Abstract
We demonstrated that trifluoperazine, a calcium-calmodulin antagonist, blocked the hyperpermeability induced by Vibrio vulnificus cytolysin in in vitro-modeled endothelium and prevented the deaths of mice. Furthermore, compared to tetracycline alone, tetracycline combined with trifluoperazine enhanced the survival rate of V. vulnificus-infected mice, indicating the role of the cytolysin as an important factor in pathogenesis.
Vibrio vulnificus is a gram-negative, halophilic bacterium that is capable of rapidly processing wound infections and septicemia (1, 2). Several V. vulnificus components and products have been suggested as virulence factors of the organism through in vitro or in vivo experiments (4, 9, 17, 19). Two of the most representative cytotoxins, cytolysin and the elastolytic protease, were considered to play major roles in V. vulnificus cytotoxicity. However, mutants with single mutations in either the cytolysin or protease gene showed no significant change in their 50% lethal doses in experimental mouse systems (15, 19). Even when both genes were knocked out, no significant change in virulence was noted (3). Consequently, key virulence factors have not yet been identified in the in vitro and in vivo cytotoxic activities of V. vulnificus. Nevertheless, it has been suggested that V. vulnificus cytolysin may be a virulent factor in mice infected orally. When V. vulnificus was administered via the oral route, its cytolysin seemed to be involved in the organism's invasion across the intestinal wall. In fact, a protease mutant is more virulent by the oral route because the cytolysin activity might be increased by the lack of the protease inactivating the cytolysin (15). Thus, cytolysin might be at least partially involved in the pathogenesis of V. vulnificus.
In most of the terminal cases involving V. vulnificus infection, patients have exhibited underlying disease, particularly cirrhosis of the liver (1, 7, 13). The infection induces septicemia and ultimately leads to death from septic shock. A hallmark of septic shock is hypotension, which is caused by extravasation of intravascular fluid through enhancement of vascular permeability. Cirrhosis shows enhanced vascular permeability. Enhanced permeability might lead more easily to hypotension, which increases the chance for the lethality of septicemia induced by V. vulnificus infection.
Anti-V. vulnificus cytolysin antibodies were detected in the blood of V. vulnificus-infected mice or humans who survived V. vulnificus disease (5), indicating that cytolysin can be produced in vivo. Cytolysin was detected in sera from V. vulnificus-infected mice (6). Indeed, the injection of V. vulnificus cytolysin in the in vivo mouse model induced pulmonary edema through enhanced vascular permeability (12). Thus, V. vulnificus cytolysin might further increase the enhanced vascular permeability of cirrhotic patients and the chance for death from septic shock. The blockage of V. vulnificus cytolysin-induced hyperpermeability might increase the survival rate of V. vulnificus-infected patients who have cirrhosis of the liver.
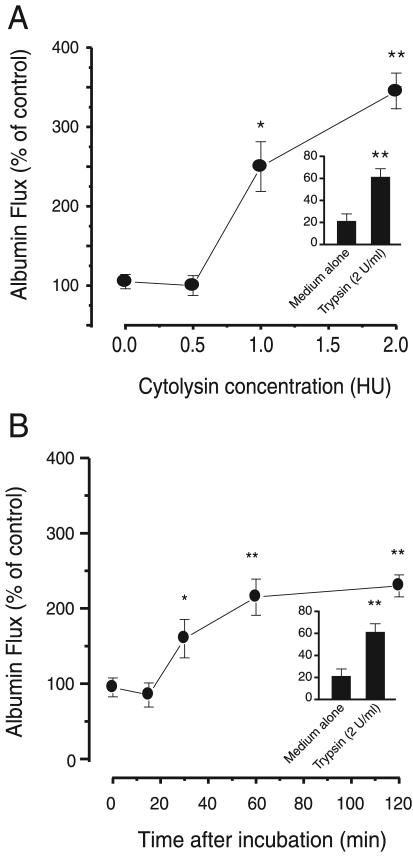
It was previously shown that V. vulnificus cytolysin induces pulmonary edema (12). That report suggested that V. vulnificus cytolysin-induced pulmonary edema is mediated by the increase of vascular permeability. To confirm this more clearly, we tested whether V. vulnificus cytolysin could change the permeability of the endothelium in an in vitro model. The in vitro endothelium was established by the monolayer culture of pulmonary endothelial cells on a polycarbonate filter of a Transwell chamber. To measure endothelial permeability, 125I-labeled albumin was applied to the upper part of the chamber with or without V. vulnificus cytolysin, and then the radioactivity of the lower chamber was determined for albumin flux. Albumin flux increased in a time- and dose-dependent manner in the presence of V. vulnificus cytolysin. Between 0.5 and 1.0 U of V. vulnificus cytolysin per ml significantly enhanced albumin flux across the endothelial cell monolayer without any cellular damage (Fig. 1A). The albumin flux reached peak levels within 60 min (Fig. 1B) in the presence of 1.0 hemolytic unit (HU) of V. vulnificus cytolysin per milliliter.
FIG. 1.
Effect of V. vulnificus cytolysin on 125I-labeled albumin flux in an endothelial monolayer. CPAE cells (5 × 105 cells) were cultured in the upper chamber of a Transwell insert for 4 days. (A) Dose dependency of V. vulnificus cytolysin-induced albumin flux. Albumin flux was determined at 60 min after the addition of various concentrations of V. vulnificus cytolysin (0.5 to 2.0 HU) and 125I-labeled albumin to the endothelial cell space of the upper chamber. (B) Time dependency of V. vulnificus cytolysin-induced albumin flux. Albumin flux was determined at the indicated times after the addition of 125I-labeled albumin and V. vulnificus cytolysin (1.0 HU) into the upper chamber. Error bars indicate standard deviations for results for three to four experiments. (A) * indicates a P of <0.005 and ** indicates a P of <0.001, compared with values for the control group. (B) * indicates a P of <0.005 and ** indicates a P of <0.001, compared with values for the control group.
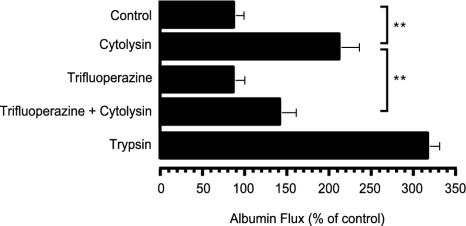
The endothelial cytoskeleton rearrangement leading to hyperpermeability is primarily regulated by intracellular calcium-signaling pathways (10). V. vulnificus cytolysin increases intracellular calcium concentrations through the influx of calcium ions into endothelial cells (8, 14). Thus, we explored whether the V. vulnificus cytolysin-induced increase of permeability is associated with the calcium-calmodulin signaling pathway. Trifluoperazine (TFP), a phenothiazine derivative of an antipsychotic drug, has been known to block the Ca2+ signal by the inhibition of the calmodulin-Ca2+-directed function with optimum concentrations between 5 and 100 μM(11, 18). The drug is relatively less toxic to cells than Ca2+-chelating agents such as EDTA, 1-(2-Amino-5-[2,7-dichloro-6-hydroxy-3-oxy-9-xanthenyl]phenoxy)-2-(2-amino-5-methylphenoxy)ethane-N,N,N′,N′-tetraacetic acid, and bix(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid/acetoxymethyl ester. Thus, we analyzed the effect of the drug on the V. vulnificus cytolysin-induced increase of permeability. Interestingly, TFP (10 μM) significantly blocked a V. vulnificus cytolysin-induced increase in albumin permeability (Fig. 2). This type of response to V. vulnificus cytolysin was similar to those of other toxins (16). Thus, these results strongly indicate that V. vulnificus cytolysin induces the calcium-calmodulin-dependent hyperpermeability of endothelial cells.
FIG. 2.
Effect of TFP on V. vulnificus cytolysin-induced albumin flux. CPAE (5 × 105) were cultured in the upper chamber of a Transwell device for 4 days, and albumin flux was determined at 60 min after the addition of 125I-labeled albumin and V. vulnificus cytolysin (1.0 HU) with or without the addition of 10 μM TFP into the upper chamber. Error bars indicate standard deviations for results for three to four experiments. **, a value different from the baseline value (P < 0.005); **, indicative of an inhibitory effect of TFP on cytolysin-induced albumin flux (P < 0.001).
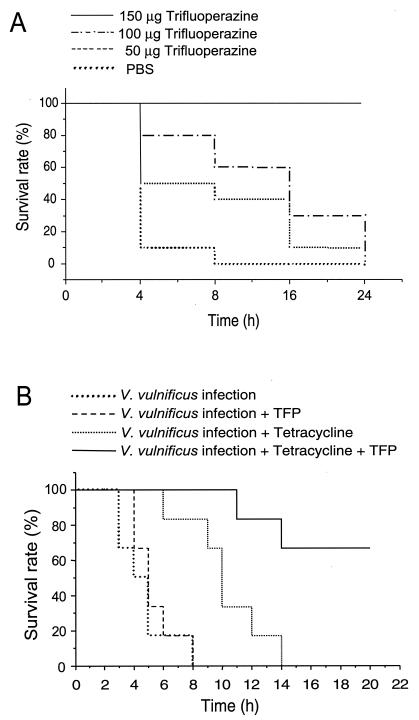
To determine whether the in vitro protective effect of TFP on cytolysin-induced hyperpermeability is implicated in vivo, we investigated whether TFP has a protective role against death induced by V. vulnificus cytolysin. Intravenous injection of V. vulnificus cytolysin (8 HU) into mice resulted in death for 100% of the mice within 24 h after injection (Fig. 3A). In contrast, administration of 50 and 100 μg of TFP into cytolysin-treated mice delayed lethality, and all mice were ultimately rescued by the administration of 150 μg of TFP. These results suggest that TFP can also prevent the deaths induced by V. vulnificus infection. Thus, instead of injecting toxin into mice, we examined whether TFP can inhibit lethality in an infection model. Mice received an intravenous injection of 50 μg of TFP or 25 μg of tetracycline 1 h after intraperitoneal injection of V. vulnificus (2 × 108 CFU). We found that the treatment of V. vulnificus-infected mice with TFP had no effect on the survival rate. However, compared to the administration tetracycline alone, TFP combined with tetracycline increased the survival rate of V. vulnificus-infected mice (Fig. 3), indicating that the cytolysin might be at least partially involved in the pathogenesis of V. vulnificus.
FIG. 3.
(A) Effect of TFP on the V. vulnificus cytolysin-induced death of mice. TFP (0 to 150 μg) was intraperitoneally injected into mice. One hour later, 8 HU of cytolysin was injected into each mouse via the tail vein. Survival was determined during the 24-h period after injection, after which there was no further loss of animal life. The survival rate of the group treated with 150 μg of TFP is significantly different from the survival rate of the control group (P < 0.001 by log rank test). (B) Effect of TFP on the V. vulnificus-induced death of mice. V. vulnificus (2 × 108 CFU) was intraperitoneally injected into mice. One hour later, tetracycline (25 μg) or TFP (50 μg) was injected into each mouse via the tail vein. Survival was determined during the 24-h period after injection, after which there was no further loss of animal life. The survival rate of the group treated with TFP and tetracycline is significantly different from the survival rate of the control group (P < 0.001 by the log rank test).
In conclusion, TFP protects against the lethality of V. vulnificus cytolysin. Furthermore, the combination of TFP and tetracycline leads to an increase in the survival of V. vulnificus-infected mice. We suggest that TFP can be used in combination with antibiotics such as tetracycline as a therapeutic agent against V. vulnificus disease.
Acknowledgments
This work was supported by a grant from the Aging and Apoptosis Research Center (R11-2002-001-01001-1) to Jong-Suk Kim.
Editor: J. T. Barbieri
REFERENCES
- 1.Blake, P. A., M. H. Merson, R. E. Weaver, D. G. Hollis, and P. C. Heublein. 1979. Disease caused by a marine Vibrio. Clinical characteristics and epidemiology. N. Engl. J. Med. 300:1-5. [DOI] [PubMed] [Google Scholar]
- 2.Chiang, S. R., and Y. C. Chuang. 2003. Vibrio vulnificus infection: clinical manifestations, pathogenesis, and antimicrobial therapy. J. Microbiol. Immunol. Infect. 36:81-88. [PubMed] [Google Scholar]
- 3.Fan, J.-J., C.-P. Shao, Y.-C. Ho, C.-K. Yu, and L.-I. Hor. 2001. Isolation and characterization of a Vibrio vulnificus mutant deficient in both extracellular metalloprotease and cytolysin. Infect. Immun. 69:5943-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray, L. D., and A. S. Kreger. 1987. Mouse skin damage caused by cytolysin from Vibrio vulnificus and by V. vulnificus infection. J. Infect. Dis. 155:236-241. [DOI] [PubMed] [Google Scholar]
- 5.Gray, L. D., and A. S. Kreger. 1989. Detection of anti-Vibrio vulnificus cytolysin antibodies in sera from mice and a human surviving V. vulnificus disease. Infect. Immun. 51:964-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray, L. D., and A. S. Kreger. 1989. Detection of Vibrio vulnificus cytolysin in V. vulnificus-infected mice. Toxicon 27:439-464. [DOI] [PubMed] [Google Scholar]
- 7.Hollis, D. G., R. E. Weaver, C. N. Baker, and C. Thornsberry. 1976. Halophilic Vibrio species isolated from blood cultures. J. Clin. Microbiol. 3:425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim, B. S., and J. S. Kim. 2002. Vibrio vulnificus cytolysin induces hyperadhesiveness of pulmonary endothelial cells for neutrophils through endothelial P-selectin: a mechanism for pulmonary damage by Vibrio vulnificus cytolysin. Exp. Mol. Med. 34:308-312. [DOI] [PubMed] [Google Scholar]
- 9.Linkous, D. A., and J. D. Oliver. 1999. Pathogenesis of Vibrio vulnificus. FEMS Microbiol. Lett. 174:207-214. [DOI] [PubMed] [Google Scholar]
- 10.McPherson, V. L., J. A. Watts, L. M. Simpson, and J. D. Oliver. 1991. Physiological effects of the lipopolysaccharide of Vibrio vulnificus on mice and rats. Microbios 67:141-149. [PubMed] [Google Scholar]
- 11.Osborn, M., and K. Weber. 1980. Damage of cellular functions by trifluoperazine, a calmodulin-specific drug. Exp. Cell Res. 130:484-488. [DOI] [PubMed] [Google Scholar]
- 12.Park, J.-W., S.-N. Ma, E.-S. Song, C.-H. Song, M.-R. Chae, B.-H. Park, H.-W. Rho, S.-D. Park, and H.-R. Kim. 1996. Pulmonary damage by Vibrio vulnificus cytolysin. Infect. Immun. 64:2873-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park, S. D., H. S. Shon, and N. J. Joh. 1991. Vibrio vulnificus septicemia in Korea: clinical and epidemiologic findings in seventy patients. J. Am. Acad. Dermatol. 24:397-403. [DOI] [PubMed] [Google Scholar]
- 14.Rho, H. W., M. J. Choi, J. N. Lee, J. W. Park, J. S. Kim, B. H. Park, H. S. Sohn, and H. R. Kim. 2002. Cytotoxic mechanism of Vibrio vulnificus cytolysin in CPAE cells. Life Sci. 70:1923-1934. [DOI] [PubMed] [Google Scholar]
- 15.Shao, C.-P., and L.-I. Hor. 2000. Metalloprotease is not essential for Vibrio vulnificus virulence in mice. Infect. Immun. 68:3569-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suttorp, N., T. Hessz, W. Seeger, A. Wilke, R. Koob, F. Lutz, and D. Drenckhahn. 1988. Bacterial exotoxins and endothelial permeability for water and albumin in vitro. Am. J. Physiol. 255:C368-C376. [DOI] [PubMed] [Google Scholar]
- 17.Testa, J., L. W. Daniel, and A. S. Kreger. 1984. Extracellular phospholipase A2 and lysophospholipase produced by Vibrio vulnificus. Infect. Immun. 45:458-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss, B., and R. M. Levin. 1978. Mechanism for selectively inhibiting the activation of cyclic nucleotide phosphodiesterase and adenylate cyclase by antipsychotic agents. Adv. Cyclic Nucleotide Res. 9:285-303. [PubMed] [Google Scholar]
- 19.Wright, A. C., and J. G. Morris, Jr. 1991. The extracellular cytolysin of Vibrio vulnificus: inactivation and relationship to virulence in mice. Infect. Immun. 59:192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]