Abstract
Oral immunization of mice with Escherichia coli-expressed Plasmodium yoelii merozoite surface protein 4/5 or the C-terminal 19-kDa fragment of merozoite surface protein 1 induced systemic antibody responses and protected mice against lethal malaria infection. A combination of these two proteins administered orally conferred improved protection compared to that conferred by either protein administered alone.
Oral administration of antigens offers a number of advantages for the delivery of malaria vaccines. Oral vaccines can be delivered outside a formal clinical setting, without the need for skilled personnel. Eliminating needles from the vaccination process can negate concerns regarding the reuse and disposal of needles and reduce the associated risks, such as the transmission of blood-borne viruses. In addition, oral vaccines appear to offer a relatively straightforward means of combining antigens into a multivalent formulation without the substantial cost and difficulties of reformulating parenteral preparations. Furthermore, if oral vaccination against malaria is feasible, oral vaccines can be further developed based on the use of transgenic plants as bioreactors for the production and possible delivery of vaccine antigens (3).
Using Plasmodium yoelii merozoite surface proteins 4 and 5 (PyMSP4/5) in an animal model system, it has been previously demonstrated that administration of Escherichia coli-expressed recombinant PyMSP4/5 (EcMSP4/5) in the presence of cholera toxin B subunit (CTB) to mice by gavage induces systemic antibody responses comparable to those achieved with parenteral immunization. The antibodies induced are predominantly immunoglobulin G1 (IgG1) and can protect mice against lethal challenge with P. yoelii (12). Here we report the oral immunogenicity of a second P. yoelii antigen, the C-terminal 19-kDa fragment of merozoite surface protein 1 (PyMSP119). We show that protection against lethal P. yoelii infection can be achieved by oral immunization with a recombinant PyMSP119 expressed by E. coli. Furthermore, an orally administered combination of recombinant PyMSP4/5 and PyMSP119 conferred improved protection compared to that conferred by either protein administered alone.
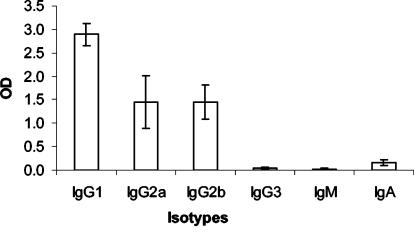
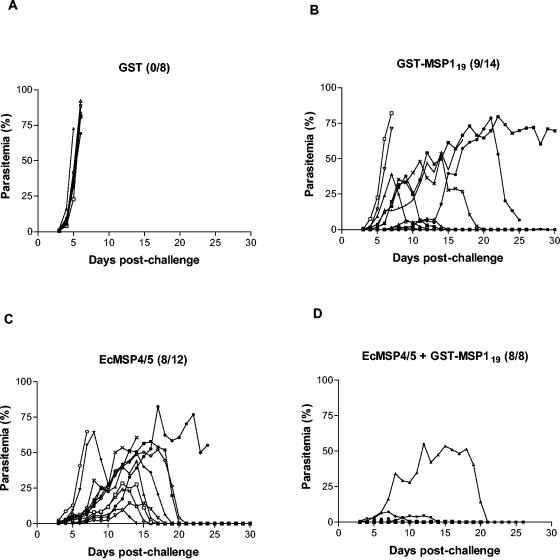
The C-terminal PyMSP119 sequence that corresponds to amino acids 1649 to 1754 and contains the two epidermal growth factor-like domains was expressed in E. coli as a glutathione S-transferase (GST) fusion (9). This fusion protein, named GST-PyMSP119, was purified from cell extracts by affinity chromatography on glutathione-agarose and eluted with reduced glutathione as described previously (11). To assess the oral immunogenicity of PyMSP119, 14 female BALB/c mice were administered by gavage an amount of GST-PyMSP119 equivalent to 25 μg of PyMSP119 in the presence of 10 μg of CTB. Six immunizations were given, at weeks 0, 1, 2, 3, 6, and 8. Sera were collected at 10 days after the sixth immunization, and the resultant antibodies were measured with an enzyme-linked immunosorbent assay as described previously (12). All mice developed high levels of PyMSP119-specific antibodies, which were comparable to those induced by intraperitoneal immunization with a Saccharomyces cerevisiae-expressed recombinant PyMSP119 (data not shown). The predominant isotype of the antibodies was IgG1, with lower IgG2a and IgG2b responses (Fig. 1), a pattern identical to that induced by oral immunization with PyMSP4/5 (12). In order to examine the protective efficacy of the induced antibodies, the immunized mice were challenged at 2 weeks after the sixth immunization with a lethal dose of 105 P. yoelii YM parasites as described previously (12). Eight mice that were immunized with GST and CTB were also challenged under the same protocol. All of these eight mice developed fulminating infections and died between days 5 and 6 postchallenge (Fig. 2A). In contrast, 9 of the 14 mice immunized with GST-PyMSP119 showed clear evidence of protective immunity and survived the challenge (Fig. 2B). There was a significant difference in the numbers of surviving mice in the groups (P = 0.0055, as determined by Fisher's exact probability test), and a significant difference was also observed in peak parasitemia levels between the two groups (P = 0.0015, as determined by the Mann-Whitney U test). These data demonstrate that recombinant PyMSP119 administered orally in the presence of CTB can induce systemic antibodies and protect a significant proportion of mice against a lethal challenge with P. yoelii.
FIG. 1.
Isotype distribution of the PyMSP119-specific antibodies raised in mice by oral immunization with GST-PyMSP119 in the presence of CTB. Sera were measured at a 1:10,000 dilution. The optical density (OD) values are the averages for individually tested sera, and the error bars indicate the standard deviations of the means.
FIG. 2.
Blood-stage parasitemia levels of mice orally immunized with GST (A), GST-PyMSP119 (B), EcMSP4/5 (C), and GST-PyMSP119 plus EcMSP4/5 (D) prior to challenge with P. yoelii YM. The survival rate (number of surviving mice/total number of mice) is shown on the top of each graph.
It is generally believed that an effective subunit malaria vaccine will include multiple protein components; however, there is limited experimental support for this proposition. It has been shown that parenteral immunization with a combination of PyMSP119 and PyMSP4/5 induces an improved level of protection compared to that induced by either protein administered alone (8). Burns et al. also showed that a combined formulation of Plasmodium chabaudi MSP1 and apical membrane antigen 1 administered parenterally results in a greater reduction in peak parasitemia levels than that achieved with either antigen alone (1). To examine whether an antigen combination could also be efficacious when administered orally, two additional groups of mice were employed: one group was treated by gavage with 25 μg of EcMSP4/5, and the other group was treated by gavage with 25 μg of EcMSP4/5 plus an amount of GST-PyMSP119 equivalent to 25 μg of PyMSP119. As shown in Fig. 2, immunization with a combination of GST-PyMSP119 and EcMSP4/5 had a dramatic effect on the levels of protection. All of the eight immunized mice survived the challenge, with peak parasitemia levels between 0.2 and 55.2%. The differences in peak parasitemia levels between different groups were statistically significant. A P value of 0.0006 (as determined by the Mann-Whitney U test) was found when the comparison was made between the group immunized with EcMSP4/5 and the group immunized with the combined antigens, and a P value of 0.036 (as determined by the Mann-Whitney U test) was found when the group immunized with GST-PyMSP119 and the group immunized with the combined antigens were compared. The survival rates in the combined-antigen group were improved compared to those in the groups immunized with either antigen alone (100% versus 66.7 and 64.3% for the EcMSP4/5 group and the GST-PyMSP119 group, respectively); however, the differences in these survival rates were not significant.
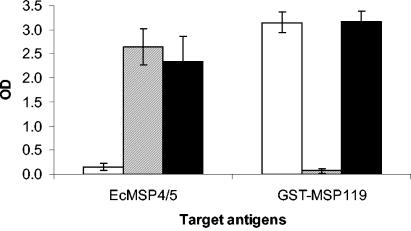
The prechallenge antibody responses were analyzed by enzyme-linked immunosorbent assay for both antigens (Fig. 3). Mice immunized with a combination of EcMSP4/5 and GST-PyMSP119 developed high levels of antibodies to both antigens. There was no difference in the antibody responses to EcMSP4/5 between the combined-antigen group and the EcMSP4/5-immunized group (P = 0.1770, as determined by the Mann-Whitney U test); similarly, no difference was observed in the antibody responses to GST-PyMSP119 between the combined-antigen group and the GST-PyMSP119-immunized group (P = 0.8112, as determined by the Mann-Whitney U test). The isotype distributions of the PyMSP4/5-specific antibodies were similar in the combined-antigen and EcMSP4/5-immunized groups, and the isotype distributions of the PyMSP119-specific antibodies were similar in the combined-antigen and PyMSP119-immunized groups (data not shown). These data demonstrated that oral immunization with a mixture of PyMSP4/5 and PyMSP119 neither boosts the antibody reactivity to either antigen nor results in antigen competition. The enhanced protection seemed to be due to an additive effect of the capacity of both antigens to induce a level of protection.
FIG. 3.
Prechallenge antibody responses of mice orally immunized with GST-PyMSP119 (empty bars), EcMSP4/5 (gray bars), and GST-PyMSP119 plus EcMSP4/5 (black bars). Sera were measured at a 1:10,000 dilution for both GST-PyMSP119 and EcMSP4/5. The optical density (OD) values are the averages for individually tested sera, and the error bars indicate the standard deviations of the means.
It is generally thought that immunity to P. yoelii is predominantly antibody mediated (4). It has been shown previously that a significant correlation between PyMSP4/5-specific antibodies and protective efficacy exists (7, 12). In general, mice with higher antibody responses showed better protection, whereas mice with lower antibody levels showed poor protection or succumbed to the malaria infection. A similar correlation was also observed in this study (data not shown). However, not all mice with high levels of antibodies were protected from parasite infection, indicating that other immune mechanisms also operated to provide the observed protection. Examination of the serum samples from the protected mice after they fully recovered showed that parasite infection neither boosted the specific antibodies to either PyMSP4/5 or PyMSP119 nor changed the pattern of isotype profiles (data not shown).
In summary, we have demonstrated that PyMSP119 is immunogenic when administered orally and offers protection against challenge with a lethal dose of P. yoelii. PyMSP119 is a leading malaria vaccine candidate whose protective efficacy when administered parenterally has been shown in a number of studies (2, 6, 9, 10). One study showed that intranasal immunization with S. cerevisiae-expressed recombinant PyMSP119 induced some degree of protective immunity, suggesting that mucosal delivery of PyMSP119 has the potential to protect against malaria via a mechanism similar to that observed following parenteral immunization (5). This study showed that significant protection can also be induced by oral immunization. Importantly, we have demonstrated that oral immunization with recombinant PyMSP4/5 plus PyMSP119 induces antibodies to both antigens and also provides enhanced protection against parasite infection. These data are consistent with those achieved by parenteral immunization (8) and provide a rationale for developing multicomponent oral vaccines.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council (NH&MRC) of Australia, the National Institutes of Health (NIH), the Howard Hughes Medical Institute International Scholars Program, and the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases.
We thank Alison Campbell for making the PyMSP119 expression construct.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Burns, J. M., Jr., P. R. Flaherty, M. M. Romero, and W. P. Weidanz. 2003. Immunization against Plasmodium chabaudi malaria using combined formulations of apical membrane antigen-1 and merozoite surface protein-1. Vaccine 21:1843-1852. [DOI] [PubMed] [Google Scholar]
- 2.Daly, T. M., and C. A. Long. 1993. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect. Immun. 61:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniell, H., S. J. Streatfield, and K. Wycoff. 2001. Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci. 6:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Good, M. F., and D. L. Doolan. 1999. Immune effector mechanisms in malaria. Curr. Opin. Immunol. 11:412-419. [DOI] [PubMed] [Google Scholar]
- 5.Hirunpetcharat, C., D. Stanisic, X. Q. Liu, J. Vadolas, R. A. Strugnell, R. Lee, L. H. Miller, D. C. Kaslow, and M. F. Good. 1998. Intranasal immunization with yeast-expressed 19 kD carboxyl-terminal fragment of Plasmodium yoelii merozoite surface protein-1 (PyMSP119) induces protective immunity to blood stage malaria infection in mice. Parasite Immunol. 20:413-420. [DOI] [PubMed] [Google Scholar]
- 6.Hirunpetcharat, C., J. H. Tian, D. C. Kaslow, N. van Rooijen, S. Kumar, J. A. Berzofsky, L. H. Miller, and M. F. Good. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 159:3400-3411. [PubMed] [Google Scholar]
- 7.Kedzierski, L., C. G. Black, and R. L. Coppel. 2000. Immunization with recombinant Plasmodium yoelii merozoite surface protein 4/5 protects mice against lethal challenge. Infect. Immun. 68:6034-6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kedzierski, L., C. G. Black, M. W. Goschnick, A. W. Stowers, and R. L. Coppel. 2002. Immunization with a combination of merozoite surface pro-teins 4/5 and 1 enhances protection against lethal challenge with Plasmodium yoelii. Infect. Immun. 70:6606-6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling, I. T., S. A. Ogun, and A. A. Holder. 1994. Immunization against malaria with a recombinant protein. Parasite Immunol. 16:63-67. [DOI] [PubMed] [Google Scholar]
- 10.Tian, J. H., L. H. Miller, D. C. Kaslow, J. Ahlers, M. F. Good, D. W. Alling, J. A. Berzofsky, and S. Kumar. 1996. Genetic regulation of protective im-mune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J. Immunol. 157:1176-1183. [PubMed] [Google Scholar]
- 11.Wang, L., C. G. Black, V. M. Marshall, and R. L. Coppel. 1999. Structural and antigenic properties of merozoite surface protein 4 of Plasmodium falciparum. Infect. Immun. 67:2193-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, L., L. Kedzierski, S. L. Wesselingh, and R. L. Coppel. 2003. Oral immunization with a recombinant malaria protein induces conformational antibodies and protects mice against lethal malaria. Infect. Immun. 71:2356-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]