Summary
Background
We aimed to present unusual cranial FDG PET/CT findings of a 56-year-old female with multiple myeloma (MM).
Case Report
Plain CT images revealed a lytic lesion in the right parietal bone, filled with an oval-shaped, large, extra-axial, extradural, intracranial mass which measured 75×75×40 mm and had smooth borders. The right parietal lobe was compressed by the mass. The maximum standardized uptake value (SUVmax) of the mass lesion was 8.94 on FDG PET/CT images. Multiple lytic lesions with an increased uptake were also detected in other calvarial bones, in several vertebras and in the proximal left femur. After seven months, a control FDG PET/CT following radiotherapy and chemotherapy revealed almost complete regression of the right parietal extra-axial mass lesion. The number, size and metabolism of lytic lesions in other bones also decreased.
Conclusions
FDG PET/CT was useful for an initial evaluation of MM lesions and was effective in monitoring the response of these lesions to therapy.
MeSH Keywords: Fluorodeoxyglucose F18, Multiple Myeloma, Positron-Emission Tomography, Skull Neoplasms
Background
Multiple myeloma (MM) is characterized by multiple osteolytic lesions due to the malignant proliferation of monoclonal plasma cells [1]. The disease usually presents in the 5th–8th decade with male predominance [2], and the percentage of MM among all cancers was reported at 1.3% [3]. Radiolabeled [18F]-2-fluoro-2-deoxy-D-glucose (FDG) is widely used in positron emission tomography (PET) for the detection of tumor metabolism [4], and bone marrow involvement in patients with MM [5]. Plain radiographs, a basic method used for both diagnosing and monitoring of MM patients, visualize lytic lesions in the skeletal system [6]. FDG PET combined with computed tomography (CT), commonly abbreviated as FDG PET/CT, is a superior imaging tool for the diagnosis and staging of MM as compared to conventional imaging tools [7]. Although cross-sectional imaging methods such as CT and magnetic resonance imaging (MRI) are considered to be sensitive in demonstrating extra-osseous dissemination in MM [8], PET/CT has been described as having a high sensitivity, specificity and prognostic value in patients with MM [7]. We present FDG PET/CT findings of an unusual MM case with a large extra-axial, extradural, intracranial mass both on admission and during remission following treatment.
Case Report
A 56-year-old female MM patient with a history of vertebral compression fracture underwent FDG PET/CT imaging. All the procedures were performed according to the World Medical Association Declaration of Helsinki (revised in 2000, Edinburgh). The patient was informed of PET/CT examination procedures, and informed consent was obtained from her. The patient fasted for 11 hours before the study, with a plasma glucose level obtained at the time of FDG administration of 94 mg/dL. F-18 FDG was injected intravenously at a dose of 370 MBq (10 mCi). Whole-body emission scanning (3−13 bed positions; acquisition time, 3 min/bed position) was performed 50 minutes after FDG administration in supine position. Scanning was performed from the head to the proximal thigh. Hybrid imaging was performed using a Discovery 610 (General Electric Medical Systems, LLC, Waukesha, WI, USA) PET/CT scanner. CT images were obtained during breath-hold using the following parameters: detector row configuration, 16×1.25 mm; tube voltage, 120–140 kVp; maximum tube current, 220 mA; beam collimation, 20.0 mm; table speed, 27.5 mm/rotation; pitch, 1.375:1; helical thickness, 3.75 mm and 512×512 matrix. We did not administer intravenous iodinated contrast media. Plain CT images revealed a lytic lesion with a narrow zone of transition in the posterolateral part of the right parietal bone (Figure 1), filled with an oval-shaped large mass measuring 75×75×40 mm with smooth borders. The lesion was hyperdense as compared to the neighbouring cerebral cortex (Figure 2). Medially and peripherally, there were small calcified structures next to the cerebral cortex suggesting a calvarial origin of the expanding lesion. Although a portion of the mass lesion extended to the scalp, it mainly demonstrated the common features of large extra-axial, extradural, intracranial lesions such as compression of the right lateral ventricle causing a slight midline shift to the left and giving rise to cerebral edema in the neighbouring right parietal lobe due to external compression. The maximum standardized uptake value (SUVmax) of the mass lesion was 8.94 on FDG PET/CT images (Figure 3). Plain CT images also demonstrated multiple, “punched out” lytic lesions in other calvarial bones, in several vertebras and in the proximal left femur. PET/CT images revealed hypermetabolism in all these lesions (SUVmax ranged between 3.67–5.45). Seven months later, a control FDG PET/CT obtained after chemotherapy and radiotherapy revealed almost complete regression of the right parietal, extra-axial, extradural mass lesion, where only a residual curvilinear hypodense soft tissue with a maximum thickness of 3.5–4.0 mm and linear calcifications remained (Figures 4, 5). Compared with the initial FDG PET/CT study, the number, size and metabolism of the lytic lesions in other calvarial bones and vertebras decreased. The hypermetabolism in the lesions of the proximal left femur also subsided.
Figure 1.
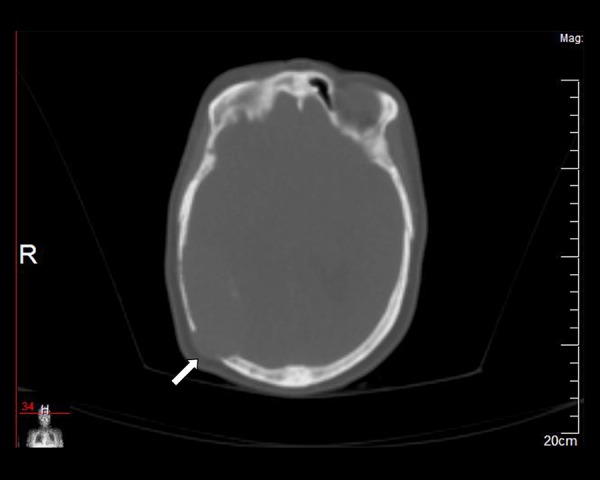
Axial plain CT (bone window settings) demonstrates a lytic lesion in the right parietal bone (arrow).
Figure 2.
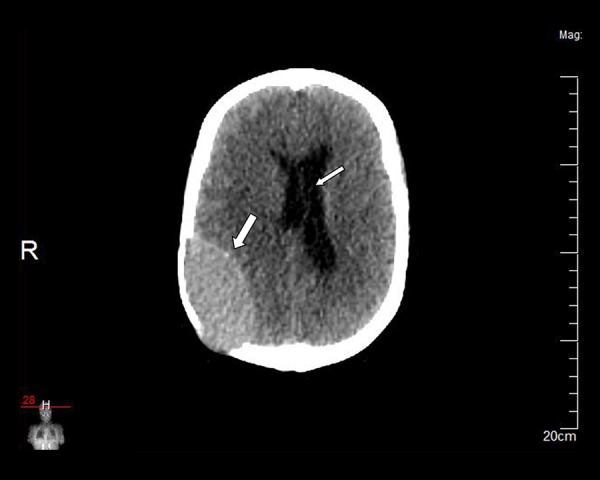
The lesion is hyperdense compared to the neighbouring cerebral cortex (soft tissue window settings) (thick arrow). Mild midline shift to the left can be seen (thin arrow).
Figure 3.
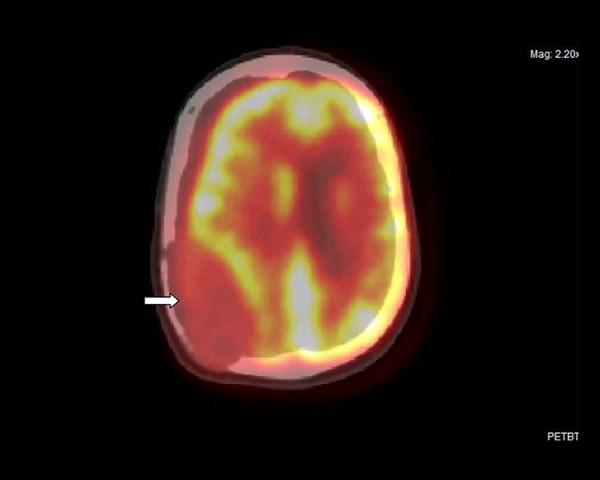
Axial fused image of the right parietal extra-axial mass lesion (arrow).
Figure 4.
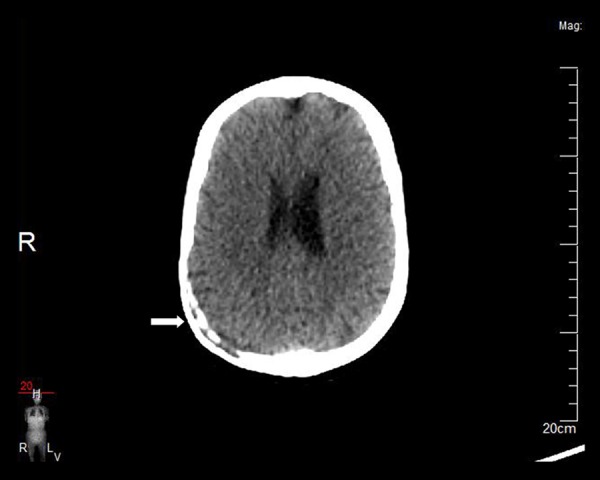
Axial plain CT obtained seven months later reveals almost complete regression of the lesion (arrow).
Figure 5.

Axial fused image obtained seven months later demonstrates no hypermetabolism in that area (arrow).
Discussion
Multiple myeloma was reported to be the most common primary malignancy of the bone in the United States [9]. Axial skeleton lesions including skull masses can occur both in the disseminated form of MM and as a solitary plasmacytoma of the bone [2]. In the disseminated form of MM, the scattered lesions are predominantly located in the axial skeleton, most frequently in the vertebras [2].
FDG PET has been proven to detect MM and its extent better than plain radiographs [10,11]. In a study including newly diagnosed MM patients, Nanni et al. [12] compared FDG PET/CT with whole-body radiography and found that FDG PET/CT detected more skeletal lesions. Bredella et al. [5] reported the sensitivity and specificity of FDG PET in demonstrating myelomatous involvement in patients with MM as 85% and 92%, respectively. They concluded that FDG PET was useful for the evaluation of the spread of disease at initial diagnosis. In a study with MM patients, the FDG-PET detection of bone marrow involvement was 100% which emphasized the significant role of FDG-PET in the evaluation of bone marrow involvement [13]. In the presented case, we were able to detect multiple lesions with an increased activity in the bone marrow of the axial skeleton and the left femur on initial admission. FDG PET/CT is said to detect small lytic bone lesions better than X-rays and was considered to be similar to MRI in identifying the involvement of vertebras and pelvis [14]. It was found that the sensitivity of FDG PET/CT in detecting bone disease of the pelvis and vertebras was the same as MRI [12]. In the presented case, we could initially made the diagnosis by FDG PET/CT without the need for MRI. However, MRI is still recommended for patients with neurological symptoms suggesting a spinal cord involvement, because it visualizes the extent of compression caused by pathological structures [6].
Many different pathological conditions such as osteomyelitis [15], eosinophilic granuloma [16], Langerhans cell histiocytosis [17], fibrous dysplasia [18], brown tumours in hyperparathyroidism [19,20] and calvarial metastases [21,22] can exhibit increased glucose metabolism and may mimic MM on PET/CT images. Therefore, in differential diagnosis of skeletal lytic lesions with increased FDG uptake such entities should also be taken into consideration and plain CT findings, in addition to FDG PET/CT data, should be carefully evaluated. In calvarial osteomyelitis, irregular bone destruction [23], pus collection in the scalp [24], empyema and fistula [25] can be demonstrated on CT. In our patient, bone destruction was relatively regular, multiple lytic lesions were not localized but scattered and no infectious lesions such as an abscess or a draining sinus were detected. Similarly to skull lesions in MM, eosinophilic granuloma also presents as a well-demarcated lytic mass on CT, but beveled edge (“hole-within-hole”) and “button sequestrum” which are typical for eosinophilic granuloma [2] could not be detected on plain CT of the presented case. Patients with fibrous dysplasia are younger and in most cases skull lesions typically look like expansile “ground glass” lesions on CT [26], which are very different from sharply demarcated “punched-out” lesions of the skull in MM [1]. Differentiating our patient’s large calvarial lesion from a brown tumour could be possible because we could not detect characteristic radiologic findings of hyperparathyroidism, such as erosions and subperiosteal bone resorption in other bones [2]. Intracranial involvement of MM is rare [27,28], and in daily pratice it is challenging to differentiate MM from multiple lytic bone metastases of the lung, breast, and renal cancer on FDG PET/CT images. Importantly, our patient did not give any history of a known or advanced malignancy and other diagnostic procedures did not reveal such a malignancy.
Conclusions
If an usually large extra-axial, extradural, intracranial mass and several lytic bone lesions with an increased metabolism are identified on initial FDG PET/CT, MM should be considered in the differential diagnosis. FDG PET/CT was useful for an initial evaluation of the number, size, effects and metabolism of MM lesions, and was effective in monitoring the response of these lesions to therapy.
Footnotes
Conflict of interest
None declared.
References
- 1.Yim Y, Moon WJ, An HS, et al. Imaging findings of various calvarial bone lesions with a focus on osteolytic lesions. J Korean Soc Radiol. 2016;74(1):43–54. [Google Scholar]
- 2.Dahnert W. Radiology Review Manual. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2007. p. 104.p. 105.p. 110.p. 124.p. 125. [Google Scholar]
- 3.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. Cancer J Clin. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 4.Lindholm P, Minn H, Leskinen-Kallio S, et al. Influence of the blood glucose concentration on FDG uptake in cancer – a PET study. J Nucl Med. 1993;34(1):1–6. [PubMed] [Google Scholar]
- 5.Bredella MA, Steinbach L, Caputo G, et al. Value of FDG PET in the assessment of patients with multiple myeloma. Am J Roentgenol. 2005;184(4):1199–204. doi: 10.2214/ajr.184.4.01841199. [DOI] [PubMed] [Google Scholar]
- 6.Dzija A. Multiple myeloma – usefulness of imaging techniques in diagnostics. Pol J Radiol. 2014;79(Suppl 1):S16–22. [Google Scholar]
- 7.Agarwal A, Chirindel A, Shah BA, Subramaniam RM. Evolving role of FDG PET/CT in multiple myeloma imaging and management. Am J Roentgenol. 2013;200(4):884–90. doi: 10.2214/AJR.12.9653. [DOI] [PubMed] [Google Scholar]
- 8.Touzeau C, Moreau P. Multiple myeloma imaging. Diagn Interv Imaging. 2013;94(2):190–92. doi: 10.1016/j.diii.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Teo HE, Peh WC. Primary bone tumors of adulthood. Cancer Imaging. 2004;4(2):74–83. doi: 10.1102/1470-7330.2004.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schirrmeister H, Bommer M, Buck AK, et al. Initial results in the assessment of multiple myeloma using 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29:361–66. doi: 10.1007/s00259-001-0711-3. [DOI] [PubMed] [Google Scholar]
- 11.Zamagni E, Nanni C, Patriarca F, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92:50–55. doi: 10.3324/haematol.10554. [DOI] [PubMed] [Google Scholar]
- 12.Nanni C, Zamagni E, Farsad M, et al. Role of 18F-FDG PET/CT in the assessment of bone involvement in newly diagnosed multiple myeloma: Preliminary results. Eur J Nucl Med Mol Imaging. 2006;33(5):525–31. doi: 10.1007/s00259-005-0004-3. [DOI] [PubMed] [Google Scholar]
- 13.Hung GU, Tsai CC, Tsai SC, Lin WY. Comparison of Tc-99m sestamibi and F-18 FDG-PET in the assessment of multiple myeloma. Anticancer Res. 2005;25:4737–41. [PubMed] [Google Scholar]
- 14.Lütje S, de Rooy JW, Croockewit S, et al. Role of radiography, MRI and FDG-PET/CT in diagnosing, staging and therapeutical evaluation of patients with multiple myeloma. Ann Hematol. 2009;88(12):1161–68. doi: 10.1007/s00277-009-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann A, Eid K, Dora C, et al. Diagnostic value of 18F-FDG PET/CT in trauma patients with suspected chronic osteomyelitis. Eur J Nucl Med Mol Imaging. 2007;34(5):704–14. doi: 10.1007/s00259-006-0290-4. [DOI] [PubMed] [Google Scholar]
- 16.Mansberg R, Ho B, Bui C, Crombie C. False positive F-18 FDG PET/CT of skeletal metastasis due to solitary eosinophilic granuloma. Mol Imaging Radionucl Ther. 2013;22(3):103–5. doi: 10.4274/Mirt.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koç ZP, Şimşek S, Akarsu S, et al. Insufficiency of bone scintigraphy in vertebral lesions of langerhans cell histiocytosis compared to f-18 fluorodeoxyglucose positron emission tomography/computed tomography and diagnostic computed tomography. Mol Imaging Radionucl Ther. 2015;24(1):21–24. doi: 10.4274/mirt.58066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stegger L, Juergens KU, Kliesch S, et al. Unexpected finding of elevated glucose uptake in fibrous dysplasia mimicking malignancy: Contradicting metabolism and morphology in combined PET/CT. Eur Radiol. 2007;17(7):1784–86. doi: 10.1007/s00330-006-0466-0. [DOI] [PubMed] [Google Scholar]
- 19.Kuwahara K, Izawa S, Murabe H, et al. Increased 18F-fluorodeoxyglucose uptake in a brown tumor in a patient with primary hyperparathyroidism. J Clin Endocrinol Metab. 2007;92(7):2408–9. doi: 10.1210/jc.2007-0591. [DOI] [PubMed] [Google Scholar]
- 20.Sager S, Aliyev A, Halac M, Oztürk T. Positron emission tomography/computed tomography imaging of brown tumors mimicking multiple skeletal metastases in patient with primary hyperparathyroidism. Indian J Endocrinol Metab. 2012;16(5):850–52. doi: 10.4103/2230-8210.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taira AV, Herfkens RJ, Gambhir SS, Quon A. Detection of bone metastases: assessment of integrated FDG PET/CT imaging. Radiology. 2007;243(1):204–11. doi: 10.1148/radiol.2431052104. [DOI] [PubMed] [Google Scholar]
- 22.Du Y, Cullum I, Illidge TM, Ell PJ. Fusion of metabolic function and morphology: sequential [18F]fluorodeoxyglucose positron-emission tomography/computed tomography studies yield new insights into the natural history of bone metastases in breast cancer. J Clin Oncol. 2007;25(23):3440–47. doi: 10.1200/JCO.2007.11.2854. [DOI] [PubMed] [Google Scholar]
- 23.Huang I, Leach JL, Fichtenbaum CJ, Narayan RK. Osteomyelitis of the skull in early-acquired syphilis: Evaluation by MR imaging and CT. Am J Neuroradiol. 2007;28(2):307–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Khare P, Gupta R, Chand P, Agarwal S. Calvarial tuberculosis presenting as cystic lesion: An unusual presentation in two patients. J Cytol. 2015;32(3):176–80. doi: 10.4103/0970-9371.168844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boubrik M, Ait Benali S. Calvarial tuberculosis: Two case reports. Arch Pediatr. 2011;18(4):397–400. doi: 10.1016/j.arcped.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Kransdorf MJ, Moser RP, Jr, Gilkey FW. Fibrous dysplasia. Radiographics. 1990;10(3):519–37. doi: 10.1148/radiographics.10.3.2188311. [DOI] [PubMed] [Google Scholar]
- 27.Cerase A, Tarantino A, Gozzetti A, et al. Intracranial involvement in plasmacytomas and multiple myeloma: a pictorial essay. Neuroradiology. 2008;50(8):665–74. doi: 10.1007/s00234-008-0390-x. [DOI] [PubMed] [Google Scholar]
- 28.Terada T. Multiple myeloma presenting as an intracranial plasmacytoma: A case report. Cases J. 2009;2:9110. doi: 10.1186/1757-1626-2-9110. [DOI] [PMC free article] [PubMed] [Google Scholar]


