Abstract
Colonization is the first step in the interaction between Streptococcus pneumoniae and its human host. To better understand the mechanisms contributing to natural carriage, a mouse model of pneumococcal colonization was developed with a clinical isolate of S. pneumoniae previously characterized in experimental colonization of humans. Similar to carriage events in humans, colonization of mice was self-limited and there was no evidence of lower respiratory tract or invasive disease. Carriage induced a serum antibody response to whole pneumococci that was associated temporally with clearance of colonization in three inbred strains of mice. Individual mice, however, did not demonstrate a correlation between the density of colonization and amounts of serum or of mucosal antibodies, including antibodies of different isotypes and antigenic specificities. The role of antibody in the clearance of carriage was then examined in mice with genetic defects in humoral immunity. xid mice, which have deficient responses to polysaccharide antigens, cleared colonization at the same rate as the parent strain. Finally, we showed that μMT mice, which lack mature B cells and fail to produce antibody, were unaffected in the density or duration of colonization. These results demonstrate that antibody is not required for clearance of pneumococcal colonization in mice.
Streptococcus pneumoniae (the pneumococcus) is responsible for a large proportion of the bacterial diseases involving the respiratory tract (acute otitis media, sinusitis, and pneumonia) as well as invasive infection (septicemia and meningitis) in humans. All pneumococcal infection, however, begins with colonization of the mucosal surface of the nasopharynx, a state far more common than host-pathogen interactions leading to disease (2, 5, 32). Interventions that affect carriage, therefore, are likely to have the greatest impact on pneumococcal disease. In fact, much of the beneficial effect that vaccination with the pneumococcal capsular polysaccharide (PnPS) has in reducing the prevalence of disease is attributable to diminished rates of colonization in populations in which immunization rates are high (8, 9, 16, 19).
Host and bacterial factors that affect the density of, susceptibility to, and duration of colonization are in general poorly understood (32). Rates of carriage in the first year of life may exceed 50% with a gradual decline with increasing age until adulthood, when the prevalence of colonization averages 5 to 10% (14, 29). Carriage surveys have shown that a given isolate may be carried for days to months before being cleared (14). Because there are 90 known pneumococcal serotypes (types), there may be simultaneous carriage of two or more strains and sequential carriage with strains of different serotypes (12, 13). The diminishing frequency of colonization with increasing age correlates with rising levels of both mucosal and serum antibody to PnPS (27, 36). This together with data showing decreased rates of carriage in vaccinated populations, in which serum antibody titers have been boosted, has led to the assumption that the immune response to the PnPS is involved in the prevention of the carrier state (5, 11, 16). It has also been suggested that preexisting type-specific antibody does not prevent acquisition of a homotypic pneumococcus but may shorten the duration of its carriage (14). This same multifamily carriage study, however, showed a rise in type-specific serum antibody in children following illness but no corresponding increase in adults following asymptomatic carriage (14). A further controversy is the contribution to human colonization of the immune response to nonpolysaccharide surface antigens. There is a reduction in colonization following mucosal immunization with combinations of pneumococcal proteins with an adjuvant in a murine model, but the role of the immune response to these proteins in clearance of the carrier state in humans has not been demonstrated (6, 18). Thus, it remains unclear if natural carriage is an immunizing event or if other host factors dictate the dynamics of transient colonization for an individual isolate.
Recently, we described the use of experimental human carriage to allow prospective study of host factors impacting on colonization in the natural host (20, 21). In the initial investigation, 6 of 14 healthy adults became colonized for 27 to 122 days following an intranasal challenge with 103 to 104 CFU of a minimally passaged type 23F clinical isolate (20). There was a minimal serum antibody response to the PnPS during experimental carriage and no correlation between the amount of PnPS-specific antibodies in serum collected prior to inoculation and the likelihood of an individual becoming colonized. All the colonized subjects, in contrast, developed a serum immunoglobulin G (IgG) and secretory IgA response to the pneumococcal surface protein A (PspA) of the inoculated strain, whereas seven of eight subjects who did not become colonized had preexisting antibody to this protein (20). This observation raised the possibility that the immune response to PspA might be protective against colonization of humans. Further analysis of the antibody response in these individuals to a variety of pneumococcal cell-surface or secreted components demonstrated that only the presence of antibody specific for PspA correlated with prevention of colonization (21). This rise in titer of strain-specific PspA antibody, however, plateaued between 2 and 3 weeks after inoculation, although carriage continued for as long as another 14 weeks (20, 21). This study, therefore, confirmed that colonization stimulates production of specific antipneumococcal antibodies but raised further questions about whether or not antibodies induced during carriage contributed to clearance of bacteria from the nasopharynx.
The human model is limited in the number of individuals that can be studied and does not allow for manipulation of the immune system. In order to determine the role of humoral immunity in the clearance of colonization, we established a mouse model that uses the same pneumococcal strain as is employed in experimental human carriage studies and which resembles the human model in many respects. Mice which demonstrated asymptomatic nasopharyngeal carriage of this strain developed a pneumococcal-specific antibody response and cleared colonization in a time frame similar to that seen in humans. Our findings suggest, however, that specific antibodies induced by colonization as well as mature B-cell function are not a requirement to clear colonization from the murine nasopharynx.
MATERIALS AND METHODS
Mice.
All mice were purchased pathogen free from Jackson Laboratories and were housed in accordance with Institutional Animal Care and Use Committee protocols. Six- to 8-week-old mice of three different genetic backgrounds (CBA/J, C57BL/6, and BALB/c) that exhibit various abilities to generate type-specific antibodies were used (23). CBA/CaHN-Btkxid/J (CBA/N), B6.129S2-Igh-6tm1Cgn/J (μMT), and B6.CB17-Prkdcscid/SzJ (SCID) mice were used to examine the impact of specific components of immunity on clearance of colonization. CBA/N mice respond poorly to T-cell-independent type 2 (TI-2) antigens such as pneumococcal polysaccharides (PnPS) due to an X-linked mutation in Burton's tyrosine kinase (1, 26, 30). μMT mice contain a targeted mutation in the heavy chain locus of C57BL/6 IgM and do not produce mature B cells or antibody (15). B6.B17-Prkdcscid/SzJ (SCID) mice in the C57BL/6 background contain a spontaneous mutation in a gene encoding the catalytic subunit of DNA-activated protein kinase, resulting in the absence of B and T cells (4). Mice were used in groups of at least five per time point for a given experimental condition.
Pneumococcal strains.
A pneumococcal type that is among the most common in human carriage and known to be relatively avirulent in mice was used in all studies (2, 24). Strain P1121, isolated from the nasopharynx of a subject enrolled in the human experimental carriage study, is a derivative of P833, the minimally passaged type 23F isolate originally obtained from a child with otitis media (20). Strain P1121 was chosen for this study because, unlike its parent, which expresses a secreted, truncated form of pneumococcal surface protein A (PspA), P1121 expresses a full-length, surface-attached PspA. P1121 also has a mixed phenotype of opaque and transparent colonies like most clinical isolates (20, 34). In addition, a spontaneously arising streptomycin-resistant mutant of P1121 (P1121strr) that had been passed once through the murine nasopharynx was used in recolonization experiments to distinguish the primary inoculum from a subsequent rechallenge.
Pneumococcal colonization.
P1121 was grown in C+Y medium (pH 6.8) until an optical density at 620 nm of 0.45 was reached. After centrifugation, the pellet was resuspended in sterile phosphate-buffered saline (PBS) to a density of 109 CFU/ml. Ten microliters (107 CFU) of the pneumococcal suspension was instilled into the nares of each mouse. After a predetermined number of days, mice were sacrificed by CO2 asphyxiation, the trachea was exposed, 200 μl of sterile PBS was instilled into it, and the lavage fluid exiting the nares was collected. The lavage fluid was then serially diluted in PBS and plated on tryptic soy plates containing catalase (4,741 U/plate) (Worthington, Lakewood, N.J.) and neomycin (20 μg/ml) to prevent the growth of contaminants. Encapsulated pneumococci were identified by their colony morphology and confirmed by quelling in the presence of type 23F antiserum (Statens Seruminstitut, Copenhagen, Denmark). Following overnight incubation at 37°C in a 5% CO2 atmosphere, colony counts were determined in duplicate with a lower limit of detection of 100 CFU/ml of lavage fluid. Blood and homogenates of excised lungs and palate were also obtained at the time of sacrifice, and the density of pneumococci in these samples was determined in cultures by plating serial dilutions for colony counting. The majority of pneumococci were consistently found in cultures of lavage fluid rather than of homogenized upper respiratory tract tissues. In addition, two anterior cervical lymph nodes and nasal-associated lymph tissue (NALT) were harvested from each mouse and incubated in Kennett's HY medium (Gibco, Carlsbad, Calif.) at 37°C and 5% CO2 for 7 days. The supernatant from the culture of tissue fragments was harvested and stored at −20°C for use in antibody studies.
In the recolonization studies, mice were initially colonized with P1121 or sham colonized with PBS. Six weeks later, the mice were challenged intranasally with doses of 107 CFU/mouse of either P1121strr or PBS. Seven days after the second inoculation, the mice were sacrificed and the density of colonization was determined as described above, using tryptic soy plates with catalase, neomycin (20 μg/ml), and streptomycin (1.0 mg/ml).
Immunization.
BALB/c mice were given an intraperitoneal immunization with a 5-μg/mouse dose of either PspA (amino acids 31 to 189 corresponding to the N-terminal 158 residues of the mature secreted protein) or PBS, both in aluminum hydroxide adjuvant (Sigma, St. Louis, Mo.). The strain-specific PspA was expressed as described previously for an Escherichia coli expression system (Novagen) (21). This PspA fragment was shown previously to be immunogenic during human colonization. Three immunizations were performed, each 2 weeks apart. Two weeks after the last immunization, the mice were challenged intranasally with P1121 (107 CFU/mouse) and sacrificed 2 weeks later. The density of colonization was determined as described above.
ELISA.
Purified strain-specific PspA was used to coat 96-well Immulon 2 high-binding plates (DYNEX Technologies) at 0.5 μg/ml in coating buffer (0.015 M Na2CO3, 0.035 M NaHCO3 [pH 9.6]). Whole P1121 was also used as the solid-phase antigen to determine the antibody titer to the pneumococcus. P1121 was streaked onto tryptic soy plates containing catalase and incubated overnight at 37°C in an atmosphere of 5% CO2. Colonies of P1121 were removed and adjusted in PBS to an optical density at 620 nm of 1.0. This solution was then diluted 1:10 in coating buffer, and after overnight incubation at 4°C, the plates were washed with PBS-Brij, blocked with 1% bovine serum albumin (Sigma) for 1 h at 37°C, and washed again. Threefold serial dilutions of sample (serum, lavage fluid, or culture supernatant) in 1% bovine serum albumin were added and then incubated overnight at 4°C. Antigen-specific antibodies were detected by goat anti-mouse immunoglobulin (heavy plus light chains)-alkaline phosphatase (Southern Biotech, Birmingham, Ala.), developed with pNPP (Sigma), and the absorbance at 415 nm was recorded after a standardized period of 30 min. Titers were determined by calculating the sample dilution at which the absorbance was equal to 0.3. Goat anti-mouse IgG-, goat anti-mouse IgA-, and goat anti-mouse IgM-alkaline phosphatase (Southern Biotech) were used to determine isotypes of specific antibodies. Antibody to the type 23F capsular polysaccharides was detected by an ELISA protocol established by the Centers for Disease Control and Prevention (7).
Statistics.
Differences in levels of colonization between different groups of mice or over time were analyzed by using an unpaired t test (two tailed). In order to determine if a correlation between antibody titer and levels of colonization existed, the correlation coefficient (r2) was determined and a t test for correlation performed. Statistical significance was defined as a P value of ≤0.05.
RESULTS
Similarities between models of pneumococcal colonization in mice and humans.
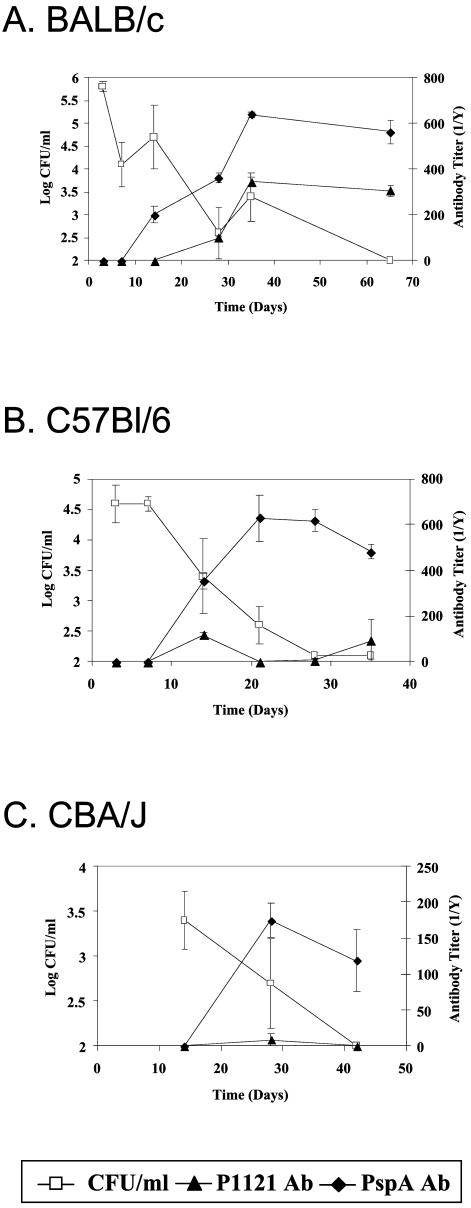
A minimally passaged type 23F pneumococcus (P1121) obtained from the nasopharynx of a subject in an experimental study of pneumococcal carriage in humans was used to inoculate mice intranasally (20). Three different inbred mouse strains (BALB/c, C57BL/6, and CBA/J) were used to study the dynamics of colonization with strain P1121. Colonization was assessed by plating serial dilutions of lavage fluid of the murine upper respiratory tract for colony counting (Fig. 1). BALB/c mice were colonized with a mean density of 7.1 × 105 CFU/ml at day 3 postinoculation and by day 65 no longer had pneumococci detected (Fig. 1A). C57BL/6 mice were colonized at a mean density of 6.6 × 104 CFU/ml at 3 days postinoculation and had no pneumococci detected by day 28 (Fig. 1B). CBA/J mice were colonized with a mean density of 2.5 × 103 CFU/ml at day 14 postinoculation and had no detectable P1121 by day 42 (Fig. 1C). No signs of pneumococcal infection were observed, and P1121 was not found in cultures of homogenized lungs or of blood. The resolution of pneumococcal carriage between days 28 and 65 postinoculation, as well as the lack of evidence of pneumococcal disease, resembles experimental pneumococcal carriage in humans in which a derivative of P1121 was cleared between days 27 and 122 (21). The similarity between this murine model and the experimental human model suggests that examination of host factors that impact colonization in mice may be relevant to naturally acquired carriage in humans.
FIG. 1.
Levels of pneumococcal colonization in comparison with serum antibody titer over time. The density of pneumococcal colonization, expressed as log CFU per milliliter of upper respiratory tract lavage fluid, was determined for BALB/c (A), C57BL/6 (B), and CBA/J (C) mice at the postinoculation day indicated. Whole bacteria (P1121) and strain-specific PspA total immunoglobulin titers were determined by ELISA. Values shown are the mean CFU per milliliter or titers ± standard errors of the means.
Characteristics of the antibody response in murine colonization.
In order to assess the contribution of antibody to the clearance of carriage, serum antibody titers were compared to the levels of colonization with strain P1121 over time. All three mouse strains demonstrated an increase in total serum immunoglobulins against P1121, measured by a whole bacterial ELISA (Fig. 1). In addition, there was a significant increase in the amount of strain-specific PspA immunoglobulins induced by colonization of BALB/c mice (Fig. 1A). No antibodies specific for type 23F polysaccharide were detected in any of the mice. Like observations during experimental human colonization, levels of specific serum antibody to PspA plateaued by days 21 to 28 postinoculation (20). For the BALB/c mice, which had the highest density and longest duration of colonization, there was a statistically significant correlation (t test for correlation, P ≤ 0.01) between the rise in P1121-specific antibody and the decline in numbers of pneumococci over time.
The antibody response to carriage does not correlate with the level of colonization in individual mice.
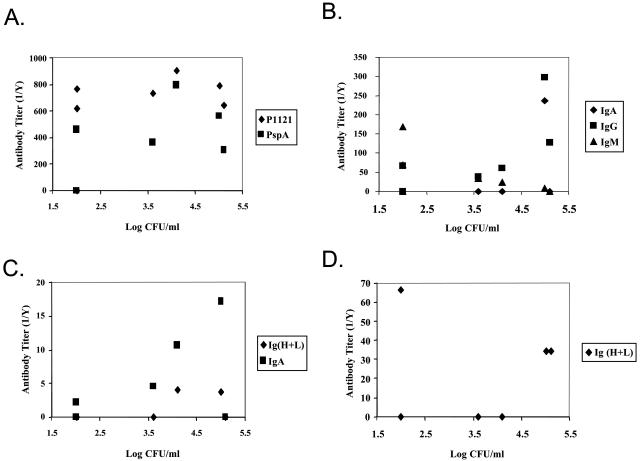
The results above suggest that the antibodies induced by colonization may be responsible for clearance of pneumococci from the murine nasopharynx. This hypothesis was examined in more detail by the comparison of antibody titers and levels of colonization in individual mice. For BALB/c and C57BL/6 mice at the time point where the density of colonization had declined significantly from the initial time point, days 35 and 21, respectively, there was no statistically significant correlation between the amounts or isotypes of antibodies and the density of pneumococci (shown for BALB/c mice in Fig. 2). In particular, no correlation was seen with total serum immunoglobulins to whole-P1121 pneumococci or type-specific PspA (Fig. 2A); P1121-specific serum IgA, IgG, or IgM (Fig. 2B); mucosal P1121-specific total immununoglobulins in lavage fluids (Fig. 2C); and P1121-specific total immunoglobulins and IgA produced locally in fragment cultures of NALT and lymph nodes (Fig. 2D). Moreover, at no time point during colonization was there a correlation between the density of P1121 in lavage fluid and the titers of P1121-specific total immunoglobulins in serum for individual animals. Thus, despite what was observed on a population level, the lack of correlation in individual animals brings into question the overall impact of antibodies in the resolution of pneumococcal colonization.
FIG. 2.
Level of colonization as a function of antibody titer in individual BALB/c mice. The density of pneumococcal colonization, expressed as log CFU per milliliter of upper respiratory tract lavage fluid, was determined for BALB/c mice at day 35 postinoculation. Total whole bacteria (P1121) and strain-specific PspA serum immunoglobulin titers (A), P1121-specific serum IgA, IgG, and IgM titers (B), P1121-specific immunoglobulin titers in upper respiratory tract lavage fluids (C), and P1121-specific IgA titers in NALT and anterior cervical lymph node culture supernatants (D) were determined by ELISA. Values shown are the mean titers from three independent determinations and log CFU per milliliter for seven individual BALB/c mice.
Colonization can be cleared without antibody to TI-2 antigens.
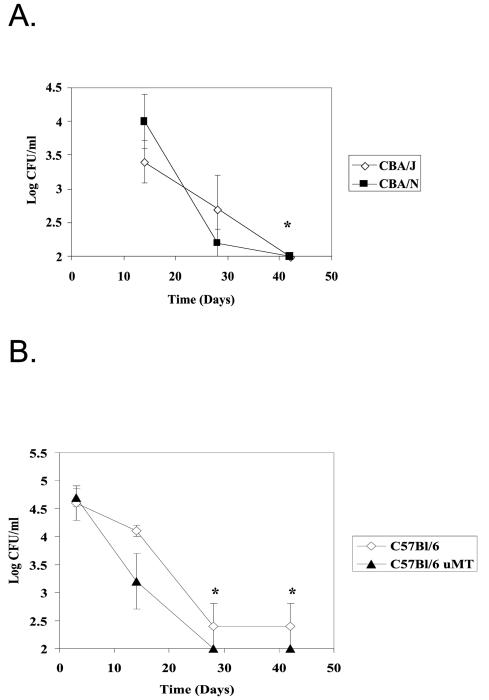
Previous studies suggested that antibody to PnPS might play a role in the duration of carriage. The role of the antibody response to T-independent antigens in the clearance of pneumococcal carriage was tested in mice that respond poorly to such antigens. By using CBA/N mice that carry the xid mutation, and comparing their colonization rates with those of CBA/J mice, we were able to observe the importance of the ability to generate antibody that recognizes PnPS and phosphorylcholine on the surface of the pneumococcus to the resolution of colonization. Over time, CBA/N mice are able to clear pneumococcal colonization and do so at the same rate as the CBA/J mice (Fig. 3A).
FIG. 3.
Comparison of the density of colonization in CBA/N (A) and μMT (B) mice over time with their respective immunocompetent parent strains. Values shown are the mean log CFU per milliliter ± standard errors of the means for groups of five mice each. The asterisks indicates a significant decrease in the density of colonization compared to the first time point at which colonization was determined (unpaired two-tailed t test, P ≤ 0.05). No significant differences in the densities of colonization were observed between CBA/N and CBA/J or μMT and C57BL/6 mice.
Colonization does not induce strain-specific protection from reacquisition.
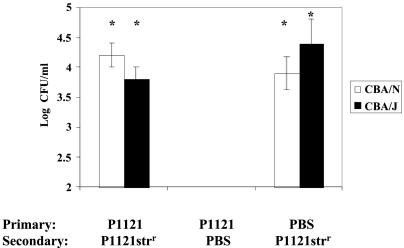
In order to study the impact of immune response on subsequent carriage, CBA/J and CBA/N mice were inoculated with P1121, allowed to clear colonization, and subsequently rechallenged with the streptomycin-resistant mutant of the same strain to distinguish it from the initial inoculum. Despite the induction of whole P1121-specific immunoglobulin, mice initially colonized and subsequently exposed to P1121strr demonstrated no significant difference in colonization density from mice not initially colonized (Fig. 4). These data suggest that although colonization can induce the production of specific antibodies, the immune response to carriage is not sufficient to preclude subsequent colonization even with the same strain. Additionally, the ability to produce antibodies to TI-2 antigens did not impact recolonization.
FIG. 4.
Effect of prior colonization on subsequent challenge with the same strain. CBA/N or CBA/J were initially inoculated with P1121 or PBS and subsequently challenged with P1121strr or PBS 6 weeks later. Values shown are the mean log CFU per milliliter ± standard errors of the means for five mice per group. The asterisk indicates a P of ≤0.05 compared to second challenge with PBS (unpaired two-tailed t test).
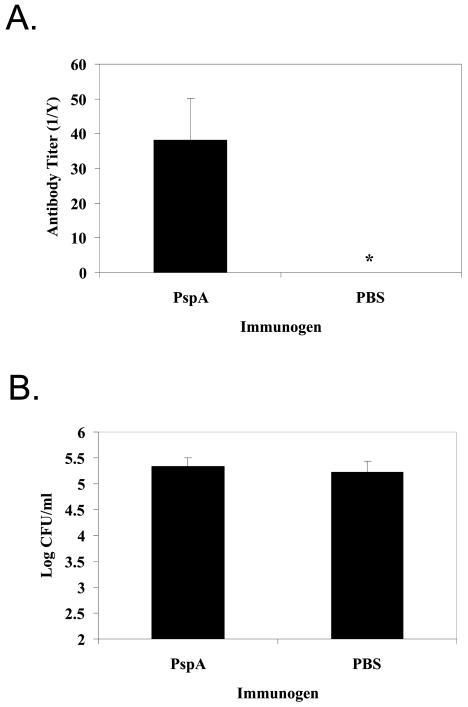
Immunization with strain-specific PspA does not reduce colonization.
To examine further the impact of specific antibody on the reduction of colonization, BALB/c mice were injected with either strain-specific PspA or PBS. Antibody to this immunogenic portion of PspA was associated with decreased susceptibility to acquisition of carriage in the human model, and antibody to PspA induced by mucosal immunization was previously reported to reduce murine colonization (6, 20, 21). After three systemic inoculations of strain-specific PspA in adjuvant, a significant rise in specific serum immunoglobulin titer was observed (data not shown). Immunized and sham-immunized mice were then challenged, and the density of colonization was determined 2 weeks later (Fig. 5B). Mice that received strain-specific PspA demonstrated statistically significant higher levels of strain-specific PspA IgA in their lavage fluids compared to mice that were injected with adjuvant alone (unpaired two-tailed t test, P ≤ 0.05) (Fig. 5A). Despite the presence of these amounts of serum and mucosal strain-specific PspA antibodies, no difference in the density of colonization was observed.
FIG. 5.
Effect of immunization on the density of colonization. BALB/c mice were immunized with either strain-specific PspA or sham immunized with adjuvant alone. PspA-specific antibody titers were determined by ELISA (A). Values shown are mean total antibody titers ± standard errors of the means for upper respiratory tract lavage fluids. The asterisks indicates a P of ≤0.05 compared to control (unpaired two-tailed t test). The density of colonization (B) was determined by plating serial dilutions of upper respiratory tract lavage fluids for colony counting. Values shown are the mean log CFU per milliliter ± standard errors of the means.
Mature B cells and antibody are not required to clear pneumococcal colonization.
In order to examine the overall role of antibody on clearance of pneumococcal colonization, levels of colonization were compared in genetically modified mice that lack mature B cells (μMT) and their parent strain (C57BL/6). Despite the presence of pneumococcal-specific antibody in the C57BL/6 parents and its total absence from μMT mice, the density of colonization in μMT mice was not significantly different from that of C57BL/6 mice through the duration of carriage (Fig. 3B). Both C57BL/6 and μMT mice showed a significant decline in the density of colonization by day 28 (unpaired two-tailed t test, P ≤ 0.05). In contrast, SCID mice remained persistently colonized over this period, suggesting that some component of the adaptive immune response other than B cells is important in the clearance of colonization (data not shown). P1121 was not detected in the lungs or blood of the immunocompromised μMT mice, which lack mature B cells, nor were any symptoms of invasive pneumococcal disease observed. These data confirm that neither antibody nor mature B cells are required for clearance of pneumococcal carriage in mice.
DISCUSSION
Microbial colonization of a mucosal surface is a result of the interplay between bacterial attributes facilitating persistence and host mechanisms promoting clearance. The focus of this study was on host factors that may impact on pneumococcal colonization. Our investigation took advantage of two previously described experimental systems, human and mouse carriage. Despite the limited host range of the pneumococcus, a murine model to study colonization of its niche in the upper respiratory tract has been described previously (35). It was unclear, however, how closely this animal model recapitulates its carriage by humans. Based on recent experience with experimental human carriage, it appears that many aspects of this murine model are in fact similar, including the minimum colonizing dose (<104 CFU) and an average duration of colonization of several weeks (10, 20, 35). This similarity allowed us to utilize the mouse model, a system more amenable to experimental manipulation than that in humans, with some degree of confidence in its biological relevance. To maximize the relationship between models, our approach employed a pneumococcal strain derived from a recent experimental human carriage study (21).
Colonization was shown to be a self-limited event in mice, as was previously demonstrated in humans. Initially, this finding seemed to be explained by the induction of specific antibody during the course of colonization. This association was valid, however, only when titers of specific antibody were related to the dynamics of colonization for a population. Our conclusion that antibody does not promote clearance is based on the following findings. (i) Among three lines of inbred animals, BALB/c mice that had the highest density and duration of colonization also generated the highest titers of serum antibody to whole bacteria as well as to the immunogenic surface protein, PspA. (ii) There was no correlation in individual mice between amounts of serum antibody of different isotypes or of antigenic specificities and the density of colonization. (iii) For individual animals, the titers of detectable mucosal antibody in washes of the upper respiratory tract or cultures of NALT paralleled those found in serum and, likewise, had no correlation with the density of colonizing bacteria. (iv) Immunodeficient mice that respond poorly to type 2 T-independent antigens (CBA/N) or completely lacking in antibody production and mature B-cell function (μMT) were able to clear colonization as effectively as their immunocompetent counterparts.
Studies of pneumococcal immunization suggest that specific immune responses against certain antigens are associated with a marked decrease in the duration of carriage and its prevalence in a population (6, 9). Such an effect on carriage is observed following the vigorous IgG response to systemically administered PnPSs conjugated to protein carriers. In contrast to the experience with vaccination, our observations in this report suggest that the humoral immune response to colonization does not significantly affect carriage in mice. It should be noted, however, that amounts of antibody generated to the type 23F PnPS during murine colonization were minimal and that among the multiple PnPS types, the 23F capsular polysaccharide may be poorly immunogenic (12, 22, 23). This lack of response to the capsular polysaccharide surface antigen of a colonizing strain correlates with the experience in both experimental and naturally acquired carriage in humans and could underlie the difference between the effects of vaccination and carriage (14, 20). Our results, therefore, suggest that carriage may not be a protective immunizing event. The susceptibility of CBA/J mice to recolonization with a streptomycin-resistant mutant of P1121 following clearance of the initial inoculum (P1121) is supportive of this conclusion. In fact, for humans there is no direct evidence that antibody induced by natural carriage is a factor influencing the dynamics of colonization.
Unlike the insignificant increase in antibody to the homotypic PnPS, there was a brisk rise in titer to the strain-specific N-terminal portion of PspA in colonized mice, although this response did not appear to affect carriage. This observation confirmed that murine pneumococcal colonization induces specific antibody as previously suggested for the natural host (27, 28). A similar rise in serum and mucosal antibody to this surface antigen was seen during experimental human colonization (20, 21). In the human study, a higher preinoculation titer of this anti-PspA antibody was associated with a reduced susceptibility to becoming colonized following intranasal challenge with an estimated 50% colonizing inoculum (20). In BALB/c mice, however, no detectable effect on subsequent colonization was induced by systemic immunization with this fragment, which generated serum and mucosal antibody. We cannot exclude the possibility that the amount or type of murine anti-PspA antibody may not be as effective as that found in the natural host.
Our findings indicate that host factors other than antibody are responsible for the gradual, steady decline in the density of colonizing pneumococci. SCID mice, which lack adaptive immunity, were persistently colonized for as long as 6 weeks postinoculation. Although SCID mice remained colonized, they still demonstrated a decline in the density of colonization over time. Together with the inability of immunocompetent CBA/J mice to prevent reacquisition of a strain previously cleared; this suggests that innate immunity may be the main contributor to the resolution of carriage. In this regard, we observed that unrelated strains of inbred mice all gradually resolved the carrier state but showed differences of ∼100-fold in the initial density of colonization following challenge with the same strain. Differences in levels of tumor necrosis factor alpha and gamma interferon expressed by different mouse strains, for example, have been previously demonstrated to be responsible for the altered resistance to a variety of bacterial infections (3, 25).
Finally, we cannot exclude an active role of the organism in the ineffectiveness of antibody generated during carriage. For instance, the expression of a capsule, a characteristic required for colonization by the pneumococcus, protects the organism from the opsonic enhancement of immunoglobulin (17). In addition, as one of the many mucosal pathogens that expresses an IgA1 protease capable of cleaving human IgA1, the pneumococcus may specifically inactivate a major portion of mucosal immunoglobulin (31, 33). Although this enzyme would not be relevant to murine immunoglobulins, there might be other examples of pneumococcal mechanisms contributing to evasion of the antimicrobial effects of the antibody response during carriage.
Acknowledgments
We thank R. Austrian for critical review.
This work was supported by grants from the U.S. Public Health Service to T.L.M. (AI51115), J.N.W. (AI44231 and AI38446), and the Bacterial Respiratory Pathogens Research Unit, University of Iowa (AI30040).
Editor: F. C. Fang
REFERENCES
- 1.Amsbaugh, D., C. Hansen, B. Prescott, P. Stashak, D. Barthold, and P. Baker. 1972. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J. Exp. Med. 136:931-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austrian, R. 1986. Some aspects of the pneumococcal carrier state. J. Antimicrob. Chemother. 18:35-45. [DOI] [PubMed] [Google Scholar]
- 3.Autenrieth, I., M. Beer, E. Bohn, S. Kaufmann, and J. Heesemann. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect. Immun. 62:2590-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blunt, T., N. Finnie, G. Taccioli, G. Smith, J. Demengeot, T. Gottlieb, R. Mizuta, A. Varghese, F. Alt, P. Jeggo, and S. Jackson. 1995. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell 80:813-823. [DOI] [PubMed] [Google Scholar]
- 5.Bogaert, D., R. de Groot, and P. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144-154. [DOI] [PubMed] [Google Scholar]
- 6.Briles, D., E. Ades, J. Paton, J. Sampson, G. Carlone, R. Huebner, E. Virolainen, E. Swiatlo, and S. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Concepcion, N., and C. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagan, R., N. Givon-Lavi, O. Zamir, M. Sikuler-Cohen, L. Guy, J. Janco, P. Yagupsky, and D. Fraser. 2002. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J. Infect. Dis. 185:927-936. [DOI] [PubMed] [Google Scholar]
- 9.Dagan, R., M. Muallem, R. Melamed, O. Leroy, and P. Yagupsky. 1997. Reduction of pneumococcal nasopharyngeal carriage in early infancy after immunization with tetravalent pneumococcal vaccines conjugated to either tetanus toxoid or diphtheria toxoid. Pediatr. Infect. Dis. J. 16:1060-1064. [DOI] [PubMed] [Google Scholar]
- 10.Ekdahl, K., I. Ahlinder, H. Hansson, E. Melander, S. Molstad, M. Soderstrom, and K. Persson. 1997. Duration of nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae: experiences from the South Swedish Pneumococcal Intervention Project. Clin. Infect. Dis. 25:1113-1117. [DOI] [PubMed] [Google Scholar]
- 11.Ghaffar, F., I. Friedland, and G. McCracken, Jr. 1999. Dynamics of nasopharyngeal colonization by Streptococcus pneumoniae. Pediatr. Infect. Dis. J. 18:638-646. [DOI] [PubMed] [Google Scholar]
- 12.Gray, B., G. Converse III, and H. Dillon, Jr. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923-933. [DOI] [PubMed] [Google Scholar]
- 13.Gundel, M. 1933. Bakteriologische und Epidemiologische Untersuchungen über die Besiedlung der oberen Atmungswege Gesunder mit Pneumokokken. Z. Hyg. Infektionskr. 114:659-704. [Google Scholar]
- 14.Gwaltney, J. J., M. Sande, R. Austrian, and J. Hendley. 1975. Spread of Streptococcus pneumoniae in families. II. Relation of transfer of S. pneumoniae to incidence of colds and serum antibody. J. Infect. Dis. 132:62-68. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 16.MacLeod, C. M., R. G. Hodges, M. Heidelberger, and W. G. Bernhard. 1945. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J. Exp. Med. 82:445-465. [PMC free article] [PubMed] [Google Scholar]
- 17.Magee, A., and J. Yother. 2001. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect. Immun. 69:3755-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malley, R., M. Lipsitch, A. Stack, R. Saladino, G. Fleisher, S. Pelton, C. Thompson, D. Briles, and P. Anderson. 2001. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect. Immun. 69:4870-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mbelle, N., R. Huebner, A. Wasas, A. Kimura, I. Chang, and K. Klugman. 1999. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J. Infect. Dis. 180:1171-1176. [DOI] [PubMed] [Google Scholar]
- 20.McCool, T., T. Cate, G. Moy, and J. Weiser. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCool, T., T. Cate, E. Tuomanen, P. Adrian, T. Mitchell, and J. Weiser. 2003. The serum IgG response to candidate vaccine antigens during experimental human pneumococcal colonization. Infect. Immun. 71:5724-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCool, T., C. Harding, N. Greenspan, and J. Schreiber. 1999. B- and T-cell immune responses to pneumococcal conjugate vaccines: divergence between carrier- and polysaccharide-specific immunogenicity. Infect. Immun. 67:4862-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCool, T., J. Schreiber, and N. Greenspan. 2003. Genetic variation influences the B-cell response to immunization with a pneumococcal polysaccharide conjugate vaccine. Infect. Immun. 71:5402-5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morch, E. 1943. Virulence of the pneumococcus types for mice, p. 157-163. In F. A. Richard (ed.), Serological studies on the pneumococci. Einar Munksgaard, Copenhagen, Denmark.
- 25.O'Brien, D., D. Briles, A. Szalai, A. Tu, I. Sanz, and M. Nahm. 1999. Tumor necrosis factor alpha receptor I is important for survival from Streptococcus pneumoniae infections. Infect. Immun. 67:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawlings, D., D. Saffran, S. Tsukada, D. Largaespada, J. Grimaldi, L. Cohen, R. Mohr, J. Bazan, M. Howard, N. Copeland, N. Jenkins, and O. Witte. 1993. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science 261:358-361. [DOI] [PubMed] [Google Scholar]
- 27.Simell, B., T. Kilpi, and H. Kayhty. 2002. Pneumococcal carriage and otitis media induce salivary antibodies to pneumococcal capsular polysaccharides in children. J. Infect. Dis. 186:1106-1114. [DOI] [PubMed] [Google Scholar]
- 28.Simell, B., M. Korkeila, H. Pursiainen, T. Kilpi, and H. Kayhty. 2001. Pneumococcal carriage and otitis media induce salivary antibodies to pneumococcal surface adhesin A, pneumolysin, and pneumococcal surface protein A in children. J. Infect. Dis. 183:887-896. [DOI] [PubMed] [Google Scholar]
- 29.Syrjanen, R., T. Kilpi, T. Kaijalainen, E. Herva, and A. Takala. 2001. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J. Infect. Dis. 184:451-459. [DOI] [PubMed] [Google Scholar]
- 30.Thomas, J., P. Sideras, C. Smith, I. Vorechovsky, V. Chapman, and W. Paul. 1993. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science 261:355. [DOI] [PubMed] [Google Scholar]
- 31.Wani, J., J. Gilbert, A. Plaut, and J. Weiser. 1996. Identification, cloning and sequencing of the immunoglobulin A1 protease gene of Streptococcus pneumoniae. Infect. Immun. 64:3967-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiser, J. 2004. Mechanisms of carriage, p. 169-182. In E. Tuomanen (ed.), The Pneumococcus. ASM Press, Washington, D.C.
- 33.Weiser, J., D. Bae, C. Fasching, R. Scamurra, A. Ratner, and E. Janoff. 2003. Antibody-enhanced pneumococcal adherence requires IgA1 protease. Proc. Natl. Acad. Sci. USA 100:415-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiser, J. N., R. Austrian, P. K. Sreenivasan, and H. R. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu, H., A. Virolainen, B. Mathews, J. King, M. Russell, and D. Briles. 1997. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb. Pathog. 23:127-137. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, Q., K. Arnaoutakis, C. Murdoch, R. Lakshman, G. Race, R. Burkinshaw, and A. Finn. 2004. Mucosal immune responses to capsular pneumococcal polysaccharides in immunized preschool children and controls with similar nasal pneumococcal colonization rates. Pediatr. Infect. Dis. J. 23:307-313. [DOI] [PubMed] [Google Scholar]