Abstract
We report that clearance of Pseudomonas aeruginosa, accumulation of neutrophils, and synthesis of tumor necrosis factor alpha and macrophage inflammatory protein 2 in the infected lung were not largely different in interleukin-18 (IL-18) knockout or transgenic mice compared with control mice. Our results suggest a limited role for IL-18 in the host defense against P. aeruginosa.
Pseudomonas aeruginosa, an important cause of nosocomial pulmonary infections, is eradicated from infected tissues mainly by the bactericidal mechanism of neutrophils (21). Upon entry of the bacteria, alveolar macrophages initiate an inflammatory response involving the recruitment of a large number of neutrophils from the circulation into the alveolar spaces (11, 20). During this process, tumor necrosis factor alpha (TNF-α) facilitates the adhesion of neutrophils to vascular endothelial cells by enhancing their expression of adhesion molecules (2, 10), and macrophage inflammatory protein 2 (MIP-2) directly induces the trafficking of these cells (17).
Interleukin-18 (IL-18), originally identified as gamma interferon (IFN-γ)-inducing factor, is secreted from activated macrophages and dendritic cells after cleavage of the precursor form by caspase-1 (14, 15). This cytokine triggers the production of IFN-γ by natural killer and type 1 helper T cells and promotes the cytolytic activity of natural killer cells against tumor cells (4, 14, 15). IL-18 is also reported to induce the secretion of TNF-α and IL-8 by human peripheral blood monocytes (16), and it contributes to the neutrophil tissue accumulation and production of TNF-α and MIP-2 in various mouse models (1, 9, 12). In the present study, we examined the role of IL-18 in the neutrophil-mediated host protective responses to P. aeruginosa infection in lungs by using mice with either genetic depletion or transgenic (Tg) overexpression of this cytokine.
Mice with a targeted disruption of the IL-18 gene (IL-18 knockout [KO] mice) were established as described previously (19) and backcrossed eight times to C57BL/6 mice. IL-18 Tg mice with a C57BL/6 background in which B cells could express mature mouse IL-18 cDNA fused with signal peptide of the mouse immunoglobulin (Ig) κ chain under the control of mouse Ig promoter were generated (5). C57BL/6 mice were purchased from Charles River Japan (Osaka, Japan) and used as a control wild-type animal for each KO mouse. We used hemizygous IL-18 Tg mice and littermate mice without expression of the transgene as the control. All experimental protocols described in the present study were approved by the Ethics Review Committee for Animal Experimentation of our university.
P. aeruginosa (PAO-1) was cultured in Trypticase soy broth (Becton Dickinson, Franklin Lake, N.J.) at 37°C, harvested at a mid-log phase, and then washed three times in phosphate-buffered saline. The inocula were prepared to concentrations of 1.3 × 107 to 4.0 × 107 CFU/ml, based on their turbidity. Fifty-microliter concentrations of live P. aeruginosa were prepared, and inoculation was done by insertion of a 25-gauge blunt needle into and parallel to the trachea.
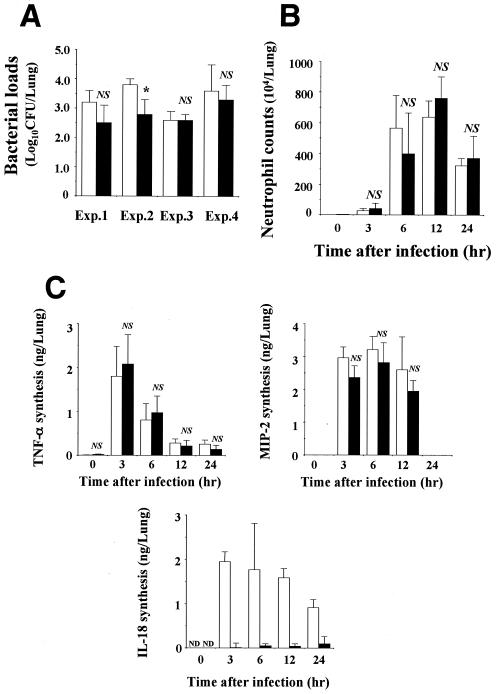
In the first experiments, we compared the clinical course of this infection in control and IL-18 KO mice. Mice were sacrificed on day 3, and lungs were separately homogenized in 5 ml of half-diluted normal saline by teasing with a stainless mesh. The homogenates (100 μl) were inoculated onto Trypticase soy agar plates, and the numbers of colonies were counted. As shown in Fig. 1A, the bacterial loads in the lungs of IL-18 KO mice were significantly lower than those of the control mice in experiment 2, suggesting an increased clearance of the bacterial pathogen. However, in experiments 1 and 4, the difference was not statistically significant. There was no such tendency in experiment 3. These results indicated that the defect in IL-18 synthesis did not have much influence on the host protection against P. aeruginosa infection.
FIG. 1.
Bacterial loads and neutrophil and cytokine responses in the lungs of IL-18 KO mice after infection with P. aeruginosa. IL-18 KO and control mice were infected intratracheally with P. aeruginosa. The counts of live bacteria (A), numbers of neutrophils (B), and concentrations of TNF-α, MIP-2, and IL-18 (C) in the lung homogenates were examined on day 3 (A) and at the indicated time points (B and C). Each bar represents the mean ± standard deviation (SD) of results from three to seven IL-18 KO mice or three to eight control mice. Similar results were obtained in three experiments (B and C). White bars, control mice; black bars, IL-18 KO mice; Exp., experiment; ND, not detected; NS, not significant; *, P < 0.05 compared with results from control mice by analysis of variance (ANOVA).
Next, we examined the influence of the IL-18 defect on the lung recruitment of neutrophils, an essential cellular component for host defense, after P. aeruginosa infection. For this purpose, leukocytes were prepared from lung homogenates, as described previously by Kawakami et al. (7). Before infection, the proportion of neutrophils in IL-18 KO and control mice was not different. As shown in Fig. 1B, there was no significant difference in the numbers of neutrophils at 3, 6, 12, and 24 h postinfection for these mice. We further examined the effect of the IL-18 defect on the synthesis of TNF-α and MIP-2 in the lungs, as measured by the respective specific enzyme-linked immunosorbent assay kit (R&D Systems, Inc., Minneapolis, Minn.). As shown in Fig. 1C, the production levels of these cytokines in control and IL-18 KO mice were not significantly different, although the level of MIP-2 was lower in the IL-18 KO mice at 3, 6, and 12 h. On the other hand, a large amount of IL-18 was produced in the lungs of control mice after infection with P. aeruginosa, whereas such production was not or only marginally detected in IL-18 KO mice. These results indicated that the defect in the synthesis of IL-18 did not result in the impairment of neutrophil-mediated host defense against this infection.
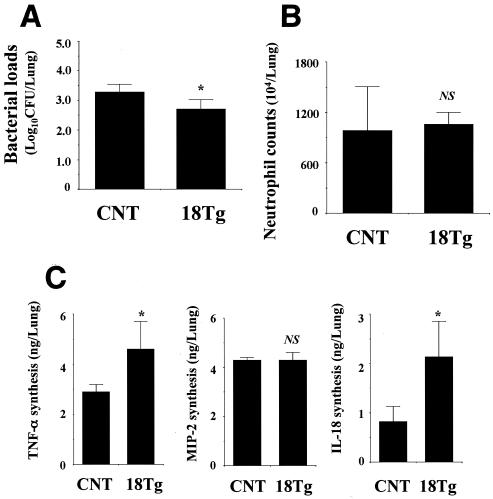
To further explore the role of IL-18 in the host defense against P. aeruginosa infection, we compared the clearance of this bacteria and neutrophil-mediated inflammatory responses for control and IL-18 Tg mice. As shown in Fig. 2A, for IL-18 Tg mice, a small reduction in the lung bacterial burdens was observed on day 3 postinfection compared with those of control mice. Neutrophils accumulated in the lungs of control and IL-18 Tg mice at comparable levels at 6 h (Fig. 2B). Similarly, there was no difference between the levels of production of MIP-2 for these groups of mice, while the production of TNF-α in the lungs significantly increased in IL-18 Tg mice compared with control mice. On the other hand, a significantly larger amount of IL-18 was produced in the lungs of IL-18 Tg mice than that produced in control mice (Fig. 2C).
FIG. 2.
Bacterial loads and neutrophil and cytokine responses in the lungs of IL-18 Tg mice after infection with P. aeruginosa. IL-18 Tg and control mice were infected intratracheally with P. aeruginosa. The counts of live bacteria (A), numbers of neutrophils (B), and concentrations of TNF-α, MIP-2, and IL-18 (C) in the lung homogenates were examined on day 3 and at 6 and 3 h postinfection, respectively. Each bar represents the mean ± SD of results from five to six IL-18 Tg mice or three to six control mice. Similar results were obtained in three experiments. CNT, control mice; 18Tg, IL-18 Tg mice; NS, not significant; *, P < 0.05 compared with results from control mice by ANOVA.
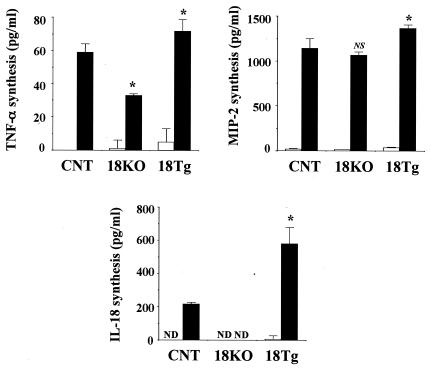
Finally, we compared the in vitro production of TNF-α and MIP-2 by lung leukocytes upon stimulation with live P. aeruginosa by control, IL-18 KO, and IL-18 Tg mice. The cells were cultured to a concentration of 1 × 106/ml with or without live P. aeruginosa (5 × 105/ml) for 12 h in RPMI 1640 medium (Nippro, Osaka, Japan) supplemented with 10% fetal calf serum (Cansera, Rexdale, Ontario, Canada), but without any antibiotics, in a 5% CO2 incubator. As shown in Fig. 3, TNF-α production was significantly lower in IL-18 KO mice and higher in IL-18 Tg mice than in control mice. In contrast, there was no difference between MIP-2 production levels in control and IL-18 KO mice, although levels were slightly higher in IL-18 Tg mice than in control mice. On the other hand, the production of IL-18 by infected lung leukocytes was not detected in IL-18 KO mice and was much higher in IL-18 Tg mice than in control mice.
FIG. 3.
In vitro synthesis of cytokines by lung leukocytes stimulated with P. aeruginosa. Lung leukocytes were cultured at a concentration of 1 × 106/ml with 5 × 105 CFU of P. aeruginosa for 12 h, and the concentrations of TNF-α, MIP-2, and IL-18 in the culture supernatants were measured. Each bar represents the mean ± SD of results from triplicate cultures. Similar results were obtained in two experiments. White bars, medium only; black bars, medium with P. aeruginosa; CNT, control; 18KO, IL-18 KO mice; 18Tg, IL-18 Tg mice; ND, not detected; NS, not significant; *, P < 0.05 compared with results from control mice by ANOVA.
In an earlier investigation, Huang and coworkers (6) reported that the administration of neutralizing anti-IL-18 antibody resulted in the decreased synthesis of IFN-γ and the impaired clearance of P. aeruginosa in the cornea, and they concluded that IL-18 contributed to host resistance to this infection through the induction of IFN-γ. In sharp contrast, Schultz and coworkers (18) indicated that deficient synthesis of IL-18 increased the bacterial clearance in the lungs, which was associated with reduced synthesis of proinflammatory cytokines. In the present study, as in the latter study, clearance of this bacterium tended to be promoted, rather than impaired, in the lungs of IL-18-deficient mice, although this change was not always significant. Although the reason for the different results remains to be clarified, a distinct mechanism(s) might be operational in different tissues, such as the cornea and lungs.
IL-18 contributes to neutrophil-mediated inflammatory responses by enhancing the production of TNF-α and MIP-2 and recruiting these cells into the tissues, as indicated in various mouse models (1, 9, 12). These earlier findings suggest the reduced synthesis of TNF-α and MIP-2 and suppression of neutrophil accumulation in the infected lungs of IL-18 KO mice, which is highly consistent with previous investigations of P. aeruginosa pneumonia and pneumococcal meningitis (18, 22). Similarly, TNF-α synthesis caused by P. aeruginosa is reduced in the corneas of anti-IL-18 antibody-treated mice (6). In our study, however, the synthesis of both cytokines in P. aeruginosa-infected lungs was not significantly different for control and IL-18 KO mice; for the latter, IL-18 synthesis was not detected at any time points. Compatible with these results, the levels of recruitment of neutrophils in the infected lung tissues were not different among these mouse strains. In animals with pneumococcal pneumonia, the synthesis of MIP-2 and accumulation of neutrophils in the lungs is reported to be higher in IL-18 KO mice than in control mice, although TNF-α is produced in equivalent amounts in these mouse strains (8). Thus, the regulation of the inflammatory response by IL-18 appears more complex than previously realized, although the precise mechanism remains to be fully understood.
In order to address the role of IL-18, we examined for the first time the effect of Tg expression of this cytokine on the clearance of P. aeruginosa in the lungs. The transgene is expressed under the control of Ig promoter (5), which indicates overproduction of IL-18 by B cells that reside in a considerable proportion in the lungs (7). In fact, this cytokine was detected at high levels in the serum and lungs of IL-18 Tg mice even before infection (data not shown). In these mice, the synthesis of MIP-2 and the recruitment of neutrophils in P. aeruginosa-infected lungs were not influenced compared to the levels detected in control mice, although bacterial clearance and TNF-α production were significantly enhanced. These results do not provide clear evidence determining the active role of IL-18 in neutrophil-mediated host resistance to P. aeruginosa infection.
In our in vitro study, TNF-α synthesis by lung leukocytes upon stimulation with P. aeruginosa was significantly lower in IL-18 KO mice than in control mice, while these cells produced a higher level of both TNF-α and MIP-2 in IL-18 Tg mice. These results are consistent with those of previous reports showing IL-18 induction of TNF-α and MIP-2 synthesis (1, 3, 9, 12, 13, 16). Nevertheless, in vivo synthesis of these cytokines, neutrophil recruitment, and clearance of bacteria in lungs were not altered in IL-18 KO mice. Although the reason for these discrepant observations is not clear, some compensatory mechanism(s) might be operating to maintain the host protective responses under an IL-18-deficient condition.
In conclusion, we demonstrated here that defective as well as exaggerated production of IL-18 did not alter the neutrophil-mediated host protective response to pulmonary infection with P. aeruginosa. Our data suggest the involvement of a more complex mechanism in IL-18-mediated regulation of host defense against infection, and this possibility should be noted in any future attempt to develop immune-based strategies with IL-18 against refractory infectious diseases caused by P. aeruginosa.
Editor: J. T. Barbieri
REFERENCES
- 1.Canetti, C. A., B. P. Leung, S. Culshaw, I. B. McInnes, F. Q. Cunha, and F. Y. Liew. 2003. IL-18 enhances collagen-induced arthritis by recruiting neutrophils via TNF-α and leukotriene B4. J. Immunol. 171:1009-1015. [DOI] [PubMed] [Google Scholar]
- 2.Collins, T., M. A. Read, A. S. Neish, M. Z. Whitley, D. Thanos, and T. Maniatis. 1995. Transcriptional regulation of endothelial cell adhesion molecules: NF-κB and cytokine-inducible enhancers. FASEB J. 9:899-909. [PubMed] [Google Scholar]
- 3.Dai, S. M., H. Matsuno, H. Nakamura, K. Nishioka, and K. Yudoh. 2004. Interleukin-18 enhances monocyte tumor necrosis factor-α and interleukin-1β production induced by direct contact with T lymphocytes: implications in rheumatoid arthritis. Arthritis Rheum. 50:432-443. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello, C. A. 1999. IL-18: a Th1-inducing, proinflammatory cytokine and new member of the IL-1 family. J. Allergy Clin. Immunol. 103:11-24. [DOI] [PubMed] [Google Scholar]
- 5.Hoshino, T., Y. Kawase, M. Okamoto, K. Yokota, K. Yoshino, K. Yamamura, J. Miyazaki, H. A. Young, and K. Oizumi. 2001. Cutting edge: IL-18-transgenic mice: in vivo evidence of a broad role for IL-18 in modulating immune function. J. Immunol. 15:7014-7018. [DOI] [PubMed] [Google Scholar]
- 6.Huang, X., S. A. McClellan, R. P. Barrett, and L. D. Hazlett. 2002. IL-18 contributes to host resistance against infection with Pseudomonas aeruginosa through induction of IFN-γ production. J. Immunol. 168:5756-5763. [DOI] [PubMed] [Google Scholar]
- 7.Kawakami, K., S. Kohno, N. Morikawa, J. Kadota, A. Saito, and K. Hara. 1994. Activation of macrophages and expansion of specific T lymphocytes in the lungs of mice intratracheally inoculated with Cryptococcus neoformans. Clin. Exp. Immunol. 96:230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauw, F. N., J. Branger, S. Florquin, P. Speelman, S. J. van Deventer, S. Akira, and T. van der Poll. 2002. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J. Immunol. 168:372-378. [DOI] [PubMed] [Google Scholar]
- 9.Leung, B. P., S. Culshaw, J. A. Gracie, D. Hunter, C. A. Canetti, C. Campbell, F. Cunha, F. Y. Liew, and I. B. McInnes. 2001. A role for IL-18 in neutrophil activation. J. Immunol. 167:2879-2886. [DOI] [PubMed] [Google Scholar]
- 10.Mackay, F., H. Loetscher, D. Stueber, G. Gehr, and W. Lesslauer. 1993. Tumor necrosis factor α (TNF-α)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. J. Exp. Med. 177:1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizgerd, J. P. 2002. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin. Immunol. 14:123-132. [DOI] [PubMed] [Google Scholar]
- 12.Netea, M. G., G. Fantuzzi, B. J. Kullberg, R. J. Stuyt, E. J. Pulido, R. C. McIntyre, Jr., L. A. Joosten, J. W. van der Meer, and C. A. Dinarello. 2000. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J. Immunol. 164:2644-2649. [DOI] [PubMed] [Google Scholar]
- 13.Netea, M. G., A. G. Vonk, M. van den Hoven, I. Verschueren, L. A. Joosten, J. H. van Krieken, W. B. van den Berg, J. W. van der Meer, and B. J. Kullberg. 2003. Differential role of IL-18 and IL-12 in the host defense against disseminated Candida albicans infection. Eur. J. Immunol. 33:3409-3417. [DOI] [PubMed] [Google Scholar]
- 14.Okamura, H., H. Tsutsui, T. Komatsu, M. Yutsudo, A. Hakura, T. Tanimoto, K. Torigoe, T. Okura, Y. Nukada, K. Hattori, K. Akita, M. Namba, F. Tanabe, K. Konishi, S. Fukuda, and M. Kurimoto. 1995. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 378:88-91. [DOI] [PubMed] [Google Scholar]
- 15.Okamura, H., H. Tsutsui, S. Kashiwamura, T. Yoshimoto, and K. Nakanishi. 1998. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv. Immunol. 70:281-312. [DOI] [PubMed] [Google Scholar]
- 16.Puren, A. J., G. Fantuzzi, Y. Gu, M. S. Su, and C. A. Dinarello. 1998. Interleukin-18 (IFNγ-inducing factor) induces IL-8 and IL-1β via TNFα production from non-CD14+ human blood mononuclear cells. J. Clin. Investig. 101:711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi, D., and A. Zlotonik. 2000. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18:217-242. [DOI] [PubMed] [Google Scholar]
- 18.Schultz, M. J., S. Knapp, S. Florquin, J. Pater, K. Takeda, S. Akira, and T. van der Poll. 2003. Interleukin-18 impairs the pulmonary host response to Pseudomonas aeruginosa. Infect. Immun. 71:1630-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda, K., H. Tsutsui, T. Yoshimoto, O. Adachi, N. Yoshida, T. Kishimoto, H. Okamura, K. Nakanishi, and S. Akira. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8:383-390. [DOI] [PubMed] [Google Scholar]
- 20.Toews, G. B., E. J. Hansen, and R. M. Strieter. 1990. Pulmonary host defenses and oropharyngeal pathogens. Am. J. Med. 88:S20-S24. [DOI] [PubMed] [Google Scholar]
- 21.Tsai, W. C., R. M. Strieter, B. Mehrad, M. W. Newstead, X. Zeng, and T. J. Standiford. 2000. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 68:4289-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwijnenburg, P. J., T. van der Poll, S. Florquin, S. Akira, K. Takeda, J. J. Roord, and A. M. van Furth. 2003. Interleukin-18 gene-deficient mice show enhanced defense and reduced inflammation during pneumococcal meningitis. J. Neuroimmunol. 138:31-37. [DOI] [PubMed] [Google Scholar]