Abstract
Legionella pneumophila utilizes a type IV secretion system (T4SS) encoded by 26 dot/icm genes to replicate inside host cells and cause disease. In contrast to all other L. pneumophila dot/icm genes, dotU and icmF have homologs in a wide variety of gram-negative bacteria, none of which possess a T4SS. Instead, dotU and icmF orthologs are linked to a locus encoding a conserved cluster of proteins designated IcmF-associated homologous proteins, which has been proposed to constitute a novel cell surface structure. We show here that dotU is partially required for L. pneumophila intracellular growth, similar to the known requirement for icmF. In addition, we show that dotU and icmF are necessary for optimal plasmid transfer and sodium sensitivity, two additional phenotypes associated with a functional Dot/Icm complex. We found that these effects are due to the destabilization of the T4SS at the transition into the stationary phase, the point at which L. pneumophila becomes virulent. Specifically, three Dot proteins (DotH, DotG, and DotF) exhibit decreased stability in a ΔdotU ΔicmF strain. Furthermore, overexpression of just one of these proteins, DotH, is sufficient to suppress the intracellular growth defect of the ΔdotU ΔicmF mutant. This suggests a model where the DotU and IcmF proteins serve to prevent DotH degradation and therefore function to stabilize the L. pneumophila T4SS. Due to their wide distribution among bacterial species and their genetic linkage to known or predicted cell surface structures, we propose that this function in complex stabilization may be broadly conserved.
Legionella pneumophila is a gram-negative bacterium that is the causative agent of a form of pneumonia known as Legionnaires' disease (16). In the environment, this pathogen is an intracellular parasite of freshwater amoebae (39). When in contact with humans, it can cause disease by replicating inside alveolar macrophages (20). L. pneumophila is able to survive and replicate inside these normally bactericidal phagocytic cells by altering their endocytic pathway to create a novel niche, the replicative phagosome, where it can grow (19, 36). Later during infection, the replicative phagosome can fuse with the lysosome prior to lysis of the host cell and release of bacteria (35).
L. pneumophila alteration of the endocytic pathway is central to its ability to cause disease and is mediated by a large number of genes called dot (defect in organelle trafficking) or icm (intracellular multiplication) that encode a type IV secretion system (T4SS) (29, 37). This family of secretion systems comprises both classical plasmid transfer systems and adapted conjugation systems used by a wide range of bacterial pathogens. Most T4SS have significant homology to the components of the Agrobacterium tumefaciens VirB complex, which exports a number of virulence factors into plant cells (6, 10). By comparison, homologs of the L. pneumophila dot/icm genes are only found in a single other pathogenic T4SS, that of Coxiella burnetii, and on the IncI plasmids ColIb-P9 and R64 (23, 32, 34).
In contrast to the other dot/icm genes, the icmF gene has at least 27 homologs present in the finished and unfinished microbial genome database (9). Organisms that contain icmF homologs represent diverse phylogeny, most notably the alpha-, beta-, and gammaproteobacteriaceae. They include plant pathogens such as A. tumefaciens, Rhizobium leguminosarum, and Xanthomonas axonopodis as well as the animal pathogens Yersinia pestis, Escherichia coli O157:H7, Vibrio cholerae, Pseudomonas aeruginosa, and Salmonella enterica (9). The only icmF homolog characterized to date is the V. cholerae gene VCA0120, which was initially described as a gene induced during V. cholerae replication in rabbit ileal loops (8). Inactivation of VCA0120 had pleiotropic effects including reduced motility, increased adherence to human intestinal epithelial cells, and enhanced conjugation as a recipient. It was speculated that these phenotypes may be the result of a change in a hypothetical cell surface structure (7, 9).
The L. pneumophila icmF gene was originally identified in a screen for Tn903dlllacZ insertion mutants defective in the ability to kill HL-60-derived macrophages (27, 28). In contrast to most of the other dot/icm genes, icmF is only partially required for L. pneumophila replication in human macrophages, though it is fully required for replication inside the more restrictive host Acanthamoeba castellanii (27, 31). Finally, a strain lacking icmF functions slightly better as a conjugal recipient of an RSF1010 plasmid when compared to a wild-type L. pneumophila strain similar to what occurs with the V. cholerae icmF mutant (7, 30). The L. pneumophila icmF gene is located at one end of dot/icm region II (27, 37) and can be found downstream of a gene previously designated dotU (34). In this paper, we characterize the role of icmF and dotU in the intracellular replication of L. pneumophila and report that they are essential for the stability of the Dot/Icm complex.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Bacterial strains are listed in Table 1. The wild-type L. pneumophila strain Lp02 (thyA hsdR rpsL) is a derivative of the serogroup 1 clinical isolate Philadelphia-1 (1). All L. pneumophila strains were cultured with N-(2-acetamido)-2-aminoethanesulfonic acid (ACES) buffered yeast extract as described previously (12, 17). Lp02 and Lp02 derivatives were cultured on media supplemented with 100 μg of thymidine/ml as needed. E. coli strains XL1-Blue and DH5α::λpir were used for cloning. Strain XL1-Blue was used for expression of His-tagged fusion proteins.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α::λpir | DH5α(λpir) tet::Mu | 22 |
| ER1821 | E. coli F−glnV44 e14-(McrA-) endA1 thi-1 Δ(mcrC-mrr)114::IS10 | New England Biolabs (Beverly, Mass.) |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr) | Stratagene (La Jolla, Calif.) |
| L. pneumophila | ||
| Lp02 | L. pneumophila Philadelphia-1 SmrrpsL hsdR thyA | 1 |
| Lp03 | Lp02 dotA mutant | 1 |
| JV1116 | Lp02 ΔdotU | This study |
| JV4015 | Lp02 ΔdotU (internal) | This study |
| JV1184 | JV1116 + pJB908 | This study |
| JV1183 | JV1116 + pJB1180 | This study |
| JV1188 | JV1116 + pJB1186 | This study |
| JV1179 | Lp02 ΔicmF | This study |
| JV1192 | JV1179 + pJB908 | This study |
| JV1194 | JV1179 + pJB1186 | This study |
| JV1181 | Lp02 ΔdotU ΔicmF | This study |
| JV1196 | JV1181 + pJB908 | This study |
| JV1199 | JV1181 + pJB1191 | This study |
| JV2572 | JV1181 + pJB1555 | This study |
| JV2573 | JV1181 + pJB1554 | This study |
| JV2574 | JV1181 + pJB2121 | This study |
| JV2575 | JV1181 + pJB2132 | This study |
| Plasmids | ||
| pKB5 | RSF1010 cloning vector thyA+, bla, mob | 1 |
| pJB908 | pKB5 ΔoriT | 33 |
| pJB1180 | pJB908, dotU+ | This study |
| pJB1186 | pJB908, icmF+ | This study |
| pJB1191 | pJB908, dotU+icmF+ | This study |
| pJB1555 | pJB908, dotH+ | This study |
| pJB1554 | pJB908, dotG+ | This study |
| pJB2121 | pJB908, dotF+ | This study |
| pJB2132 | pJB908, dotH+dotG+dotF+ | This study |
| pSR47S | R6K suicide vector (Kanr, sacB) | 25 |
| pJB1149 | pSR47S with dotU flanking regions | This study |
| pJB1720 | pSR47S with dotU internal regions | This study |
| pJB1156 | pSR47S with icmF flanking regions | This study |
| pJB1168 | pSR47S with dotU icmF flanking regions | This study |
| pQE30 | Expression vector for His-tagged proteins | Qiagen |
| pJB1537 | pQE30 with dotU | This study |
| pJB1544 | pQE30 with icmF | This study |
Strain and plasmid construction.
The suicide plasmids pJB1149, pJB1720, pJB1156, and pJB1168, used to create the ΔdotU strains JV1116 and JV4015, the ΔicmF strain JV1179, and the ΔdotU ΔicmF strain JV1181, were constructed as follows. Five hundred-base pair regions flanking the relevant open reading frames or internal to the dotU gene were PCR amplified from Lp02 chromosomal DNA with primers engineered to have SalI, BamHI, or EagI restriction sites. The resulting products were cut with SalI/BamHI or BamHI/EagI, cloned in a three-piece ligation into the SalI/NotI sites of the suicide vector pSR47S (25), and confirmed by sequencing. Next, each plasmid was transformed into the wild-type L. pneumophila strain Lp02 where it integrated into the chromosome via homologous recombination to generate a merodiploid strain. Merodiploids were resolved by using a counterselection based on sucrose sensitivity, and resulting colonies were screened for the absence of the relevant gene via PCR, thus generating strains JV1116, JV4015, JV1179, and JV1181. Two different ΔdotU strains (JV1116 and JV4015) were constructed because ΔdotU in strain JV1116 appeared to have a slight polar effect on IcmF expression. Though JV1116 can be fully complemented for intracellular growth by dotU, indicating that levels of IcmF in this strain are sufficient, we chose to use a second ΔdotU strain (JV4015) for IcmF localization experiments, since it had no polar effect on IcmF expression.
The cloning vector pJB908 (33) was used to create dotU, icmF, dotU icmF, dotH, dotG, dotF, and dotHGF complementing clones. To construct plasmids pJB1180 and pJB1186, the dotU or icmF open reading frames were PCR amplified from Lp02 chromosomal DNA with primers containing KpnI/BamHI or XbaI/SalI restriction sites, cloned into the KpnI/BamHI or XbaI/SalI sites of pJB908, and confirmed by sequencing. The dotU icmF double complementing clone pJB1191 was constructed by inserting the pJB1186 XbaI/SalI (icmF) fragment into the XbaI/SalI sites of pJB1180, thus placing the icmF open reading frame downstream of dotU. The dotH, dotG, and dotF genes were each PCR amplified from Lp02 chromosomal DNA with primers containing KpnI/BamHI, BamHI/XbaI, or XbaI/SalI restriction sites, respectively. Products were cloned into pJB908, confirmed by sequencing, and named pJB1555, pJB1554, and pJB2121. The dotH dotG dotF triple complementing clone pJB2132 was constructed by first cloning the XbaI/SalI (dotF) fragment from pJB2121 into pJB1555 and then adding the pJB1554 BamHI/XbaI (dotG) fragment, so that the final construct contained the dotH, dotG, and dotF open reading frames in that order.
For DotU and IcmF protein purification and generation of antibodies, the dotU and icmF open reading frames were PCR amplified with primers containing BamHI or BamHI/SalI restriction sites, cloned on a BamHI (dotU) or a BamHI/SalI (icmF) fragment into the His6 expression vector pQE30 (Qiagen, Valencia, Calif.), and confirmed by sequencing. The plasmids were named pJB1537 (His-DotU) and pJB1544 (His-IcmF).
Cell fractionation.
One hundred optical density at 600 nm (OD600) units of bacterial cells were grown to mid-exponential phase, harvested, and stored at −20°C until needed. For preparation of membrane fractions, cells were resuspended in 2.5 ml of cold 20 mM Tris (pH 8.0), 5 mM EDTA. Lysozyme was added to 100 μg/ml, and cells were incubated on ice for 1 h and then lysed by sonication on ice (applied in 30-s bursts for a total of 4 min). The lysate was cleared by 10 min of centrifugation at 10,000 × g at 4°C, and a sample (representing the total protein fraction) was taken. The lysate was then subjected to ultracentrifugation for 1 h at 100,000 × g at 4°C to pellet the membrane fractions. The supernatant (soluble protein fraction) was removed and transferred to a new tube while the membrane pellet (total membrane fraction) was washed in cold 20 mM Tris (pH 8.0). Both fractions were centrifuged for 30 min at 100,000 × g at 4°C, the membrane pellet was resuspended in the original volume, and samples were taken (33).
Intracellular growth assays.
The human monocytic cell line U937 was passaged, and cells were differentiated as described previously (26). Mouse bone marrow macrophages (BMM) were prepared from female A/J mice as described previously (5). In growth assays not requiring isopropyl-β-d-thiogalactopyranoside (IPTG) induction of gene expression, approximately 109 L. pneumophila cells were harvested from a 2-day 37°C patch, resuspended in sterile deionized water, and diluted 1:4,000 (for U937 cells) or 1:1,000 (for BMM) in RPMI 1640 (BioWhittaker, Walkersville, Md.) tissue culture media prior to infection. In growth assays where IPTG induction was used, L. pneumophila cells were grown in broth to exponential phase, back diluted into media containing 100 μM IPTG for overexpression of dotH, dotG, or dotF, and grown to early stationary phase. Approximately 109 bacteria were pelleted, washed one time in sterile deionized water, resuspended in 1 ml of sterile deionized water, and then diluted 1:4,000 into RPMI. In all cases, 0.5 ml of an L. pneumophila cell suspension was added to a monolayer of differentiated U937 cells (1 × 106 per well) or mouse BMM (5 × 105 per well) in 24-well dishes and the mixture was coincubated for 1 h at 37°C in 5% CO2. Medium containing extracellular bacteria was then aspirated, and cell monolayers were washed two times with 0.5 ml of RPMI (containing 100 μM IPTG, where needed). Cell monolayers were maintained at 37°C in 5% CO2 for 3 days, and each day, bacteria were recovered and plated to determine the total number of CFU in a given well. Because L. pneumophila cannot replicate in RPMI, daily quantitation of CFU allows an accurate representation of bacterial intracellular growth over time.
Antibody generation and affinity purification.
His-DotU and His-IcmF were expressed by using plasmids pJB1537 and pJB1544, purified on nickel-nitrilotriacetic acid-agarose (Qiagen), and injected into rabbits (Cocalico, Inc., Reamstown, Pa.) for generation of polyclonal antibodies. DotL antibodies were generated similarly (J. P. Vogel et al., unpublished data). DotB antibodies were raised as described previously; DotB, DotU, and IcmF antibodies were affinity purified as described previously (33). DotF, DotG, DotH, DotI, DotN, and DotO antibodies were provided by Ralph Isberg. Isocitrate dehydrogenase (ICDH) antibodies were provided by Linc Sonenshein.
Conjugation and sodium sensitivity assays.
Conjugation assays were performed as described previously (37). Briefly, ∼1 × 109 L. pneumophila donor cells containing plasmid pKB5 were grown to stationary phase, mixed with ∼1 × 1010 E. coli recipient cells (strain ER1821), and applied to a 45-mm-pore-size hemagglutinin filter (Millipore, Bedford, Mass.) on a prewarmed agar plate. After a 2-h incubation at 37°C, cells were resuspended in sterile deionized water and plated on selective media to quantitate the total number of donors and recipients in the reaction mixture. Sodium sensitivity was determined by plating dilutions of stationary-phase L. pneumophila cells on charcoal yeast extract thymidine plates with or without 0.65% mM NaCl, as previously described (4, 38).
RESULTS
dotU and icmF are conserved among diverse species.
L. pneumophila DotU (GenBank accession no. AAQ10306) and IcmF (GenBank accession no. T18341) have orthologs in a wide range of gram-negative bacterial species, with more than 27 orthologs for each protein now reported in GenBank. Notably, in every organism where an icmF ortholog is found, a dotU ortholog also exists. The IcmF orthologs share 19 to 24% identity over their entire length and are typically of a size similar to that of L. pneumophila IcmF (973 amino acids) (Table 2). The same is true for the corresponding DotU orthologs, which are close in size to that of L. pneumophila DotU (261 amino acids). In some cases, however, DotU orthologs are slightly larger; an example is the R. leguminosarum ImpK protein, which contains an amino-terminal domain homologous to DotU and a carboxy-terminal domain homologous to OmpA. In addition, some species contain multiple copies of DotU and IcmF (Table 2) (15).
TABLE 2.
L. pneumophila DotU and IcmF homologs exist in a wide range of other bacteria
| Homolog no. | Organism | IcmF locus no. | No. of IcmF amino acids | E value (% identity) | DotU locus no. | No. of DotU amino acids | E value (% identity) |
|---|---|---|---|---|---|---|---|
| 1 | V. cholerae | VCA120 | 1,181 | 2e−35 (19) | VCA115 | 257 | 1e−09 (23) |
| 2 | E. coli O157:H7 VT2 Sakai | Z0250 | 1,144 | 7e−32 (19) | Z0255 | 253 | 2e−07 (23) |
| 3 | Rhizobium | ImpL | 1,158 | 1e−28 (21) | ImpK | 510 | 5e−05 (24) |
| 4 | Salmonella serovar Typhimurium LT2 | STM0285 (SciS) | 1,289 | 6e−15 (20) | SciP | 434 | 6e−05 (24) |
| 5 | A. tumefaciens | AGR_L_1062 | 1,159 | 3e−31 (19) | AGR_L_1060 | 506 | 6e−05 (23) |
| 6 | P. aeruginosa | PA0077 | 1,101 | 4e−40 (20) | PA0078 | 449 | 3e−11 (29) |
| 7 | P. aeruginosa | PA1669 | 1,175 | 3e−44 (19) | PA1668 | 289 | 6e−11 (28) |
| 8 | P. aeruginosa | PA2361 | 1,271 | ND (13)a | PA2362 | 252 | ND (17) |
| 9 | Y. pestis CO92 | YPO3603 | 1,177 | 4e−36 (21) | YPO3598 | 255 | 3e−03 (19) |
| 10 | Y. pestis | YPO0515 | 1,150 | 1e−22 (19) | YPO0514 | 536 | 8e−05 (27) |
| 11 | Y. pestis | YPO2724 | 1,275 | 2e−11 (18) | None | NAb | NA |
| 12 | Y. pestis | YPO1482 | 1,140 | 3e−05 (22) | None | NA | NA |
| 13 | X. axonopodis pv citri strain 306 | XAC4119 | 1,176 | 3e−24 (19) | XAC4120 | 451 | 3e−14 (30) |
ND, not detectable by BLAST search.
NA, not applicable.
The L. pneumophila dotU and icmF genes are located at one end of dot/icm region II, which encodes structural components of the T4SS (29, 37) (Fig. 1). In contrast, the dotU and icmF homologs in other organisms can be found in a conserved cluster of genes that encode proteins known as IcmF-associated homologous proteins (IAHPs) (9). Three such loci have been characterized to date, and these are found in V. cholerae, R. leguminosarum, and S. enterica (Fig. 1) (2, 7, 15). Each locus contains approximately a dozen proteins with various degrees of conservation, and some IAHP clusters also contain a ClpB homolog (15). The majority of these proteins are predicted to localize to the membrane and likely constitute a macromolecular complex of unknown function.
FIG. 1.
IcmF and DotU orthologs are found in IAHP gene clusters. The L. pneumophila (L.p.) dotU and icmF genes (shown by red arrows) are located adjacent to a large number of dot/icm genes (shown by blue arrows) and are immediately flanked by genes with no role in type IV secretion (shown by white arrows). The IAHPs from V. cholerae (V.c.), R. leguminosarum (R.l.), and S. enterica (S.e.) all contain orthologs to dotU and icmF (shown by red arrows). The IAHP clusters contain a conserved core set of proteins (shown by solid green arrows) and less well conserved proteins that are found only in a subset of IAHP loci (indicated by stippled or checkered green arrows). Finally, most of these loci also contain a clpB homolog (shown by a black arrow).
DotU and IcmF are partially required for intracellular growth.
To test whether dotU, like icmF, plays a role in L. pneumophila replication, we compared the growth of the wild-type strain Lp02 with a strain containing an in-frame deletion of dotU (JV1116) in U937 macrophages. The wild-type strain grew more than 1,000-fold in 3 days. In contrast, the ΔdotU strain was partially defective in replication, with 10- to 100-fold-fewer CFU than strain Lp02. This is in contrast to most dot/icm mutant strains, including the dotA null mutant strain Lp03, which are completely defective for replication (Fig. 2A). JV1116 (ΔdotU) intracellular growth was restored to wild-type levels by a dotU complementing clone and could not be restored with an icmF complementing clone (Fig. 2A). This indicated that the ΔdotU mutant JV1116 can be fully complemented for intracellular growth and that the intracellular growth defect we observed was not due to polarity on the downstream icmF gene.
FIG. 2.
Intracellular growth of dotU and icmF mutants in U937 monocytes. (A) The wild-type L. pneumophila strain Lp02 (squares), the dotA mutant Lp03 (diamonds), and the ΔdotU strain JV1116 containing either the empty vector pJB908 (circles), the dotU complementing clone pJB1180 (triangles), or the icmF complementing clone pJB1186 (inverted triangles) were assayed for growth in U937 cells. Mean numbers of CFU are plotted as a function of time. (B) The wild-type L. pneumophila strain Lp02 (squares), the dotA mutant Lp03 (diamonds), and the ΔicmF strain JV1179 containing either the empty vector pJB908 (circles) or the icmF complementing clone pJB1186 (triangles) were assayed for growth in U937 cells. (C) The wild-type L. pneumophila strain Lp02 (squares), the dotA mutant Lp03 (diamonds), and the ΔdotU ΔicmF strain JV1181 containing either the empty vector pJB908 (circles) or the dotU icmF complementing clone pJB1191 (triangles) were assayed for growth in U937 cells. Assays were done in triplicate. Error bars indicate standard deviations of the means. d, day.
To compare the L. pneumophila requirement for dotU and icmF in U937 cells, we tested an ΔicmF strain (JV1179) and found that it also has a partial growth defect in this cell line. This phenotype could be fully complemented by expression of icmF from a plasmid (Fig. 2B). Thus, the dotU and icmF mutants have similar intracellular growth phenotypes. In addition, a dotU icmF double deletion strain (JV1181) likewise has a partial growth defect in U937s, indicating that the DotU and IcmF proteins may work together to perform a common function (Fig. 2C).
The intracellular replication phenotypes of the ΔdotU, ΔicmF, and ΔdotU ΔicmF strains were also characterized in mouse BMM, a more stringent host (21). The growth defect observed for the single mutants was more severe in mouse BMM than in U937s (Fig. 3A and B). However, the mutants were still able to replicate at low levels compared to the replication-deficient dotA null mutant Lp03. Finally, the dotU icmF double deletion strain appeared similar to the single mutants, confirming the observation in U937 cells (Fig. 3C).
FIG. 3.
Intracellular growth of dotU and icmF mutants in mouse BMM. (A) The wild-type L. pneumophila strain Lp02 (squares), the dotA mutant Lp03 (diamonds), and the ΔdotU strain JV1116 containing either the empty vector pJB908 (circles), the dotU complementing clone pJB1180 (triangles), or the icmF complementing clone pJB1186 (inverted triangles) were assayed for growth in BMM. Mean numbers of CFU are plotted as a function of time. (B) The wild-type L. pneumophila strain Lp02 (squares), the dotA mutant Lp03 (diamonds), and the ΔicmF strain JV1179 containing either the empty vector pJB908 (circles) or the icmF complementing clone pJB1186 (triangles) were assayed for growth in BMM. (C) The wild-type L. pneumophila strain Lp02 (squares), the dotA mutant Lp03 (diamonds), and the ΔdotU ΔicmF strain JV1181 containing either the empty vector pJB908 (circles) or the dotU icmF complementing clone pJB1191 (triangles) were assayed for growth in BMM. Assays were done in triplicate. Error bars indicate standard deviations of the means. d, day.
DotU and IcmF localize to the L. pneumophila membrane.
Examination of the DotU and IcmF predicted protein sequences reveals few hints as to their potential functions. IcmF is a large protein that contains a potential nucleotide binding motif (GXXXXGKS/T) (40). In addition, it appears to possess several transmembrane domains, consistent with localization to the bacterial inner membrane. DotU is a smaller protein that does not contain any obvious functional motifs and that is also predicted to localize to the membrane. To confirm DotU and IcmF intracellular localization, L. pneumophila extracts were fractionated into cytoplasm and membrane samples and subjected to Western blots with polyclonal DotU and IcmF antibodies. Although IcmF protein was readily detected in cell extracts from an early-stationary-phase culture, we failed to detect any DotU (data not shown). Subsequent analysis of whole-cell samples taken at various stages of L. pneumophila growth revealed that DotU is present in the exponential phase but not in the stationary phase, consistent with rapid processing or degradation upon entry into stationary phase (Fig. 4). Similarly, IcmF is also absent in the late stages of L. pneumophila growth. In contrast to DotU, however, complete disappearance of full-length IcmF does not appear to occur until late in stationary phase and happens gradually (Fig. 4). This type of growth-phase-related fluctuation in protein levels is atypical for most Dot/Icm proteins, which appear to be constitutively expressed (Vogel et al., unpublished) (Fig. 4, DotL; see also Fig. 7, DotB).
FIG. 4.
DotU and IcmF disappear at different points in stationary-phase L. pneumophila. Equivalent OD600 units of cells from L. pneumophila wild-type strain Lp02 were taken at various time points during growth in broth, from early exponential (E) through late stationary (S) phase. Lanes 1 through 6 correspond to culture OD600s of 2.5, 2.8, 3.1, 3.2, 3.4, and 3.4, respectively. Cells became motile between OD600s of 2.8 and 3.1, just prior to entering the stationary phase. Cell lysates were used for DotU, IcmF, DotL, or ICDH Western blots. Solid arrowheads point to bands that correspond to full-length proteins while the open arrowhead indicates a smaller reactive species likely to represent processed or partially degraded IcmF protein. The molecular masses of relevant markers (in kilodaltons) are shown on the left.
FIG. 7.
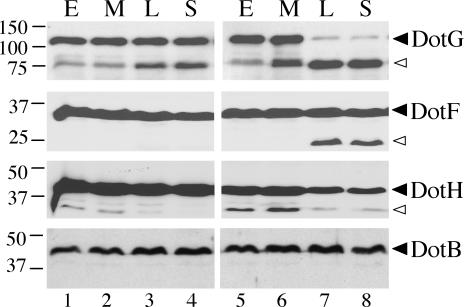
DotG, DotF, and DotH protein levels are altered in a ΔdotU ΔicmF strain. Equivalent OD600 units of cells from the L. pneumophila wild-type strain Lp02 (lanes 1 to 4) or the ΔdotU ΔicmF strain JV1181 (lanes 5 to 8) were taken at the early (E), mid-exponential (M), late exponential (L), and stationary (S) phases of growth and used as total protein samples for DotG, DotF, or DotH Western blots. The same samples were used for a DotB Western blot as a negative control. The molecular masses of relevant markers (in kilodaltons) are shown on the left. Solid arrowheads point to bands that correspond to full-length proteins while open arrowheads indicate smaller reactive species likely to represent processed or partially degraded proteins. Results are representative of those of several experiments.
Based on the above-described result, cell fractions generated from exponential cells were used to determine the intracellular localization of DotU and IcmF. As predicted, both DotU and IcmF were found to be present almost entirely in the membrane fraction (Fig. 5, lanes 1 to 3). Levels and membrane localization of IcmF were not affected by the absence of dotU (Fig. 5, lanes 7 to 9), confirming that this ΔdotU strain was not polar on the downstream icmF gene. In contrast, the loss of icmF had a strong effect on DotU protein levels (Fig. 5, lanes 4 to 6). This result is consistent with a DotU/IcmF protein-protein interaction, as interacting proteins often stabilize each other (13, 18).
FIG. 5.
Subcellular localization of DotU and IcmF. (A) Total protein (T, lanes 1, 4, and 7), total soluble protein (S, lanes 2, 5, and 8), and total membrane protein (M, lanes 3, 6, and 9) fractions were taken from equivalent OD600 units of three L. pneumophila strains and were subjected to DotU and IcmF Western blots. Strains included the wild-type Lp02 strain (lanes 1 to 3), the ΔicmF strain JV1179 (lanes 4 to 6), and the ΔdotU strain JV4015 (lanes 7 to 9). The molecular masses of relevant markers (in kilodaltons) are shown on the left. Results are representative of those of several experiments.
The dotU and icmF genes are partially required for additional dot/icm-associated phenotypes.
The observation that dotU and icmF-like genes are not normally associated with T4SS but are instead found in diverse organisms that lack T4SS genes (Table 2) (2, 9, 15) suggests that they are not likely to be core components of the L. pneumophila T4SS. Nevertheless, ΔdotU ΔicmF mutants were incapable of wild-type intracellular growth, a process that requires a functional Dot/Icm complex. This phenotype was not due to a general defect in replication, as these strains could grow normally outside of host cells in AYET broth (data not shown). To determine whether the intracellular growth defect of the dotU and icmF mutants was due to an effect on the T4SS, we examined these mutant strains for additional dot/icm-dependent phenotypes. First, the ΔdotU, ΔicmF, and ΔdotU ΔicmF strains were tested for the ability to transfer an RSF1010 plasmid to an E. coli recipient. While the wild-type strain Lp02 could transfer a plasmid at a frequency of ∼5 × 10−6 recipients per donor cell, strains JV1116 (ΔdotU), JV1179 (ΔicmF), and JV1181 (ΔdotU ΔicmF) did so with a 10-fold-reduced frequency. Strain Lp03 (dotA null mutant) was incapable of transferring a plasmid above the limit of detection, ∼1 × 10−9 (Fig. 6A). Thus, dotU and icmF appear to be partially required for Dot/Icm-mediated plasmid transfer.
FIG. 6.
Conjugation and salt resistance phenotypes of dotU and icmF mutants. (A) The following L. pneumophila strains were assayed for the ability to transfer an RSF1010 plasmid to E. coli recipient cells: wild-type strain Lp02 (column 1), dotA mutant strain Lp03 (column 2), ΔdotU strain JV1116 (column 3), ΔicmF strain JV1179 (column 4), and ΔdotU ΔicmF strain JV1181 (column 5). The conjugation frequency was calculated as the number of E. coli recipients per L. pneumophila donor cell. Assays were done in triplicate. Error bars indicate the standard deviations of the means. (B) The plating efficiency on 0.65% NaCl was determined for strains used in panel A, and is shown here as the percentage of NaCl-resistant CFU in a cell population. The strains were wild-type Lp02 (column 1), dotA mutant Lp03 (column 2), the ΔdotU strain JV1116 (column 3), the ΔicmF strain JV1179 (column 4), and the ΔdotU ΔicmF strain JV1181 (column 5). Assays were done in triplicate. Error bars indicate standard deviations of the means.
Next, the dotU and icmF mutants were characterized for sodium sensitivity. Wild-type L. pneumophila is normally highly sodium sensitive, whereas mutations in the Dot/Icm machinery confer sodium resistance. The plating efficiencies of strains JV1116 (ΔdotU), JV1179 (ΔicmF), and JV1181 (ΔdotU ΔicmF) on medium with sodium were found to be 10- to 100-fold reduced compared to the dotA null mutant strain Lp03 but approximately 100-fold greater than the wild-type strain Lp02 (Fig. 6B), indicating a partial role for DotU and IcmF in sodium resistance. Both the sodium sensitivity and plasmid transfer deficiencies of strain JV1181 (ΔdotU ΔicmF) could be fully complemented (data not shown). Because the dotU and icmF mutant strains are partially defective in intracellular growth, plasmid transfer, and sodium sensitivity, we predicted that the DotU and IcmF proteins may directly affect the Dot/Icm complex.
Mutations in dotU and icmF affect stability of the DotH, DotG, and DotF proteins.
To check for effects of DotU and IcmF on the Dot/Icm complex, we performed Western blots against Lp02 and JV1181 (ΔdotU ΔicmF) whole-cell extracts with a panel of Dot/Icm antibodies. We found that the DotH, DotG, and DotF proteins were affected for stability in the dotU icmF mutant strain compared to wild-type strain Lp02 (Fig. 7). Altered protein levels were only apparent in whole-cell extracts taken from late-exponential- or stationary-phase cultures, reminiscent of the DotU processing previously observed. The fact that DotHGF destabilization correlates with DotU processing suggests that the latter event may be required for the normal activity of DotU.
The DotG protein was the most profoundly altered by the absence of dotU and icmF and could only be found as an ∼75-kDa breakdown product in the late stages of in vitro growth. While levels of full-length DotF were not markedly reduced in the ΔdotU ΔicmF strain, an ∼25-kDa breakdown product not seen in the wild-type strain appeared in the late stages of growth (Fig. 7). In contrast, levels of full-length DotH protein were reduced in the late stages of JV1181 (ΔdotU ΔicmF) growth compared to those of Lp02, but this did not correlate with a visible increase in breakdown product (Fig. 7). All observed stability effects were identical in the ΔdotU, ΔicmF, and ΔdotU ΔicmF strains and could be complemented (data not shown). Notably, these effects were specific to a subset of Dot/Icm proteins, as the majority were not destabilized in the ΔdotU ΔicmF strain; specifically, levels of DotB, DotI, DotL, DotN, and DotO were not altered. Thus, DotU and IcmF affect the stability of a subset of Dot/Icm proteins.
Overexpression of dotH suppresses the intracellular growth defect of a dotU icmF mutant.
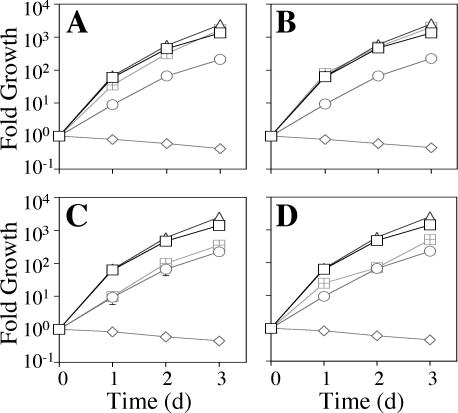
To determine whether the dot/icm phenotypes of the ΔdotU ΔicmF strain were caused only by the reduced levels of DotH, DotG, and DotF, we expressed the corresponding genes in strain JV1181 (ΔdotU ΔicmF) and assayed intracellular growth. We found that simultaneous overexpression of dotHGF was sufficient to eliminate the ΔdotU ΔicmF intracellular growth defect in U937 cells (Fig. 8A). Furthermore, overexpression of just the dotH gene alone was able to suppress the growth defect, whereas this effect was not seen with just the dotF or dotG complementing clones (Fig. 8B to D).
FIG. 8.
Suppression of ΔdotU ΔicmF intracellular growth defect by overexpression of DotH. (A) The wild-type L. pneumophila strain Lp02 (squares), the dotA mutant Lp03 (diamonds), and the ΔdotU ΔicmF strain JV1181 containing either the empty vector pJB908 (circles), the dotU icmF complementing clone pJB1191 (triangles), or the dotHGF complementing clone pJB2132 (hatched squares) were assayed for growth in U937 cells. Mean numbers of CFU are plotted as a function of time. (B) The wild-type L. pneumophila strain Lp02 (squares), the dotA mutant Lp03 (diamonds), and the ΔdotU ΔicmF strain JV1181 containing either the empty vector pJB908 (circles), the dotU icmF complementing clone pJB1191 (triangles), or the dotH complementing clone pJB1555 (hatched squares) were assayed for growth in U937 cells. (C) The wild-type L. pneumophila strain Lp02 (squares), the dotA mutant Lp03 (diamonds), and the ΔdotU ΔicmF strain JV1181 containing either the empty vector pJB908 (circles), the dotU icmF complementing clone pJB1191 (triangles), or the dotG complementing clone pJB1554 (hatched squares) were assayed for growth in U937 cells. (D) The wild-type L. pneumophila strain Lp02 (squares), the dotA mutant Lp03 (diamonds), and the ΔdotU ΔicmF strain JV1181 containing either the empty vector pJB908 (circles), the dotU icmF complementing clone pJB1191 (triangles), or the dotF complementing clone pJB2121 (hatched squares) were assayed for growth in U937 cells. Growth curves were determined in the presence of 100 μM IPTG. Assays were done in triplicate. Error bars indicate standard deviations of the means. d, day.
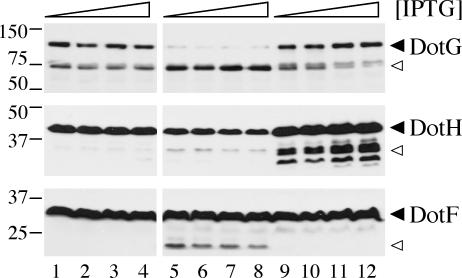
One explanation for this is that DotU and IcmF directly affect only DotH stability, and the decreased levels of DotG and DotF result indirectly from lowered levels of DotH. Therefore, we tested whether overexpression of dotH in the ΔdotU ΔicmF strain could restore wild-type levels of DotG and DotF. We compared DotH, DotG, and DotF protein levels in the wild-type strain Lp02, the ΔdotU ΔicmF strain, or the ΔdotU ΔicmF strain containing the dotH complementing clone pJB1555, each exposed to a range of IPTG concentrations. Expression of dotH with all concentrations of IPTG resulted in overproduction of DotH (Fig. 9, compare lanes 9 to 12 with lanes 5 to 8). Moreover, expression of dotH restored wild-type levels of DotG and eliminated the apparent degradation of DotF. These findings suggest the DotU and IcmF proteins work together to stabilize the Dot/Icm complex by maintaining proper levels of functional DotH, which is required for the stability of DotG and DotF.
FIG. 9.
Suppression of ΔdotU ΔicmF protein aberrations by overexpression of DotH. L. pneumophila wild-type strain Lp02 (lanes 1 to 4) or ΔdotU ΔicmF strain JV1181 containing either the empty vector pJB908 (lanes 5 to 8) or the dotH complementing clone pJB1555 (lanes 8 to 12) was grown in the presence of 0, 10, 100, or 1,000 μM IPTG to stationary phase. Whole-cell samples (equivalent OD600 units) were taken and subjected to DotH, DotG, or DotF Western blots. The molecular masses of relevant markers (in kilodaltons) are shown on the left. Arrowheads are as described in the legend to Fig. 7.
DISCUSSION
We have shown here that the dotU gene, adjacent to icmF on the L. pneumophila chromosome, is required for the optimal intracellular growth of this organism. Loss of either dotU alone, icmF alone, or both genes causes a similar impairment in intracellular replication of L. pneumophila. We have observed that several of the Dot/Icm proteins, DotH, DotG, and DotF, appear to be significantly destabilized in a ΔdotU ΔicmF mutant strain. This destabilization can be suppressed by overproduction of a single gene, dotH, indicating that the DotH protein is likely a key factor in the stability of a putative DotHGF subcomplex. Furthermore, overexpression of dotH was sufficient to abolish the intracellular growth defect of the ΔdotU ΔicmF mutant strain, suggesting that destabilization of DotH is the critical factor inhibiting intracellular replication in the ΔdotU ΔicmF background. Since DotH, DotG, and DotF are components of the Dot/Icm secretion machinery and since this machinery is known to be of critical importance for alteration of the endocytic pathway, it is reasonable to conclude that the ΔdotU ΔicmF intracellular growth defect likely results from mistargeting of L. pneumophila cells early in infection.
The icmF gene was originally identified in a screen for L. pneumophila mutants defective in the ability to kill HL-60-derived macrophages (27, 28). dotU, immediately upstream of icmF, was first identified via a transposon insertion that impaired the ability of L. pneumophila to cause disease in a guinea pig model of infection (11). However, since the insertion was not complemented, it remained possible that the phenotype was simply due to a polar effect on the downstream icmF gene (11). In this report, we have generated a nonpolar ΔdotU strain that displays a partial intracellular growth defect similar to an ΔicmF strain. The ΔdotU ΔicmF double mutant has a phenotype identical to that of either single mutant, which suggests that the two proteins may function together. This notion is supported by several facts. First, the two genes appear to be carried on a single operon, a trait often indicative of genes that perform a similar activity. Second, the proteins colocalize to the L. pneumophila membrane. Finally, loss of the IcmF protein affects the stability of DotU, a trait common among proteins that interact (13, 18).
It was a formal possibility that the intracellular growth defect seen in dotU icmF mutants was independent of the T4SS. However, several lines of evidence exist to the contrary. First, the ΔdotU ΔicmF mutant is partially resistant to low levels of sodium added to the media, a trait shared with other dot/icm mutants. Second, the ΔdotU ΔicmF mutant is partially defective for transfer of a plasmid, a second trait shared with the other dot/icm mutants. Finally, the ΔdotU ΔicmF mutant is able to suppress the lethality caused by the loss of the dotL gene (Vogel et al., unpublished). In certain strains of L. pneumophila, loss of the DotL protein has been observed to be a lethal event that can be suppressed by inactivation of the majority of the dot/icm genes. Thus, suppression of ΔdotL lethality is an additional dot/icm phenotype (Vogel et al., unpublished). Taken together, these data suggest that while DotU and IcmF are not likely to be core components of the Dot/Icm complex, they somehow modify it.
Consistent with this, we observed degradation of at least three Dot/Icm proteins in the ΔdotU ΔicmF strain background: DotF, DotG, and DotH. Although DotH is predicted to localize to the inner membrane by sequence analysis, it has been shown to be extruded on the L. pneumophila cell surface during infection (41). DotF has been shown to interact with several Dot/Icm secreted substrates by a two-hybrid system (24). DotG has limited similarity to the A. tumefaciens VirB10 protein, a core component of the VirB T4SS, in which it forms a subcomplex with VirB9 and VirB11 (14). Based on the homology of DotG to VirB10 and the data presented here, it is possible that DotF, DotG, and DotH form a similar subcomplex in L. pneumophila.
Overexpression of dotH alone was sufficient to suppress the destabilization of the Dot/Icm complex due to loss of dotU and icmF, resulting in restored levels of DotG and DotF and elimination of the ΔdotU ΔicmF intracellular growth defect. This suggests that DotH may be the key component of a proposed DotHGF subcomplex. Analysis of ΔdotH, ΔdotG, and ΔdotF strains is in keeping with this. Levels of DotH protein are unaffected by mutations in dotG or dotF while levels of DotF are mildly affected by mutations in dotH but not dotG, and finally, levels of DotG are drastically affected by mutations in either dotH or dotF (Vogel et al., unpublished). Therefore, DotU and IcmF may act directly on DotH, the disruption of which would then affect the DotG and DotF proteins.
The loss of dotU/icmF results in degradation of components of the Dot/Icm complex at the transition from exponential phase into stationary phase. Based on this growth phase specificity, it is apparent that dotU and icmF are critical to L. pneumophila precisely at the time when it becomes virulent (3). Curiously, DotU itself also appears to be processed and/or degraded at the transition into the virulent state, suggesting that this event may be important in DotU and/or IcmF function.
Data shown here are consistent with a role for L. pneumophila DotU and IcmF as accessory factors to the type IV secretion machinery, protecting it from degradation (Fig. 10). One possibility is that they assist in the assembly of a functional Dot/Icm complex and that in their absence the complex misassembles, leading to partial proteolysis. Alternatively, DotU and IcmF could be critical components for maintaining the assembled complex in a stable and active form, thus protecting it from destabilization and proteolysis in the stationary phase. A third possibility is that these proteins function to regulate the Dot/Icm complex, controlling a switch from an inactive to an active T4SS in response to growth phase; DotU degradation and processing could be the key event in this process. Finally, these proteins could be involved in recycling of the L. pneumophila T4SS.
FIG. 10.
Model of how DotU and IcmF prevent destabilization of the Dot/Icm complex. The DotU and IcmF proteins localize to the inner membrane where they likely work together to shield one or more components of the Dot/Icm complex from degradation by an as yet unidentified protease. In the absence of DotU/IcmF, the subcomplex DotH, DotG, and DotF is selectively targeted for proteolysis.
One of the most intriguing features of DotU and IcmF is their high degree of conservation among diverse bacterial species. In many organisms, dotU and icmF orthologs are found in IAHP loci, which have been proposed to encode cell surface structures (2, 9, 15). Inactivation of the icmF homolog in the V. cholerae IAHP locus appeared to have pleiotropic effects on cell surface structures (7, 9). Deletion of the S. enterica IAHP locus resulted in a slightly decreased ability to invade eukaryotic cells (15). Although the precise function of these IAHP loci remains unknown, they appear to encode a cell surface organelle. In fact, the R. leguminosarum IAHP locus (designated imp), involved in nodule formation, has been shown to encode a temperature-dependent secretion system (2). Thus, similar to the case with L. pneumophila, the dotU and icmF orthologs are genetically linked to macromolecular membrane-spanning complexes. Whether for T4SS or IAHP clusters, DotU and IcmF proteins are likely to serve a related function as novel accessory factors that maintain the stability of membrane complexes.
Acknowledgments
We thank Patrick Bardill and Carr Vincent for critical analysis of the manuscript, Ralph Isberg for providing Dot/Icm antibodies, and Linc Sonenshein for providing ICDH antibodies.
J.A.S. was supported by the Washington University, Department of Internal Medicine, Infectious Diseases Training Grant no. 5 T32 AI07172-22. J.P.V. was supported by the Whittaker Foundation, the American Lung Association, and NIH grant AI48052-02.
Editor: J. T. Barbieri
REFERENCES
- 1.Berger, K. H., and R. R. Isberg. 1993. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7:7-19. [DOI] [PubMed] [Google Scholar]
- 2.Bladergroen, M. R., K. Badelt, and H. P. Spaink. 2003. Infection-blocking genes of a symbiotic Rhizobium leguminosarum strain that are involved in temperature-dependent protein secretion. Mol. Plant-Microbe Interact. 16:53-64. [DOI] [PubMed] [Google Scholar]
- 3.Byrne, B., and M. S. Swanson. 1998. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catrenich, C. E., and W. Johnson. 1989. Characterization of the selective inhibition of growth of virulent Legionella pneumophila by supplemented Mueller-Hinton medium. Infect. Immun. 57:1862-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celada, A., P. W. Gray, E. Rinderknecht, and R. D. Schreiber. 1984. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J. Exp. Med. 160:55-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christie, P. J., and J. P. Vogel. 2000. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 8:354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das, S., A. Chakrabortty, R. Banerjee, and K. Chaudhuri. 2002. Involvement of in vivo induced icmF gene of Vibrio cholerae in motility, adherence to epithelial cells, and conjugation frequency. Biochem. Biophys. Res. Commun. 295:922-928. [DOI] [PubMed] [Google Scholar]
- 8.Das, S., A. Chakrabortty, R. Banerjee, S. Roychoudhury, and K. Chaudhuri. 2000. Comparison of global transcription responses allows identification of Vibrio cholerae genes differentially expressed following infection. FEMS Microbiol. Lett. 190:87-91. [DOI] [PubMed] [Google Scholar]
- 9.Das, S., and K. Chaudhuri. 2003. Identification of a unique IAHP (IcmF associated homologous proteins) cluster in Vibrio cholerae and other proteobacteria through in silico analysis. In Silico Biol. 3:287-300. [PubMed] [Google Scholar]
- 10.Ding, Z., K. Atmakuri, and P. J. Christie. 2003. The outs and ins of bacterial type IV secretion substrates. Trends Microbiol. 11:527-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelstein, P. H., M. A. Edelstein, F. Higa, and S. Falkow. 1999. Discovery of virulence genes of Legionella pneumophila by using signature tagged mutagenesis in a guinea pig pneumonia model. Proc. Natl. Acad. Sci. USA 96:8190-8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feeley, J. C., R. J. Gibson, G. W. Gorman, N. C. Langford, J. K. Rasheed, D. C. Mackel, and W. B. Baine. 1979. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J. Clin. Microbiol. 10:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez, D., G. M. Spudich, X. R. Zhou, and P. J. Christie. 1996. The Agrobacterium tumefaciens VirB7 lipoprotein is required for stabilization of VirB proteins during assembly of the T-complex transport apparatus. J. Bacteriol. 178:3168-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finberg, K. E., T. R. Muth, S. P. Young, J. B. Maken, S. M. Heitritter, A. N. Binns, and L. M. Banta. 1995. Interactions of VirB9, -10, and -11 with the membrane fraction of Agrobacterium tumefaciens: solubility studies provide evidence for tight associations. J. Bacteriol. 177:4881-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folkesson, A., S. Lofdahl, and S. Normark. 2002. The Salmonella enterica subspecies I specific centisome 7 genomic island encodes novel protein families present in bacteria living in close contact with eukaryotic cells. Res. Microbiol. 153:537-545. [DOI] [PubMed] [Google Scholar]
- 16.Fraser, D. W., T. R. Tsai, W. Orenstein, W. E. Parkin, H. J. Beecham, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, J. E. McDade, C. C. Shepard, and P. S. Brachman. 1977. Legionnaires' disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297:1189-1197. [DOI] [PubMed] [Google Scholar]
- 17.Gabay, J. E., and M. A. Horwitz. 1985. Isolation and characterization of the cytoplasmic and outer membranes of the Legionnaires′ disease bacterium (Legionella pneumophila). J. Exp. Med. 161:409-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hapfelmeier, S., N. Domke, P. C. Zambryski, and C. Baron. 2000. VirB6 is required for stabilization of VirB5 and VirB3 and formation of VirB7 homodimers in Agrobacterium tumefaciens. J. Bacteriol. 182:4505-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz, M. A. 1983. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158:2108-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horwitz, M. A., and S. C. Silverstein. 1980. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J. Clin. Investig. 66:441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi, A. D., and M. S. Swanson. 1999. Comparative analysis of Legionella pneumophila and Legionella micdadei virulence traits. Infect. Immun. 67:4134-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolter, R., M. Inuzuka, and D. R. Helinski. 1978. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell 15:1199-1208. [DOI] [PubMed] [Google Scholar]
- 23.Komano, T., T. Yoshida, K. Narahara, and N. Furuya. 2000. The transfer region of IncI1 plasmid R64: similarities between R64 tra and Legionella icm/dot genes. Mol. Microbiol. 35:1348-1359. [DOI] [PubMed] [Google Scholar]
- 24.Luo, Z. Q., and R. R. Isberg. 2004. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. USA 101:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merriam, J. J., R. Mathur, R. Maxfield-Boumil, and R. R. Isberg. 1997. Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect. Immun. 65:2497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearlman, E., A. H. Jiwa, N. C. Engleberg, and B. I. Eisenstein. 1988. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb. Pathog. 5:87-95. [DOI] [PubMed] [Google Scholar]
- 27.Purcell, M., and H. A. Shuman. 1998. The Legionella pneumophila icmGCDJBF genes are required for killing of human macrophages. Infect. Immun. 66:2245-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadosky, A. B., L. A. Wiater, and H. A. Shuman. 1993. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect. Immun. 61:5361-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segal, G., M. Purcell, and H. A. Shuman. 1998. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. USA 95:1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segal, G., and H. A. Shuman. 1998. Intracellular multiplication and human macrophage killing by Legionella pneumophila are inhibited by conjugal components of IncQ plasmid RSF1010. Mol. Microbiol. 30:197-208. [DOI] [PubMed] [Google Scholar]
- 31.Segal, G., and H. A. Shuman. 1999. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun. 67:2117-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segal, G., and H. A. Shuman. 1999. Possible origin of the Legionella pneumophila virulence genes and their relation to Coxiella burnetii. Mol. Microbiol. 33:669-670. [DOI] [PubMed] [Google Scholar]
- 33.Sexton, J. A., J. S. Pinkner, R. Roth, J. E. Heuser, S. J. Hultgren, and J. P. Vogel. 2004. The Legionella pneumophila PilT homologue DotB exhibits ATPase activity that is critical for intracellular growth. J. Bacteriol. 186:1658-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sexton, J. A., and J. P. Vogel. 2002. Type IVB secretion by intracellular pathogens. Traffic 3:178-185. [DOI] [PubMed] [Google Scholar]
- 35.Sturgill-Koszycki, S., and M. S. Swanson. 2000. Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J. Exp. Med. 192:1261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogel, J. P., H. L. Andrews, S. K. Wong, and R. R. Isberg. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279:873-876. [DOI] [PubMed] [Google Scholar]
- 38.Vogel, J. P., C. Roy, and R. R. Isberg. 1996. Use of salt to isolate Legionella pneumophila mutants unable to replicate in macrophages. Ann. N. Y. Acad. Sci. 797:271-272. [DOI] [PubMed] [Google Scholar]
- 39.Wadowsky, R. M., L. J. Butler, M. K. Cook, S. M. Verma, M. A. Paul, B. S. Fields, G. Keleti, J. L. Sykora, and R. B. Yee. 1988. Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Appl. Environ. Microbiol. 54:2677-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watarai, M., H. L. Andrews, and R. R. Isberg. 2001. Formation of a fibrous structure on the surface of Legionella pneumophila associated with exposure of DotH and DotO proteins after intracellular growth. Mol. Microbiol. 39:313-329. [DOI] [PubMed] [Google Scholar]