Abstract
Toll-like receptor (TLR) signal transduction is a central component of the innate immune response to pathogenic challenge. Although recent studies have begun to elucidate differences in acquired immunity in tissues of the human female reproductive tract, there is a relative paucity of work regarding innate defense mechanisms. We investigated TLR mRNA and protein expression in tissues of the human female reproductive tract. Constitutive mRNA expression of TLRs 1 to 6 was observed in fallopian tubes, uterine endometrium, cervix, and ectocervix. Furthermore, transcripts of the signaling adapter MyD88 and the accessory molecule CD14 were also detected in all tissues assayed. Quantitative analysis of TLR2 mRNA levels revealed highest expression of this molecule in fallopian tube and cervical tissues, followed by endometrium and ectocervix. In contrast to TLR2, TLR4 expression declined progressively along the tract, with highest expression in the upper tissues (fallopian tubes and endometrium), followed by cervix and ectocervix. In addition to mRNA, protein expression of TLR2 and TLR4 was also documented in these tissues. These data suggest that TLRs are differentially expressed in distinct compartments of the female reproductive tract and may provide insight regarding the regulation of inflammation and immunity within the tract.
The human female reproductive tract is immunologically unique in that it must tolerate allogeneic sperm and permit implantation and fetal development while simultaneously detecting and responding to a broad diversity of sexually transmitted pathogens. To accommodate these disparate functions, the female reproductive tract relies upon both innate and acquired immune defense mechanisms. Thus, the epithelial cells, macrophages, lymphocytes, and dendritic cells present within the female genital tract possess unique features which permit them to respond to a distinct microbiotic milieu (13). To mediate these responses, the innate immune system has evolved to recognize distinct products of microbial metabolism present on a wide variety of microorganisms. These conserved products, termed pathogen-associated molecular patterns, are recognized by a repertoire of invariant pattern recognition receptors (8, 13).
Many functions of innate immunity are transduced through pattern recognition receptors; recent studies impute a critical role to one particular group of pattern recognition receptors, the evolutionarily conserved mammalian Toll-like receptor (TLR) family of proteins (14). These type I transmembrane receptors are characterized by a leucine-rich extracellular domain and a conserved intracellular activation domain, which is homologous to the interleukin-1 receptor and has thus been designated the Toll/interleukin-1 receptor domain (3). Binding of an activated Toll/interleukin-1 receptor domain to the adapter protein MyD88 initiates a signaling cascade, leading to NF-κB activation and the subsequent production of proinflammatory and immunoregulatory cytokines, chemokines, and costimulatory molecules (3).
Of the 10 cloned mammalian TLRs, TLR2 and TLR4 are the best characterized with respect to innate responses to bacteria. TLR4, in association with accessory molecules MD-2 and CD14, is the signal transduction receptor for gram-negative bacterial lipopolysaccharide and heat shock proteins (16, 18, 19, 20, 24, 32). A broader range of microbial products activate immune responses through engagement of TLR2, including peptidoglycan from gram-positive bacteria (15), bacterial lipopeptides (15), and zymosan (17).
Given the microbial diversity of the human female reproductive tract and the significant role that TLRs play in mediating innate immune recognition, we investigated Toll-like receptor expression in the fallopian tube, uterus, cervix, and ectocervix. Constitutive expression of TLRs 1 to 6 as well as the accessory molecule CD14 and the molecular adapter MyD88 was observed throughout the female reproductive tract. With real-time reverse transcription-PCR, we demonstrated distinct patterns of TLR2 and TLR4 mRNA expression in these tissues. TLR2 and TLR4 proteins were also detected in each of these tissues, indicating efficient translation of these transcripts. These findings definitively establish TLR expression within the human female genital tract and implicate TLR-mediated immune surveillance as an important component of defense in the female reproductive tract.
MATERIALS AND METHODS
Reproductive tract tissues.
Human tissues were obtained immediately following surgery from patients from whom informed consent was obtained. Donors ranged in age from 26 to 81 years and were treated for a diversity of gynecologic maladies. All tissues used in these studies were distal to sites of pathology and were determined to be unaffected with disease at the gross anatomical level. Approval to use human tissues was obtained from the Human Experimentation Committee in accordance with the human experimentation guidelines of the U.S. Department of Health and Human Services.
RNA isolation and reverse transcription-PCR.
Total RNA was isolated from tissue by sequential extraction with Trizol reagent (Invitrogen) followed by purification with RNeasy mini columns (Qiagen). RNA integrity was confirmed by agarose gel electrophoresis, and RNA was quantified by spectrophotometric analysis. Prior to amplification, all RNA samples were treated with RNase-free DNase I (Qiagen) to preclude genomic DNA contamination. With 500 ng of RNA as the template for each reaction, first-strand cDNA was synthesized with random primers and SuperScript II Moloney murine leukemia virus reverse transcriptase (Invitrogen). PCR analysis was performed with Taq DNA polymerase for 35 cycles in a PCR Express Gradient thermocycler (Hybaid) with the primer pairs delineated in Table 1. Cycling conditions were as follows: 2 min of initial denaturation at 95°C, followed by 35 cycles, each of which consisted of 30 s at 94°C, 30 s at 57°C, and 45 s at 72°C, followed by a final extension at 72°C for 5 min. Reactions were amplified in the absence of reverse transcriptase as negative controls. A nontemplate control reaction was also included to ensure lack of DNA contamination; 10 μl of PCR product was electrophoresed on a 1.5% agarose gel with 0.5% ethidium bromide and photographed under UV light.
TABLE 1.
PCR primers used for amplification of pattern recognition receptors
| Primer | Forward sequence (5′-3′) | Reverse sequence (5′-3′) |
|---|---|---|
| TLR1 | ATTTCATATAGTGGGCACGATTC | AAAGAGATT GTGATGGGCAAAGT |
| TLR2 | GGCCAGCAAATTACCTGTGTG | AGGCGGACATCCTGAACCT |
| TLR3 | CCTGGTTTGTTAATTGGATTAACGA | TGAGGTGGAGTGTTGCAAAGG |
| TLR4 | CAGAGTTTCCTGCAATGGATCA | GCTTATCTGAAGGTGTTGCACAT |
| TLR5 | TGCCTTGAAGCCTTCAGTTATG | CCAACCACCACCATGATGAG |
| TLR6 | GAAGAAGAACAACCCTTTAGGATAGC | AGGCAAACAAAATGGAAGCTT |
| MyD88 | GAGCGTTTCGATGCCTTCAT | CGGATCATCTCCTGCACAAA |
| MD2 | ATTCCAAGGAGAGATTTAAAGCAATT | CAGATCCTCGGCAAATAACTTCTT |
| CD14 | CGCTCCGAGATGCATGTG | TTGGCTGGCAGTCCTTTAGG |
TaqMan PCR.
For quantification of TLR2 and TLR4 expression in tissues of the female reproductive tract, TaqMan PCR analysis was employed; 0.5 μl per well of cDNA was transferred into i-Cycler 96-well plates (Bio-Rad), and TaqMan Master Mix (Applied Biosystems) was added in accordance with the manufacturer's instructions. The primer pairs used for amplification of TLR2 were (5′-3′) GGC CAG CAA ATT ACC TGT GTG and AGG CGG ACA TCC TGA ACC T, and those for TLR4 were (5′-3′) CAG AGT TTC CTG CAA TGG ATC A and GCT TAT CTG AAG GTG TTG CAC AT.
TaqMan MGB probes (labeled with fluorescent reporter dye 6FAM) were used for analysis of TLR2 (TCC ATC CCA TGT GCG TGG CC) and TLR4 (CGT TCA ACT TCC ACC AAG AGC TGC CT). Input cDNA was normalized with a validated predeveloped assay reagent β-actin primer probe pair (Applied Biosystems) as an internal control. Amplification was performed on an iCycler with an optical unit (Bio-Rad) that permits real-time monitoring of increased PCR product concentration. Threshold cycle number was determined with the Opticon software and levels of TLR mRNA expression were normalized to β-actin levels with the formula 2 − −(Et−Rt), where Rt is the mean threshold cycle for the reference gene (β-actin) and Et is the mean threshold cycle for the experimental gene. Relative fluorescence units (RFU) were assigned to these values, and these data were used to generate the expression profiles delineated in the text. The thermal profile for TaqMan PCR consisted of 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Amplicon accumulation was measured during the extension phase. All reactions were performed in duplicate. Data were analyzed with the iCycler analysis software, version 2.3. (Bio-Rad).
Immunoprecipitation and immunoblotting.
Cell lysates were prepared by mechanical disruption of snap-frozen tissue in T-PER lysis reagent (25 mM Bicine, 150 mM NaCl, pH 7.6) (Pierce) with a mortar and pestle. Samples were centrifuged at 10,000 rpm for 5 min to pellet cell debris, and supernatants were collected, aliquoted, and stored at −80°C. Protein concentrations were determined by the BCA protein assay (Pierce). TLR2 was immunoprecipitated from 1 mg of each protein lysate with a rabbit polyclonal anti-TLR2 antibody (H-175) (Santa Cruz Biotechnology). Immunoprecipitation of TLR4 was performed with a mouse monoclonal anti-TLR4 antibody (HTA 125) (Santa Cruz Biotechnology). Immune complexes were captured with protein A/G-Sepharose beads (Pierce) for 2 h at 4°C, and the beads were washed four times with 100 mM NaCl.
To ensure the specificity of protein-antibody interaction, lysates were incubated with beads in the absence of antibody as well as with an irrelevant P3 immunoglobulin G1 isotype control (Caltag). Proteins were resolved by sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis (PAGE) and electrotransferred to nitrocellulose membrane in Tris-glycine buffer, with 20% methanol. Immunoblots were washed with 1× phosphate-buffered saline-0.05% Tween 20 and blocked in 5% milk for 1 h at room temperature. Membranes were then probed with goat polyclonal antibody N-17 (Santa Cruz Biotechnology) for detection of TLR2, followed by donkey anti-goat horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology). TLR4 was detected by probing immunoprecipitates with rabbit polyclonal anti-TLR4 antibody H-80 (Santa Cruz Biotechnology), followed by goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Bio-Rad). Reactive antigens were visualized with Supersignal chemiluminescence substrate (Pierce).
Statistical Analysis.
Data are represented as mean ± standard deviation. Comparisons among tissue types were made with paired tissue samples derived from the same patient. The sample size for each comparison is indicated in the upper right quadrant of each figure. For each paired graphical representation, the RFU value of the tissue type represented in the first column was set to 1 (divided by itself), and the values of the second column are represented as a fraction of the first-column values. Statistical analysis was performed on the RFU values of each pair of columns with a paired t test with two-tailed P values. Statistical significance was achieved at P ≤ 0.05.
RESULTS
Tissues of the human female reproductive tract express TLR family members 1 to 6 as well as adaptor molecule MyD88 and accessory molecules CD14 and MD2.
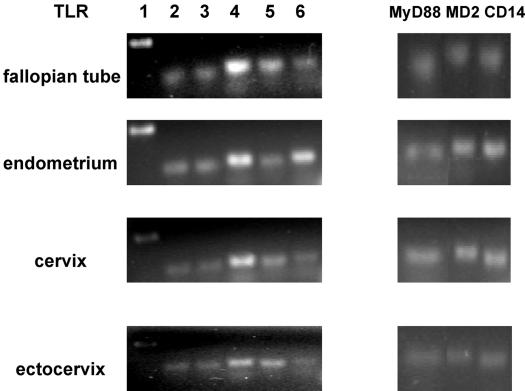
Although previous work has described the distribution of TLRs 2 and 4 in the mouse genital tract (4, 6), a comprehensive analysis of TLR mRNA expression in human tissues of the female reproductive tract has not been reported. Given the fundamental role attributed to TLRs in mediating innate immunity and the broad scope of microbial challenges encountered by the female reproductive tract, initial studies examined the TLR mRNA expression profiles of the fallopian tube, uterine endometrium, cervix, and ectocervix. Total RNA was extracted from homogenized whole tissue and analyzed for TLR expression by reverse transcription-PCR. As presented in Fig. 1, mRNA expression of TLRs 1 to 6 was detected in all four tissue types.
FIG. 1.
mRNA expression of TLRs, MyD88, MD-2, and CD14 in tissues of the female reproductive tract. Total RNA was extracted from fallopian tube, endometrial, cervical and ectocervical tissues, reverse transcribed, and amplified with the primers described in Table 1 for expression of pattern recognition receptors. For each tissue, the data shown are representative of gene expression observed in 10 different patient samples.
In addition to ligand engagement, recruitment of the adaptor molecule MyD88 is required for most TLR-mediated signal transduction (25, 26). Moreover, biochemical and genetic evidence indicates that complex formation with the bacterial coreceptor CD14 is necessary for maximal activation of TLR2 and TLR4 signaling (10, 32) and suggests a similar requirement for MD-2 association (for MyD88-dependent TLR4 signaling) (24). Significantly, all tissues analyzed demonstrated mRNA expression of these molecules (Fig. 1).
mRNA expression levels of TLR2 and TLR4 in human female reproductive tract tissues.
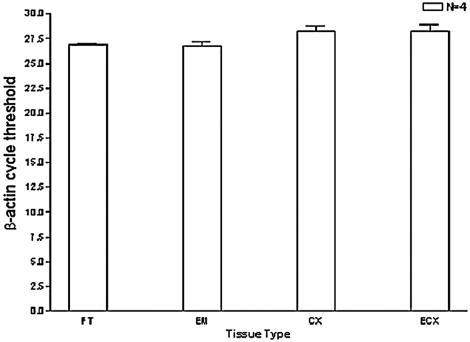
Bacterial infection within the female genital tract can have potentially devastating consequences, including chronic inflammation and infertility (11, 23). Innate immunity mediated by TLR2 and TLR4 signaling may provide a means of detecting and responding to these pathogens. As our preliminary studies confirmed mRNA expression of TLR2 and TLR4 in female reproductive tract tissue, we next quantified transcript levels of these receptors with TaqMan PCR. To determine if quantitative differences in mRNA expression levels were evident in different regions of the female reproductive tract, tissues derived from the same patient were compared in pairs, and statistical analysis performed. TLR mRNA expression levels were normalized to β-actin; we validated utilization of this gene by analyzing relative expression among tissues of the human female reproductive tract. The data shown in Fig. 2 (in which β-actin mRNA expression of each tissue type is represented by threshold cycle) indicated minimal variability in β-actin mRNA levels.
FIG. 2.
Validation of β-actin as a normalization control. Total RNA was extracted from each female reproductive tract tissue, reverse transcribed, and analyzed by TaqMan PCR with validated β-actin primers and probe (as described in the text). Comparisons were made with tissue samples derived from the same patient to maintain internal consistency; therefore, four individual patients from whom fallopian tube (FT), endometrium (EM), cervix (CX), and ectocervix (ECX) samples were obtained are represented in this study. All reactions were performed in triplicate.
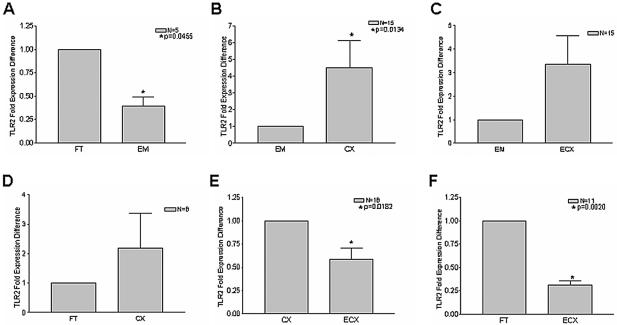
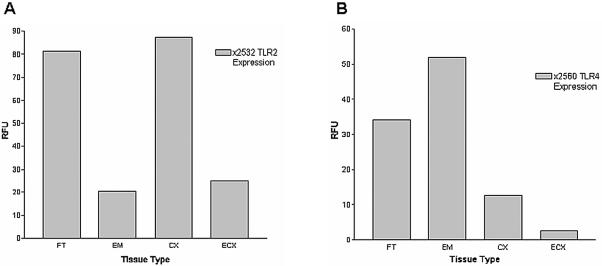
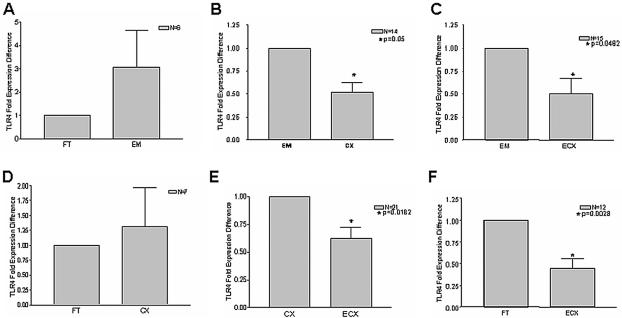
Figure 3 shows TaqMan real-time quantitative PCR analysis of TLR2 expression in tissues of the human female reproductive tract. Striking and significant differences were observed with respect to TLR2 mRNA levels in some of the tissues examined. In particular, TLR2 expression was significantly lower in endometrium than in either fallopian tube or cervix (Fig. 3A and 3B). While the mean for endometrial TLR2 expression was also lower than that of ectocervix (Fig. 3C), this was not statistically significant. There was also no significant difference between fallopian tube and cervical TLR2 expression (Fig. 3D), but the disparity in mRNA levels between cervix and ectocervix—as well as between fallopian tube and ectocervix—was significant (Fig. 3E and F). Although normalization was essential for the above comparison of composite data for TLR2 expression in multiple samples from different tissues (Fig. 3), these differences are perhaps better appreciated by examination of RFU values without normalization, as presented for a representative patient sample (see Fig. 5A).
FIG. 3.
TLR2 mRNA is most highly expressed in fallopian tubes and cervix. Total RNA was isolated from the fallopian tube (FT), endometrium (EM), cervix (CX), and ectocervix (ECX) as described in the text, and reverse transcription was performed with random hexamers. Comparisons among tissue types were made with paired tissue samples derived from the same patients. The sample size for each comparison is indicated in the upper right quadrant of each figure. Real-time PCR analysis of relative TLR2 mRNA expression was performed in duplicate with TaqMan TLR2- and β-actin-specific primers and probes. Samples incubated in the absence of reverse transcriptase were analyzed as well to control for genomic DNA contamination.
FIG. 5.
Representative analysis of TLR2 and TLR4 expression profiles. (A) Total RNA was extracted from fallopian tube (FT), endometrium (EM), cervix (CX), and ectocervix (ECX) samples of patient 2532 and reverse transcribed with random hexamers. TLR2 levels were quantified by real-time analysis with Taqman TLR2 primers and probes as in Fig. 3. (B) TLR4 mRNA levels were quantified in fallopian tube (FT), endometrium (EM), cervix (CX), and ectocervix (ECX) samples derived from patient 2560 by real-time Taqman PCR as described for Fig. 4. Reactions were standardized against β-actin and are expressed in relative fluorescence units (RFU). Values shown are the means of duplicate PCR runs, which never varied by more than 3%.
To determine whether or not TLR4 transcript levels mirrored those observed with TLR2, real-time quantitative PCR analysis was also performed on these tissues with TLR4-specific primers. Intriguingly, a distinct expression profile was observed. In contrast to TLR2, mean TLR4 mRNA levels in endometrium tended to be higher than for other tissues and were significantly different when comparing endometrium to cervix and ectocervix (Fig. 4B and C). Moreover, there was a progressive decline in TLR4 expression moving down the reproductive tract from endometrium to cervix to ectocervix (Fig. 4B, C, and E). In fact, expression of TLR4 in ectocervix was lower than that in any other tissue (Fig. 4 C, E and F). In contrast to TLR2 expression, there was no significant difference between fallopian tube and endometrial TLR4 mRNA levels (Fig. 4A). Representative data from a single patient are shown in Fig. 5B.
FIG. 4.
TLR4 mRNA expression declines along tissues of the female reproductive tract. cDNA was synthesized by reverse transcription from total RNA, and relative TLR4 mRNA levels were assessed by real-time PCR analysis with normalization to β-actin controls. Each reaction was performed in duplicate. Comparisons among tissue types were made with paired tissue samples of the fallopian tube (FT), endometrium (EM), cervix (CX), and ectocervix (ECX) derived from the same patients. The sample size for each comparison is indicated in the upper right quadrant of each panel.
Expression of TLR2 and TLR4 protein in tissues of the female reproductive tract.
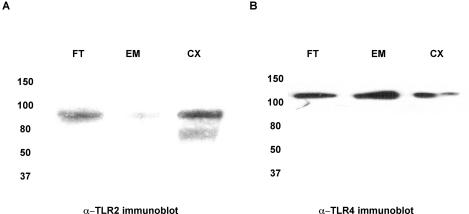
Consistent with their roles in immune surveillance, we detected expression of TLR2 and TLR4 transcripts in tissues of the upper and lower female reproductive tract. To confirm proper posttranscriptional processing and expression of these molecules, TLR2 and TLR4 were immunoprecipitated from a random sampling of fallopian tube, endometrium, and cervix and examined by immunoblot analysis. As demonstrated in Fig. 6, TLR2 and TLR4 proteins were expressed, to variable extents, in all three tissue types. In each immunoblot, a specific band was detected that corresponded to the reported full-length moieties of TLR2 and TLR4. The specificity of each antigen-antibody interaction was confirmed by simultaneous immunoprecipitation with a P3 control antibody, followed by Western blot analysis for TLR2 and TLR4. Neither TLR2 nor TLR4 was nonspecifically immunoprecipitated by the P3 antibody (data not shown).
FIG. 6.
Detection of TLR2 and TLR4 proteins in upper and lower tissues of the female reproductive tract. Whole-cell lysates were generated from total tissue of the fallopian tube (FT), endometrium (EM), cervix (CX), and ectocervix (ECX) and quantified with the BCA kit. One milligram of each of these lysates was immunoprecipitated with either (A) anti-TLR2 polyclonal antibody (H-175) or (B) mouse monoclonal anti-TLR4 antibody (HTA 125), and subsequently probed with (A) goat polyclonal antibody N-17 for detection of TLR2 or (B) rabbit polyclonal anti-TLR4 antibody H-80. Data shown are representative of three separate immunoprecipitations from three different tissues.
DISCUSSION
Recent studies have suggested that upper and lower tissues of the female reproductive tract are phenotypically compartmentalized with respect to acquired immunity. For example, functional studies with a model of Chlamydia trachomatis infection in mice indicated preferential recruitment of CD4+ T lymphocytes to upper regions of the genital tract during infection (9). In addition, Yeaman et al. demonstrated the existence of unique CD8+-T-cell-rich lymphoid aggregates in human endometrium (33). In contrast, relatively little is known about innate immune responses within the human female reproductive tract. Our findings are the first to characterize TLR expression in nontumorigenic complex tissues of the human female genital tract. We observed constitutive in vivo expression of TLRs 1 to 6, MyD88, and the accessory molecules CD14 and MD-2 in fallopian tube, endometrium, cervix, and ectocervix. Real-time quantitative PCR demonstrated variable expression of TLR2 and TLR4 among tissues of the upper and lower tract. These differences likely occur through a variety of mechanisms and may reflect adaptation of the innate immune response to the distinct microbial milieu encountered by each tissue.
In this regard, it is imperative to consider the unique microenvironment of each female reproductive tract compartment. The nonsterile lower tissues of the tract, the vagina and ectocervix, harbor a variety of commensal bacteria which maintain low vaginal pH (reviewed in reference 21). In addition to sexually transmitted pathogenic challenges, these tissues are subject to secondary contamination as a result of their proximity to rectal flora. The cervix, while not exposed to the same breadth of microbiota as the vagina and ectocervix, is considered an immunologically dynamic site; cervical mucus provides an impediment to sperm and bacterial infiltration of the uterus (5). The most common cervical pathogens are the gram-negative bacteria Neisseria gonorrhoeae and Chlamydia trachomatis (21). The uppermost section of the female reproductive tract consists of the fallopian tube and endometrium. Although infection of these sites is uncommon, ascending pathogens (particularly N. gonorrhoeae and C. trachomatis) may breach the mucosal barrier of the cervix, resulting in adverse reproductive consequences, including the development of pelvic inflammatory disease, tubal factor infertility, and ectopic pregnancy (2, 12, 27, 31).
Given the roles imputed to TLR2 and TLR4 in the regulation of innate immunity and response to bacterial challenge, we examined quantitative expression of these pattern recognition receptors in tissues of the female genital tract. Our findings indicate that TLR2 is most highly expressed in fallopian tubes and cervix, with lower levels present in endometrium and ectocervix. TLR2 is activated by a broad spectrum of pathogen-associated molecular patterns, including peptidoglycan from gram-positive bacteria, bacterial lipoproteins, mycobacterial cell wall lipoarabinomannan, and yeast cell walls (15, 17, 25). This diversity of ligands may account for the importance of its elevated expression in fallopian tube and cervical tissue; less apparent is the reduced TLR2 expression observed in the endometrium. It is possible that this expression pattern is related to the role of the endometrium in the maintenance of an environment hospitable to fetal implantation. Thus, TLR2 expression may be maintained at a low level in the healthy uterus but be upregulated in response to pathogenic challenge as a defense mechanism that can be mobilized to protect the fetus from infection during pregnancy and labor. In this model, TLR4 levels may be sufficiently high to preclude infection of endometrium by organisms that are commonly encountered. TLR2 expression, while constitutively low, would be inducible postinfection. In fact, studies conducted by Watari et al. have shown that TLR2 expression is augmented by tumor necrosis factor alpha (29). Thus, TLR4 engagement, which results in the production of tumor necrosis factor alpha, would precipitate a more vigorous inflammatory response subsequent to increased expression of TLR2.
Intriguingly, TLR4 mRNA was most highly expressed in fallopian tube and endometrium, tissues most likely to be challenged by gram-negative N. gonorrhoeae and C. trachomatis (21). Increased innate surveillance at these sites (manifested by increased TLR4 expression) may provide a means of ensuring sterile conditions while conferring protection from microbial challenge. The tissues of the lower tract, the cervix and ectocervix, which maintain a first line of defense for the female reproductive tract, are exposed to a much broader variety of infectious agents. The decreased expression of TLR4 associated with cervix and ectocervix is no doubt reflective of the complex microbiota associated with these tissues. Clearly, it is essential that these tissues develop a mechanism for selectively responding to pathogens while avoiding chronic inflammation due to immune responses to commensal bacteria. Limiting TLR4 levels may provide a means of regulating sensitivity to bacterial components in the lower tract.
Previous work conducted with primary human vaginal, endocervical, and ectocervical epithelial cells detected mRNA expression of TLRs 1, 2, 3, 5, and 6 but failed to observe expression of TLR4 and MD-2 (7). Although these cells were unresponsive to protein-free preparations of lipopolysaccharide from Escherichia coli as well as lipooligosaccharide from N. gonorrhoeae, they did mount inflammatory responses to whole gram-negative bacteria and bacterial lysates (7). While this study differs from ours in that we evaluated TLR expression in total tissue lysate as opposed to a single cell type, these data are significant in light of our discovery that while TLR4 expression is low in cervical tissue, TLR2 mRNA levels are relatively high (comparable to those observed in fallopian tube). It is possible that under conditions of low TLR4 expression, TLR2 is activated, either because of redundant or alternative ligand recognition. This model is supported by a recent report from Darville et al., in which recognition of C. trachomatis was shown to be mediated by TLR2 in TLR4 knockout mice (4). In addition, TLR4-deficient gingival epithelial cells respond to gram-negative bacteria via TLR2 ligands (1).
Recruitment of the adaptor protein MyD88 is critical for most TLR-mediated signal transduction and activation of NF-κB and mitogen-activated protein kinase (25). Although MyD88-independent signaling does occur, MyD88 is required for lipopolysaccharide-induced tumor necrosis factor alpha production (30). Our studies demonstrate ubiquitous expression of MyD88 throughout the female reproductive tract, which is consistent with its role as an intracellular adaptor for multiple and varied receptors. Furthermore, CD14 and MD-2 are necessary for fully efficient microbial recognition by TLR2 and TLR4; we detected constitutive expression of both these transcripts in fallopian tube, endometrium, cervix, and ectocervix. Thus, the tissues of the female reproductive tract possess the full complement of adaptor and accessory molecules required for optimal TLR2- and TLR4-mediated signal transduction.
In addition to mRNA expression, we also demonstrated the presence of TLR2 and TLR4 protein in human fallopian tube, endometrium, and cervix. It is notable that detection of these receptors required an initial immunoprecipitation step prior to immunoblotting, indicating low constitutive expression of these proteins within the female reproductive tract. These data are not surprising, as surface expression of TLRs in monocytes has been shown to be limited to a few thousand molecules per cell (28). These relatively low TLR protein expression levels imply that these receptors function as extremely efficient signal transducers.
With a murine model, Renshaw et al. demonstrated an inverse correlation between macrophage TLR expression and aging (22). Similarly, we analyzed TLR2 and TLR4 mRNA expression levels in tissues from 28 donors, ranging in age from 26 to 81 years (summarized in Table 2). In contrast to observations in mice, there was not a statistically significant difference in TLR mRNA expression levels with respect to age for our samples. There was similarly no significant correlation between receptor expression and menstrual stage. These findings may be due to higher inherent TLR expression variability among human subjects than in inbred mice and suggest that evaluation of a much larger population will be necessary to reveal potential expression differences that might exist in relation to age or hormonal status. It is also important to note that our experiments were conducted with heterogeneous cell populations and thus do not exclude the possibility that TLR expression might differ with age and/or hormonal status in distinct cell types.
TABLE 2.
Summary of patient population
| Menstrual stage | Patient no. | Age (yr) | Diagnosis |
|---|---|---|---|
| Postmenopausal | 2497 | 48 | Endometrial hyperplasia |
| 2561 | 58 | Endometrial hyperplasia | |
| 2549 | 65 | Prolapse | |
| 2574 | 50 | Fibroids | |
| 2490 | 54 | Prolapse | |
| 2515 | 54 | Menorrhagia | |
| 2583 | 81 | Prolapse | |
| 2500 | 58 | Prolapse | |
| Proliferative | 2560 | 48 | Fibroids |
| 2506 | 26 | Pelvic pain | |
| 2567 | 42 | Pelvic mass | |
| 2536 | 30 | Pelvic pain | |
| 2535 | 45 | Dysmenorrhea | |
| 2522 | 28 | Cervical cancer | |
| 2527 | 38 | Pelvic pain | |
| 2551 | 50 | Menorrhagia | |
| Secretory | 2531 | 41 | Menorrhagia |
| 2521 | 34 | Menorrhagia | |
| 2577 | 44 | Fibroids | |
| 2502 | 48 | Prolapse | |
| 2544 | 37 | Dysmenorrhea | |
| 2542 | 35 | Dysmenorrhea | |
| 2573 | 41 | Dysmenorrhea | |
| 2539 | 38 | Secretory | |
| Unknown | 2579 | 47 | Fibroids |
| 2511 | 38 | Prolapse | |
| 2564 | 51 | Endometrial hyperplasia | |
| 2532 | 58 | Ovarian mass |
In conclusion, we have demonstrated that TLRs 1 to 6 are constitutively expressed in the human female reproductive tract. The pattern recognition receptors TLR2 and TLR4 show quantitatively distinct mRNA expression profiles among tissues of the female genital tract, and transcripts of these messages are expressed as proteins within the fallopian tubes, endometrium, and cervix. This study is the first to comprehensively examine in vivo expression of TLRs 1 to 6 within the human female reproductive tract. An enhanced understanding of innate immune mechanisms within the female reproductive tract and their role in bacterial recognition may provide insight into the pathogenesis of sexually transmitted diseases and the deleterious sequelae associated with genital tract inflammation.
Acknowledgments
This work was supported by NIH grant AI51877.
Editor: J. D. Clements
REFERENCES
- 1.Asai, Y., Y. Ohyama, K. Gen, and T. Ogawa. 2001. Bacterial fimbriae and their peptides activate human gingival epithelial cells through Toll-like receptor 2. Infect. Immun. 69:7387-7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlow, R. E., I. D. Cooke, O. Odukoya, M. Heatley, J. Jenkins, G. Narayansingh, S. S. Ramsewak, and A. Eley. 2001. The prevalence of Chlamydia trachomatis in fresh tissue specimens from patients with ectopic pregnancy or tubal factor infertility as determined by PCR and in-situ hybridisation. J. Med. Microbiol. 50:902-908. [DOI] [PubMed] [Google Scholar]
- 3.Bowie, A., and L. A. O'Neill. 2000. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J. Leukoc. Biol. 67:508-514. [DOI] [PubMed] [Google Scholar]
- 4.Darville, T., J. M. O'Neill, C. W. Andrews, Jr., U. M. Nagarajan, L. Stahl, and D. M. Ojcius. 2003. Toll-like receptor-2, but not toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J. Immunol. 171:6187-6197. [DOI] [PubMed] [Google Scholar]
- 5.Eggert-Kruse, W., Reimann-Andersen, J., G. Rohr, S. Pohl, W. Tilgen, and B. Runnebaum. 1995. Clinical relevance of sperm morphology assessment using strict criteria and relationship with sperm-mucus interaction in vivo and in vitro. Fertil. Steril. 63:612-624. [DOI] [PubMed] [Google Scholar]
- 6.Elovitz, M. A., Z. Wang, E. K. Chien, D. F. Rychlik, and M. Phillippe. 2003. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am. J. Pathol. 163:2103-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fichorova, R. N., A. O. Cronin, E. Lien, D. J. Anderson, and R. R. Ingalls. 2002. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J. Immunol. 168:2424-2432. [DOI] [PubMed] [Google Scholar]
- 8.Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 9.Kelly, K. A., J. C. Walker, S. H. Jameel, H. L. Gray, and R. G. Rank. 2000. Differential regulation of CD4 lymphocyte recruitment between the upper and lower regions of the genital tract during Chlamydia trachomatis infection. Infect. Immun. 68:1519-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirschning, C. J., H. Wesche, T. Ayres, and M. Rothe, 1998. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 188:2091-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Land, J. A., and J. L. Evers. 2002. Chlamydia infection and subfertility. Best. Pract. Res. Clin. Obstet. Gynaecol. 16:901-912. [DOI] [PubMed] [Google Scholar]
- 12.McGee, Z. A., R. L. Jensen, C. M. Clemens, D. Robinson, A. P. Johnson, and C. R. Gregg. 1999. Gonococcal infection of human Fallopian tube mucosa in organ culture: relationship of mucosal tissue TNF-alpha concentration to sloughing of ciliated cells. Sex. Transm. Dis. 26:160-165. [DOI] [PubMed] [Google Scholar]
- 13.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov, R., and C. A. Janeway, Jr. 2002. Decoding the patterns of self and nonself by the innate immune system. Science 296:298-300. [DOI] [PubMed] [Google Scholar]
- 15.Michelsen, K. S., A. Aicher, M. Mohaupt, T. Hartung, S. Dimmeler, C. J. Kirschning, and R. R. Schumann. 2001. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J. Biol. Chem. 276:25680-25686. [DOI] [PubMed] [Google Scholar]
- 16.Nagai, Y., S. Akashi, M. Nagafuku, M. Ogata, Y. Iwakura, S. Akira, T. Kitamura, A. Kosugi, M. Kimoto, and K. Miyake. 2002. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3:667-672. [DOI] [PubMed] [Google Scholar]
- 17.Nishiguchi, M., M. Matsumoto, T. Takao, et al. 2001. Mycoplasma fermentans lipoprotein M161Ag-induced cell activation is mediated by Toll-like receptor 2: role of N-terminal hydrophobic portion in its multiple functions. J. Immunol. 166:2610-2616. [DOI] [PubMed] [Google Scholar]
- 18.Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 164:558-561. [DOI] [PubMed] [Google Scholar]
- 19.Poltorak, A., X. He, I. Smirnova, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 20.Poltorak, A., I. Smirnova, X. He, et al. 1998. Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol. Dis. 24:340-355. [DOI] [PubMed] [Google Scholar]
- 21.Quayle, A. J. 2002. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J. Reprod. Immunol. 57:61-79. [DOI] [PubMed] [Google Scholar]
- 22.Renshaw, M., J. Rockwell, C. Engleman, A. Gewirtz, J. Katz, and S. Sambhara. 2002. Cutting edge: impaired Toll-like receptor expression and function in aging. J. Immunol. 169:4697-4701. [DOI] [PubMed] [Google Scholar]
- 23.Romero, R., J. Espinoza, T. Chaiworapongsa, and K. Kalache. 2002. Infection and prematurity and the role of preventive strategies. Semin. Neonatol. 7:259-274. [DOI] [PubMed] [Google Scholar]
- 24.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeuchi, O., and S. Akira. 2002. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr. Top. Microbiol. Immunol. 270:155-167. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi, O., K. Takeda, K. Hoshino, O. Adachi, T. Ogawa, and S. Akira. 2000. Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int. Immunol. 12:113-117. [DOI] [PubMed] [Google Scholar]
- 27.Van Voorhis, W. C., L. K. Barrett, Y. T. Sweeney, C. C. Kuo, and D. L. Patton. 1997. Repeated Chlamydia trachomatis infection of Macaca nemestrina Fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect. Immun. 65:2175-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visintin, A., A. Mazzoni, J. H. Spitzer, D. H. Wyllie, S. K. Dower, and D. M. Segal. 2001. Regulation of Toll-like receptors in human monocytes and dendritic cells. J. Immunol. 166:249-255. [DOI] [PubMed] [Google Scholar]
- 29.Watari, M., H. Watari, I. Nachamkin, and J. F. Strauss. 2000. Lipopolysaccharide induces expression of genes encoding pro-inflammatory cytokines and the elastin-degrading enzyme, cathepsin S, in human cervical smooth-muscle cells. J. Soc. Gynecol. Investig. 7:190-198. [DOI] [PubMed] [Google Scholar]
- 30.Weighardt, H., Kaiser- S. Moore, R. M. Vabulas, C. J. Kirschning, H. Wagner, and B. Holzmann. 2002. Cutting edge: myeloid differentiation factor 88 deficiency improves resistance against sepsis caused by polymicrobial infection. J. Immunol. 169:2823-2827. [DOI] [PubMed] [Google Scholar]
- 31.Wiesenfeld, H. C., S. L. Hillier, M. A. Krohn, A. J. Amortegui, R. P. Heine, D. V. Landers, and R. L. Sweet. 2002. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet. Gynecol. 100:456-463. [DOI] [PubMed] [Google Scholar]
- 32.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 33.Yeaman, G. R., P. M. Guyre, M. W. Fanger, J. E. Collins, H. D. White, W. Rathbun, K. A. Orndorff, J. Gonzalez, J. E. Stern, and C. R. Wira. 1997. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J. Leukoc. Biol. 61:427-435. [PubMed] [Google Scholar]