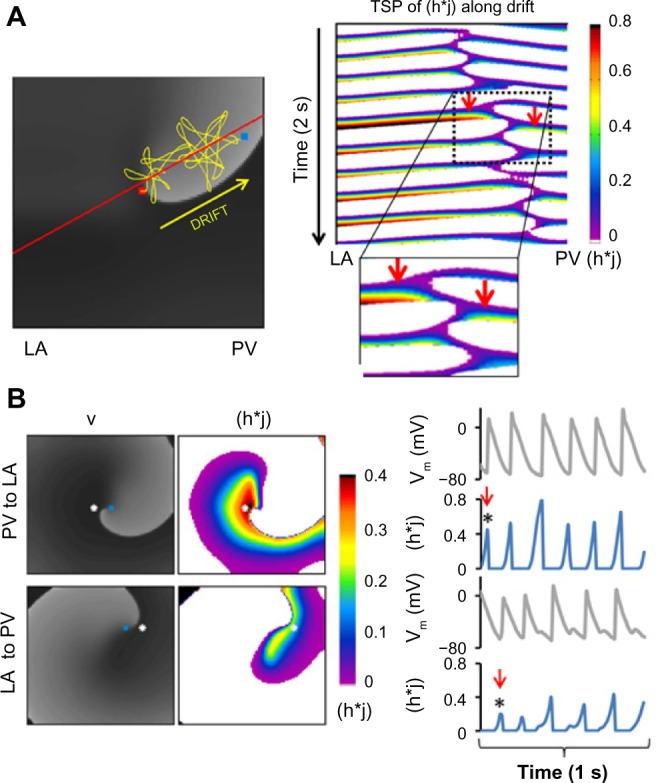
Heterogeneous sodium availability during rotor drift in a flat model. (A) Left: voltage snapshot (gray scale) with superimposed trajectory of rotor SP (yellow tracing) drifting from the center of the model (red dot) toward its final location at the PV edge (blue dot). Right: color coded time–space plot for h·j along the rotor drift direction (red solid line in voltage snapshot). Insert is a magnified view showing that h*J close to the rotor core is larger on the LA side than on the PV side (red arrows). (B) Left: snapshots of voltage (gray scale) and h·j (color coded) maps at two instances: when the rotor wave front near the core is directed from PV to LA (top) and about half cycle later and when it is directed from LA to PV (bottom). Blue dots represent the tip of the rotor and white dots are 4 mm from tip, toward LA (top) and toward PV (bottom). Right: Single pixel time series showing simultaneous voltage and h·j at the white dots. Red arrows indicate the times of the voltage and h·j snapshots on the left side of the panel (reproduced from Ref. 19).
Figure 5B shows snapshots of the voltage and h·j at a moment when the wave front near the rotor tip is propagating toward the LA (top) and a half cycle later, when that wave front is propagating toward the PV (bottom). The h·j snapshots clearly demonstrate that the wave propagating toward the LA is facing a higher h·j (red) as compared with the wave propagating toward the PV. The h·j gradient during the drift is further confirmed by plotting the time course of the voltage and h·j at 2 pixels flanking momentarily the SP of the rotor: one on the LA side and the other on the PV side, 4 mm away from the tip. Those dynamical plots show that the peak h·j for each cycle at the LA side is always >0.4 and at the PV side is always <0.4. As illustrated, the alternating SP drift is larger during propagation toward the PV (low h·j) than during its propagation toward the LA (high h·j). The result is a net drift toward the lower h·j, ie, less excitable, region. Importantly, it is noticeable that the presence of heterogeneity at the core area is critical for the rotor drift; in the absence of heterogeneity at the core, a rotor may be stable.19
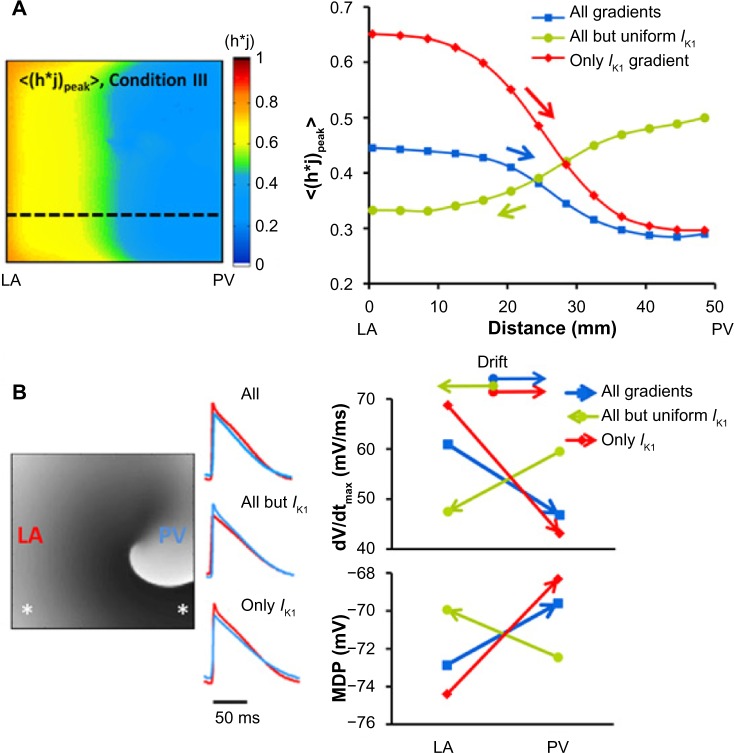
Figure 6 summarizes the relationship between rotor drift and spatial distribution of excitability factors during reentry in the three conditions simulated. Figure 6A shows for illustration purposes a map with the distribution of the time-averaged h·j peak values for the model with greatest excitability gradient (Condition III) and shows that those values are about twofold larger in the LA edge as compared with those at the PV edge (0.8 and 0.4, respectively). The graph in Figure 6A presents the spatial profiles of the h·j and the drift direction for the three conditions. It is seen that the two conditions with lowest density of IK1 at the PV edge (blue and red) have profiles with reduced h·j at that edge as well, in contrast to the condition without the IK1 gradient, where h·j is maximal at the PV edge. Overall, the directionality of the drift in the three conditions as indicated by the superimposed arrows is fully consistent with the rotor attraction by any region with lowest h·j values where the steepest h·j gradient (red) corresponds to the fastest drifting rotor, as indicated in Figure 4B (red).
Figure 6.
Gradients of sodium availability and AP measurements during rotor drift predict drift direction. (A) Left: map of averaged (h·j)peak (<(h·j)peak>) during rotor activity under Condition III (only IK1) on a flat model is showing a LA to PV decrease. Right: PV-LAJ spatial profiles of <(h·j)peak> for the three conditions modeled. Superimposed arrows indicate direction of rotor drift for each condition, consistently toward the region of lowest <(h·j)peak> (lowest sodium availability). (B) AP measurements of excitability during reentry. Left: A voltage snapshot and APs from the LA (red) and PV (blue) edges (asterisks on map) for the three conditions simulated. Right: upstroke velocity (dV/dtmax) and MDP at the LA and PV region with direction of drift (arrows) for the three conditions. Drift is consistently toward lowest upstroke velocity and most positive MDP regions (reproduced from Ref. 19).
Next, Figure 6B quantifies metrics associated with the AP measurements of excitability: we focus on the dV/dtmax and MDP, which plays a role in determining the availability of INa during the membrane depolarization. On the left side, we present samples of aligned pairs of APs recorded in locations (asterisks) near the LA (red) and PV (blue) edges during reentry in the three conditions. As can be appreciated, each condition presents a distinct heterogeneity in its APs as quantified on the right side of the panel, but for the three conditions, the drift direction is toward the regions with lowest excitability as determined by the slowest dV/dtmax and most positive MDP.22
Figures 3 and 4 demonstrated the important role of IK1 in determining the direction of the rotor drift in the PV-LAJ. In Figure 7 we study the effect of various relevant current–voltage relationships of IK1 on such drift direction. Figure 7A shows four relationships between the current density and transmembrane voltage (I–V relationship) for different IK1 levels, including up- and downregulation, as well as their corresponding APs showing different APDs and MDPs. The four I–V relationships were incorporated in a PV-LAJ model with Condition I, and rotor activity as well as AP parameters were tracked. As can be observed from Figure 7B, increasing or decreasing the IK1 increases or decreases the average rotor frequency, respectively. Figure 7C demonstrates that h·j profiles across the PV-LAJ vary in both levels and gradient directions as a consequence of altering the I–V relationships. In particular, it is noticeable that the rotor drifts toward the PV for a broad range of IK1 levels, whereas a reversal in the drift and h·j gradient direction occurs only at a 75% reduction in IK1 (a behavior similar to that of a uniform IK1 shown under Condition II in Fig. 4).
Figure 7.
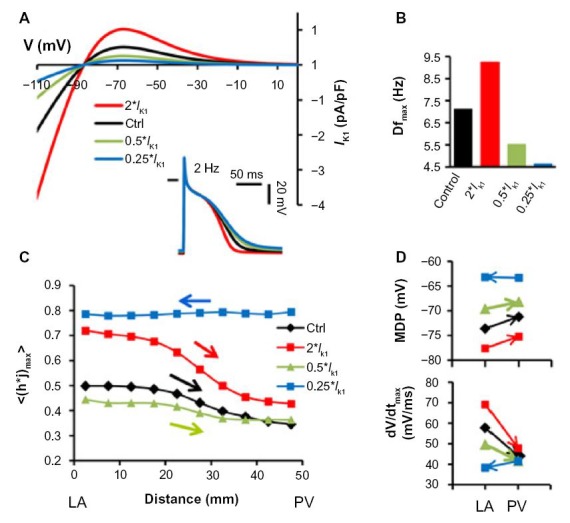
Sensitivity of the rotor drift direction to IK1 I–V profile. (A) IK1 I–V curves for normal (control) and other levels of IK1 density. Steady-state APs induced at 2 Hz are illustrated. (B) Rotation frequency in LA homogenous model for each IK1 density level. (C) Average peak INa availability profiles show drift toward lower excitability (arrows) in a flat model with Condition I. (D) Analysis of MDP and dV/dtmax for each profile at single pixels in the LA and PV (4 mm from boundaries) under Condition I (reproduced from Ref. 19).
Up- and downregulation of IK1 also hyperpolarize and depolarize MDP as well as increase and decrease upstroke speed, respectively (Fig. 4), with consistent drifts toward higher MDP and lower upstroke velocity. Overall, the consequences of altering the I–V properties of IK1 in the heterogeneous models on MDP and upstroke velocity are complex and suggest a nonmonotonic dependency. Nevertheless, further alterations in extracellular potassium concentration in the range of 4–7 mM, as well as alteration in the I–V profile to resemble closely those of Kir2.1 or Kir2.3, affect excitability and rotation frequency but do not alter direction of h·j gradients and drift directions toward the PV.19
Discussion
The findings presented here suggest the following: (1) the heterogeneous distribution of transmembrane currents in the PV-LAJ plays a major role in the preferential localization of rotors near or at the PVs, and the spatial dispersion of IK1 is particularly important in determining such attraction. (2) Rotor drift in the PV-LAJ can be attributed to an excitability (maximum INa availability) gradient in the vicinity of the rotor pivoting point.
Mechanisms of AF and rotor dynamics
Our understanding of AF in individual patients would benefit from knowledge of how driving rotors form and then become stable or unstable, under the conditions of multifactorial substrate heterogeneity.25,26 Paroxysmal AF in patients and in isolated normal sheep hearts has been found often to depend on fast rotors localized to the posterior wall of the LA and the PVs region with fibrillatory conduction toward the rest of the atria.1,2 However, the ionic properties underlying those rotors’ formation and drift remain unclear.7,27 Moreover, recent studies have indicated that in some patients, rotors that drive the AF may reside outside of the PV area.4 Our simulations here demonstrate that the spatial distribution of ionic currents found in the canine PV-LAJ is conducive to attracting rotors to the PV region. In addition, we also demonstrate that this attraction toward the LA can be reversed or arrested, if certain ionic currents are altered, which in turn may explain the variability in the location of rotors found in different patients. However, AF may involve several coexisting rotors at any given moment. In these cases, in addition to the drift imposed by the underlying substrate, the faster rotors can also exert an overriding influence on the slower rotors,28 and the combination of these two factors on rotor dynamics warrants further investigation.
Substrate heterogeneity and rotor drift
In addition to the role of restitution characteristics of cells in rotor stability,29 simulations with gradient of excitability showed spiral wave drifting in the direction of the region exhibiting lower excitability and velocity, with additional perpendicular component depending on the rotor chirality,8 excitability, and repolarization,9 regardless of the details of the initial conditions.30 Simulations using more biophysically detailed ionic models found that for a fixed gradient in APD imposed by linearly varying potassium currents, the velocity of the drift of a rotor is a function of the magnitude of the gradient10 and a steep gradient in APD can lead to conduction block of premature beats.31 Our simulations in a flat and cylindrical (to account for a drift not fully aligned with the gradient direction) models19 are in agreement with all those previous studies but refine the previous prediction that rotors would drift toward regions with longer APD9,32,33 to only >80% repolarization measured at frequencies close to the rotor frequency. Our simulations also show (Figs. 6 and 7) the drift predictability of various measures of excitability, which includes MDP, dV/dtmax, and h·j (INa availability).
IK1, IKr, and rotor dynamics during fibrillation
IK1 and IKr density gradients in the dog PV-LAJ are found to be opposing each other.14 As recent studies have shown that these two currents are important in rotor dynamics and AF,12,13,34–37 the ionic mechanisms leading to the propensity of the PV region to favor rotor activity16–18 became complex. We show for the first time19 a clear propensity of the currents distribution in the PV-LAJ to attract rotors to the PVs and the dominant role for the IK1 dispersion over all currents, and in particular IKr, in determining the localization of a rotor in that area and open the possibility that interplay between IK1 and IKr may be important for the differential localization of rotors in AF.
Comparing with other recent studies, the drift toward low IK1 in our study is fully consistent with the simulations by Kneller et al.21, Comtois et al.38, and Comtois and Nattel39 who studied the effect of artificial heterogeneity in the inward rectifier IK,ACh on AF dynamics. Their simulations also suggest that while rotors accelerate their rotation frequency with increasing IK,ACh,27 the low IK,ACh regions are the ones that attracts rotors.21 Other studies also described rotor attractions to long APD regions, albeit with a sharp heterogeneities.40 A recent study by Sekar et al.41 utilized circular monolayers with overexpression of IK1 either in a central circular island or in its periphery to show that rotors stably pivot around the island regardless of the relative level of IK1. In that study, the gradient, however, was very sharp relative to the size of the rotor core, and the preparation was highly symmetrical, which may explain why that study did not show a preferential anchoring of rotors to either low or high inward rectifying K+ current levels as observed in this and other studies.21,38,42 Finally, in a recent study in cardiomyocytes monolayers with heterogeneous IKr expression, stable rotors localized to the region with highest expression of IKr.37 Those stable rotors did not drift as in our simulations since they reside in a relatively uniform large region, in accordance with our simulations presented elsewhere.19
Limitations
We study a specific set of membrane kinetic models (CRN-K) with a Boltzmann distribution of the current densities across the PV-LAJ preferred for extensive validation of propagation properties22,43 over a more recent and detailed model requiring adjustments.44,45 Experimental or clinical data on ionic properties and dispersion in the atria are scarce; we focused on the effect of reported ionic currents data for the dog. However, attraction or repulsion of rotors by the PVs at the PV-LAJ may be affected by factors other than those studied here. For example, the heterogeneity in the intrinsic cellular properties may have different effective heterogeneity in refractory and excitability30 depending on structural intercellular coupling,46 fibrosis,47 or the size of the medium.31,39,48,49 Further, the drift of rotors may be influenced by accumulations of intra- or extracellular ions, as has been shown to occur in AF.50 Our study ruled out that PV narrow funnel-like anatomy reverses the ionic-induced attraction to the PVs19; however, additional anatomical factors such as wall thickness,51,52 the fiber bundles,53 or fibrosis7 may also regulate the drift of rotors, possibly even counteracting the drift trend caused by the ionic gradients. To mitigate these limitations, we focused in our study on conditions relevant only to paroxysmal AF, prior to any remodeling and fibrosis, and incorporated various possible I–V relationships to substantiate our conclusions regarding the IK1 dominance and drift prediction.19 Our study nevertheless should be considered only as a first step in elucidating the concept of heterogeneity-induced drift and needs to be tested in future experimental studies.
Conclusions
Consistent with experimental and clinical studies on paroxysmal AF, simulations in an ionically heterogeneous model of the PV-LAJ showed rotor attraction toward the PVs. Our simulations suggest that IK1 heterogeneity across the PV-LAJ is dominant compared to other currents in conveying the drift direction through its effect on refractoriness and excitability.
Abbreviations
- AP
action potential
- APD
action potential duration
- AF
atrial fibrillation
- Condition I
all currents varied spatially in the model
- Condition II
all currentsexcept IK1varied spatially in the model
- Condition III
only IK1 varied spatially in the model
- CRN-K
Courtemanche-Ramirez-Nattel and Kneller
- CV
conduction velocity
- DF
dominant frequency
- dVdtmax
maximum upstroke velocity
- Gx
maximum conductance of channel x
- h·j
the fraction of sodium channels available for activation
- h·jpeak
the maximum fraction of sodium channels available for activation during a cycle
- IK1
inward rectifier potassium current
- IK,ACh
acetylcholine modulated inward rectifier potassium current
- IKr
rapid delayed rectifier potassium current
- IKs
slow delayed rectifier potassium current
- Ito
outward transient potassium current
- INa
sodium current
- ICaL
L-type calcium current
- LA
left atrium
- LV
left ventricle
- MDP
minimum diastolic potential
- PVs
pulmonary veins
- PV-LAJ
LA and PV junction
- RV
right ventricle
- SP
singularity point. (Used to indicate the pivoting location of a rotor)
- S1, S2
two sequential stimulations at particular times and locations
- TSP
timespace plot
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1395 words, excluding any confidential comments to the academic editor.
FUNDING: Supported in part by the National Heart, Lung, and Blood Institute grants (P01-HL039707, P01-HL087226, and R01-HL118304), the Coulter Foundation from the Biomedical Engineering Department (University of Michigan), the Gelman Award from the Cardiovascular Division (University of Michigan), and the Leducq Foundation. The author confirms that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: OB reports receiving grants and non-financial support from Medtronic; non-financial support from St. Jude Medical; personal fees from Biosense Webster, Boston Scientific and Aurora Medical; consultant fees from Acutus Medical; OB is co-founder, Scientific Officer and has equity in Rhythm Solutions, Inc; OB is the Research and Development Director of Volta Medical, None of these contributed to the published work. OB holds patents issued for: method and algorithm for spatially identifying cardiac sources of cardiac fibrillation; and atrial fibrillation classification using power measurement. He has patents pending for: catheter and method for detection of electrical activity in an organ; a catheter and method to localize ectopic and reentrant activity in the heart; method and system for mapping and analyzing cardiac electrical activity; apparatus, system, kit and method for heart mapping; singular value decomposition of optically-mapped cardiac rotors and fibrillatory activity.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: OB. Wrote the first draft of the manuscript: OB. Developed the structure and arguments for the paper: OB. Made critical revisions and approved final version: OB. The author reviewed and approved of the final manuscript.
REFERENCES
- 1.Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–9. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 2.Atienza F, Almendral J, Moreno J, et al. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation. 2006;114:2434–42. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 3.Cuculich PS, Wang Y, Lindsay BD, et al. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–72. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. J Am Coll Cardiol. 2012;60:628–36. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherry EM, Ehrlich JR, Nattel S, Fenton FH. Pulmonary vein reentry – properties and size matter: insights from a computational analysis. Heart Rhythm. 2007;4:1553–62. doi: 10.1016/j.hrthm.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–97. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka K, Zlochiver S, Vikstrom KL, et al. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res. 2007;101:839–47. doi: 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 8.Pertsov AM, Davidenko JM, Salomonsz R, Baxter WT, Jalife J. Spiral waves of excitation underlie reentrant activity in isolated cardiac muscle. Circ Res. 1993;72:631–50. doi: 10.1161/01.res.72.3.631. [DOI] [PubMed] [Google Scholar]
- 9.Fast VG, Pertsov AM. Drift of a vortex in the myocardium. Biophysics. 1990;35:489. [PubMed] [Google Scholar]
- 10.Ten Tusscher KH Panfilov AV. Reentry in heterogeneous cardiac tissue described by the luo-rudy ventricular action potential model. Am J Physiol Heart Circ Physiol. 2003;284:H542–8. doi: 10.1152/ajpheart.00608.2002. [DOI] [PubMed] [Google Scholar]
- 11.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 12.Hou L, Deo M, Furspan P, et al. A major role for herg in determining frequency of reentry in neonatal rat ventricular myocyte monolayer. Circ Res. 2010;107:1503–11. doi: 10.1161/CIRCRESAHA.110.232470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noujaim SF, Pandit SV, Berenfeld O, et al. Up-regulation of the inward rectifier K+ current (I K1) in the mouse heart accelerates and stabilizes rotors. J Physiol. 2007;578:315–26. doi: 10.1113/jphysiol.2006.121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cha TJ, Ehrlich JR, Zhang L, Chartier D, Leung TK, Nattel S. Atrial tachycardia remodeling of pulmonary vein cardiomyocytes: comparison with left atrium and potential relation to arrhythmogenesis. Circulation. 2005;111:728–35. doi: 10.1161/01.CIR.0000155240.05251.D0. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich JR, Cha TJ, Zhang LM, et al. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J Physiol. 2003;551:801–13. doi: 10.1113/jphysiol.2003.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemola K, Chartier D, Yeh YH, et al. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation. 2008;117:470–7. doi: 10.1161/CIRCULATIONAHA.107.737023. [DOI] [PubMed] [Google Scholar]
- 17.Po SS, Li Y, Tang D, et al. Rapid and stable re-entry within the pulmonary vein as a mechanism initiating paroxysmal atrial fibrillation. J Am Coll Cardiol. 2005;45:1871–7. doi: 10.1016/j.jacc.2005.02.070. [DOI] [PubMed] [Google Scholar]
- 18.Arora R, Verheule S, Scott L, et al. Arrhythmogenic substrate of the pulmonary veins assessed by high-resolution optical mapping. Circulation. 2003;107:1816–21. doi: 10.1161/01.CIR.0000058461.86339.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvo CJ, Deo M, Zlochiver S, Millet J, Berenfeld O. Attraction of rotors to the pulmonary veins in paroxysmal atrial fibrillation: a modeling study. Biophys J. 2014;106:1811–21. doi: 10.1016/j.bpj.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol. 1998;44:H301–21. doi: 10.1152/ajpheart.1998.275.1.H301. [DOI] [PubMed] [Google Scholar]
- 21.Kneller J, Zou R, Vigmond EJ, Wang Z, Leon LJ, Nattel S. Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circ Res. 2002;90:E73–87. doi: 10.1161/01.res.0000019783.88094.ba. [DOI] [PubMed] [Google Scholar]
- 22.Pandit SV, Berenfeld O, Anumonwo J, et al. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J. 2005;88(6):3806–21. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelband H, Bush HL, Rosen MR, Myerburg RJ, Hoffman BF. Electrophysiologic properties of isolated preparations of human atrial myocardium. Circ Res. 1972;30:293–300. doi: 10.1161/01.res.30.3.293. [DOI] [PubMed] [Google Scholar]
- 24.Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res. 2001;52:226–35. doi: 10.1016/s0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- 25.Jalife J. Experimental and clinical AF mechanisms: bridging the divide. J Interv Card Electrophysiol. 2003;9:85–92. doi: 10.1023/a:1026251517004. [DOI] [PubMed] [Google Scholar]
- 26.Oral H. Mechanisms of atrial fibrillation: lessons from studies in patients. Prog Cardiovasc Dis. 2005;48:29–40. doi: 10.1016/j.pcad.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Sarmast F, Kolli A, Zaitsev A, et al. Cholinergic atrial fibrillation: I(K,Ach) gradients determine unequal left/right atrial frequencies and rotor dynamics. Cardiovasc Res. 2003;59:863–73. doi: 10.1016/s0008-6363(03)00540-6. [DOI] [PubMed] [Google Scholar]
- 28.Davidenko JM, Salomonsz R, Pertsov AM, Baxter WT, Jalife J. Effects of pacing on stationary reentrant activity. theoretical and experimental study. Circ Res. 1995;77:1166–79. doi: 10.1161/01.res.77.6.1166. [DOI] [PubMed] [Google Scholar]
- 29.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–88. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 30.Wellner M, Pertsov AM, Jalife J. Spiral drift and core properties. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1999;59:5192–204. doi: 10.1103/physreve.59.5192. [DOI] [PubMed] [Google Scholar]
- 31.Sampson KJ, Henriquez CS. Simulation and prediction of functional block in the presence of structural and ionic heterogeneity. Am J Physiol. 2001;281:H2597–603. doi: 10.1152/ajpheart.2001.281.6.H2597. [DOI] [PubMed] [Google Scholar]
- 32.Davidenko JM, Pertsov AM, Salomonsz R, Baxter WT, Jalife J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature. 1991;355:349–51. doi: 10.1038/355349a0. [DOI] [PubMed] [Google Scholar]
- 33.Dhamoon AS, Pandit SV, Sarmast F, et al. Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res. 2004;94:1332–9. doi: 10.1161/01.RES.0000128408.66946.67. [DOI] [PubMed] [Google Scholar]
- 34.Noujaim SF, Stuckey JA, Ponce-Balbuena D, et al. Specific residues of the cytoplasmic domains of cardiac inward rectifier potassium channels are effective antifibrillatory targets. FASEB J. 2010;24(11):4302–12. doi: 10.1096/fj.10-163246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ Res. 2013;112:849–62. doi: 10.1161/CIRCRESAHA.111.300158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amit G, Kikuchi K, Greener ID, Yang L, Novack V, Donahue JK. Selective molecular potassium channel blockade prevents atrial fibrillation. Circulation. 2010;121:2263–70. doi: 10.1161/CIRCULATIONAHA.109.911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell K, Calvo CJ, Mironov S, Herron T, Berenfeld O, Jalife J. Spatial gradients in action potential duration created by regional magnetofection of hERG are a substrate for wavebreak and turbulent propagation in cardiomyocyte monolayers. J Physiol. 2012;590:6363–79. doi: 10.1113/jphysiol.2012.238758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Comtois P, Sakabe M, Vigmond EJ, et al. Mechanisms of atrial fibrillation termination by rapidly unbinding Na+ channel blockers: insights from mathematical models and experimental correlates. Am J Physiol Heart Circ Physiol. 2008;295:H1489–504. doi: 10.1152/ajpheart.01054.2007. [DOI] [PubMed] [Google Scholar]
- 39.Comtois P, Nattel S. Impact of tissue geometry on simulated cholinergic atrial fibrillation: a modeling study. Chaos. 2011;21:013108. doi: 10.1063/1.3544470. [DOI] [PubMed] [Google Scholar]
- 40.Pashaei A, Bayer J, Meillet V, Dubois R, Vigmond E. Computation and projection of spiral wave trajectories during atrial fibrillation: a computational study. Card Electrophysiol Clin. 2015;7:37–47. doi: 10.1016/j.ccep.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Sekar RB, Kizana E, Cho HC, et al. IK1 heterogeneity affects genesis and stability of spiral waves in cardiac myocyte monolayers. Circ Res. 2009;104:355–64. doi: 10.1161/CIRCRESAHA.108.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samie FH, Berenfeld O, Anumonwo J, et al. Rectification of the background potassium current: a determinant of rotor dynamics in ventricular fibrillation. Circ Res. 2001;89:1216–23. doi: 10.1161/hh2401.100818. [DOI] [PubMed] [Google Scholar]
- 43.Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm. 2008;5:846–54. doi: 10.1016/j.hrthm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grandi E, Pandit SV, Voigt N, et al. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res. 2011;109:1055–66. doi: 10.1161/CIRCRESAHA.111.253955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deo M, Ruan Y, Pandit SV, et al. KCNJ2 mutation in short QT syndrome 3 results in atrial fibrillation and ventricular proarrhythmia. Proc Natl Acad Sci U S A. 2013;110:4291–6. doi: 10.1073/pnas.1218154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bub G, Shrier A, Glass L. Global organization of dynamics in oscillatory heterogeneous excitable media. Phys Rev Lett. 2005;94:028105. doi: 10.1103/PhysRevLett.94.028105. [DOI] [PubMed] [Google Scholar]
- 47.Ashihara T, Haraguchi R, Nakazawa K, et al. The role of fibroblasts in complex fractionated electrograms during persistent/permanent atrial fibrillation: implications for electrogram-based catheter ablation. Circ Res. 2012;110:275–84. doi: 10.1161/CIRCRESAHA.111.255026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viswanathan PC, Shaw RM, Rudy Y. Effects of IKr and IKs heterogeneity on action potential duration and its rate dependence: a simulation study. Circulation. 1999;99:2466–74. doi: 10.1161/01.cir.99.18.2466. [DOI] [PubMed] [Google Scholar]
- 49.Sampson KJ, Henriquez CS. Electrotonic influences on action potential duration dispersion in small hearts: a simulation study. Am J Physiol Heart Circ Physiol. 2005;289:H350–60. doi: 10.1152/ajpheart.00507.2004. [DOI] [PubMed] [Google Scholar]
- 50.Miyata A, Dowell JD, Zipes DP, Rubart M. Rate-dependent [K+](o) accumulation in canine right atria in vivo: electrophysiological consequences. Am J Physiol Heart Circ Physiol. 2002;283:H506–17. doi: 10.1152/ajpheart.00721.2001. [DOI] [PubMed] [Google Scholar]
- 51.Zhao J, Butters TD, Zhang H, et al. An image-based model of atrial muscular architecture: effects of structural anisotropy on electrical activation. Circ Arrhythm Electrophysiol. 2012;5:361–70. doi: 10.1161/CIRCEP.111.967950. [DOI] [PubMed] [Google Scholar]
- 52.Yamazaki M, Mironov S, Taravant C, et al. Heterogeneous atrial wall thickness and stretch promote scroll waves anchoring during atrial fibrillation. Cardiovasc Res. 2012;94:48–57. doi: 10.1093/cvr/cvr357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klos M, Calvo D, Yamazaki M, et al. Atrial septopulmonary bundle of the posterior left atrium provides a substrate for atrial fibrillation initiation in a model of vagally mediated pulmonary vein tachycardia of the structurally normal heart. Circ Arrhythm Electrophysiol. 2008;1:175–83. doi: 10.1161/CIRCEP.107.760447. [DOI] [PMC free article] [PubMed] [Google Scholar]