Abstract
The Lyme disease spirochete, Borrelia burgdorferi, causes persistent mammalian infection despite the development of vigorous immune responses against the pathogen. To examine spirochetal phenotypes that dominate in the hostile immune environment, the mRNA transcripts of four prototypic surface lipoproteins, decorin-binding protein A (DbpA), outer surface protein C (OspC), BBF01, and VlsE, were analyzed by quantitative reverse transcription-PCR under various immune conditions. We demonstrate that B. burgdorferi changes its surface antigenic expression in response to immune attack. dbpA expression was unchanged while the spirochetes decreased ospC expression by 446 times and increased BBF01 and vlsE expression up to 20 and 32 times, respectively, under the influence of immune pressure generated in immunocompetent mice during infection. This change in antigenic expression could be induced by passively immunizing infected severe combined immunodeficiency mice with specific Borrelia antisera or OspC antibody and appears to allow B. burgdorferi to resist immune attack.
Lyme disease, caused by Borrelia burgdorferi, is a complex multisystem disorder that can result in arthritis, neurological abnormalities, carditis, and cutaneous lesions such as erythema migrans and acrodermatitis chronica atrophicans (48). B. burgdorferi infection in humans and mice may persist despite the development of vigorous immune responses against the pathogen (47). B. burgdorferi has approximately 150 lipoprotein genes (8, 16), and some may be anchored to the spirochetal outer membrane to form an antigenic layer that protects the bacterium from, and directly interacts with, the environment (19). Surface-exposed lipoproteins may render the spirochetes susceptible to specific lipoprotein antibodies in vitro, in the arthropod vector, and in the mammalian host (11, 22, 32, 37, 43, 45). While lipoproteins may stimulate innate responses via Toll-like receptors 1 and 2, enhancing both humoral and cellular immune responses to B. burgdorferi (2, 52), little is known about how this extracellular bacterium survives in the hostile immune environment during mammalian infection.
B. burgdorferi adapts to diverse environments in the tick and mammal during its life cycle, in part by selective gene expression. Environmental cues such as temperature (42, 49), pH (7), nutrients or chemicals (3, 53), and others (5, 39, 44) influence B. burgdorferi gene expression in vitro, and the cultivation of B. burgdorferi in dialysis membrane chambers implanted into rat peritoneal cavities up-regulates several spirochetal genes (1). The most dramatic modifications that occur as B. burgdorferi migrates from ticks to a mammal involve lipoprotein gene expression, such as the down-regulation of outer surface protein A (OspA) and the up-regulation of OspC that occurs during tick feeding (38, 46). These changes occur among populations of spirochetes; however, individual spirochetes that express OspA, OspC, both, or neither may be detected (38). Investigating how changes in surface antigenic expression of B. burgdorferi contribute to its resistance to immune attack during persistent mammalian infection will provide insights into the pathogenesis of Lyme disease.
B. burgdorferi exhibits tissue-specific gene expression during mammalian infection (36). It persistently expresses ospC during infection of immunodeficient mammals (27, 29). The development of OspC antibody, however, preferentially selects for spirochetes that do not abundantly express OspC in immunocompetent mice. It is likely that antibodies to many lipoprotein antigens can place an immune selection pressure on spirochetes that express these antigens (29). We now examine spirochetal phenotypes that are selected for under the influence of host responses by quantitatively analyzing the mRNA transcripts of four prototypic surface-exposed lipoproteins, decorin-binding protein A (DbpA) (18, 20), OspC (33, 51), BBF01 (13, 15), and VlsE (Vmp-like sequence, expressed) (25, 54), during murine infection.
MATERIALS AND METHODS
Spirochete and mouse strains.
B. burgdorferi B31 clone 5A11 (a gift from Steven Norris, University of Texas, Houston) was cultivated in Barbour-Stoenner-Kelly H complete medium at 33°C (Sigma Chemical Co., St. Louis, Mo.). BALB/c wild-type and BALB/c background severe combined immunodeficiency (SCID) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). B-cell-deficient mice on a BALB/c background were generated as previously described (9). All mice were 4 to 8 weeks old when they were infected.
Mouse inoculation and passive immunization.
Ten wild-type, 10 B-cell-deficient, and 70 SCID mice were given one single intradermal injection of 105 cultured spirochetes. All of the infected wild-type and B-cell-deficient mice and 30 of the infected SCID mice were sacrificed at 12 days to 4 months postinfection. The rest of the SCID mice were used for passive immunization experiments. Heart, joint, and skin tissues (not from the inoculation site) were harvested and immediately frozen in liquid nitrogen. Frozen samples were stored at −70°C until DNA and RNA were isolated.
OspC monoclonal antibody preparation and passive immunization.
The hybridoma cell line B5 was generated as described previously (34). Secreted OspC monoclonal antibody isotyped as immunoglobulin G2a (IgG2a) is able to protect mice against a tick-transmitted infection with B. burgdorferi B31 and to reduce ospC expression in infected SCID mice. The antibody was purified from mouse ascites fluid with use of a protein G column (Pierce Chemical Company, Rockford, Ill.). Purity and concentration were assessed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the Bio-Rad protein assay kit (Bio-Rad Laboratories, Richmond, Calif.). Ten SCID mice were infected for 4 months as described above, and each subcutaneously received 24 μg of OspC monoclonal antibody every 2 days for three doses. Another 10 mice were each given three 24-μg doses of purified healthy mouse IgG2a (Sigma) as a control. All animals were sacrificed 3 days later after the last passive immunization. Heart, joint, and skin tissues were collected and stored as described above.
Anti-Borrelia serum preparation and passive immunization.
To prepare anti-Borrelia sera, BALB/c mice were infected with cultured B31 5A11 spirochetes as described above. Blood was drawn between 2 and 4 months postinfection, and sera were isolated, pooled, and stored at −20°C. To prepare prebled sera, blood was collected from uninfected BALB/c mice and sera were isolated, pooled, and stored. Ten SCID mice were infected for 4 months as described above, and each subcutaneously received 100 μl of anti-Borrelia sera every 2 days for six doses. Another 10 infected mice were each given six 100-μl doses of prebled murine sera as a control. All animals were sacrificed 3 days later after the last passive immunization. Heart, joint, and skin tissues were collected and stored as described above.
RNA and DNA preparation.
Frozen heart, joint, and skin samples were transferred in liquid nitrogen and ground thoroughly with a mortar and pestle. An appropriate amount of tissue powder was transferred into a 500-μl polypropylene tube for DNA preparation with use of the DNeasy Mini kit according to the manufacturer's instructions (Qiagen Inc., Valencia, Calif.). RNA was isolated from the remaining powder with use of Trizol reagent (Invitrogen Life Technologies, Carlsbad, Calif.). To ensure that there was no DNA contamination, RNA preparations were first digested in solution with RNase-free DNase I (Life Technologies, Inc., Gaithersburg, Md.) at 37°C for 2 h and then loaded onto the RNeasy Mini columns and further treated with RNase-free DNase I (Qiagen) for an additional 20 min at room temperature. Doubly digested samples were repurified and analyzed for potential DNA contamination by PCR amplification of the flaB gene.
cDNA preparation.
The DNA-free RNA preparation was first annealed with the reverse oligonucleotide primer mixture of flaB (5′-ATTCCAAGCTCTTCAGCTG-3′), dbpA (5′-CATTGCTGAAAATTCACCAC-3′), ospC (5′-CAGCATCAGTAACACCTTC-3′), BBF01 (5′-CCCTTGAGTAAGGAAACTAC-3), and vlsE (5′-CGTCGTACTACTTATATCGC-3′) genes at 65, 60, 55, 50, and 45°C each for 1 min, in the presence of reverse transcription (RT) buffer (Invitrogen). Deoxynucleoside triphosphates and SuperScript II RNase H− reverse transcriptase (Invitrogen) were added, RT was conducted at 42°C for 1 h, and then the reaction mixture was inactivated at 95°C for 5 min according to the manufacturer's instructions.
Quantitative PCR (qPCR) DNA concentration standards.
flaB, dbpA, ospC, and BBF01 DNA concentration standards were prepared from cultured B. burgdorferi. B31 5A11 spirochetes were grown to stationary phase in Barbour-Stoenner-Kelly H complete medium, counted in a Petroff-Hausser counter (Hausser Scientific Partnership, Horsham, Pa.), and harvested by centrifugation at 15,000 × g for 10 min. Resultant pellets were digested with proteinase K (Qiagen) at 55°C for 2 h, inactivated at 95°C for 10 min, and diluted at 100 to 105 spirochetal DNA copies/μl.
To generate an actin DNA concentration standard, primers (forward, 5′-TGAGCGGTTCCGGTGTCC-3′; reverse, 5′-CAGTGAGGCCAGAATGGA-3′) were designed to amplify a 292-bp internal fragment of the actin gene by PCR with use of murine DNA as a template. To prepare a vlsE DNA concentration standard, a spirochetal DNA preparation was PCR amplified to generate a 330-bp internal fragment within the 5′-conserved region of the vlsE gene with use of primers (forward, 5′-TTTCATTATAAGGAGACGATGA-3′; reverse, 5′-CGTCGTACTACTTATATCGC-3′). Taq polymerase was purchased from Roche Diagnostics Co. (Indianapolis, Ind.). A PCR program with the following parameters was used: 95°C for 5 min; 95°C for 40 s, 50°C for 1 min, and 72°C for 40 s, 50 cycles; and 72°C for 10 min. PCR products were purified using the Quick PCR product purification kit (Qiagen). Purity was examined by agarose gel electrophoresis. DNA concentrations were determined by measuring the optical density at a 260-nm wavelength, converted to copy number, and diluted at 102 to 107 DNA copies/μl for actin DNA quantification and at 100 to 105 DNA copies/μl for vlsE cDNA quantification. The vlsE DNA concentration standard was prepared in this way since the qPCR efficiency of spirochetal DNA as a template was very low, probably because of this gene being too close to the right telomere of lp28-1 (54).
qPCR.
qPCR analyses were performed using the iCycler (Bio-Rad Laboratories, Hercules, Calif.). The Platinum Taq DNA polymerase High Fidelity kit was purchased from Invitrogen. The sequences of primers and internal probes of flaB, dbpA, ospC, BBF01, vlsE, and actin genes are listed in Table 1. Taqman TAMRA probes were ordered from Applied Biosystems (Foster City, Calif.). Amplification was performed in a 50-μl final volume in individual wells of a 96-well iCycler iQ PCR plate (Bio-Rad). Twelve wells of each plate were assigned for DNA standards at six different concentrations in duplicate. Each cDNA or DNA sample was amplified in duplicate for the flaB, dbpA, ospC, BBF01, vlsE, or actin gene. A PCR program with the following parameters was used: 95°C for 5 min and 50 cycles of 95°C for 30 s and 60°C for 1 min. The mean cDNA copy numbers of flaB, dbpA, ospC, BBF01, and vlsE transcripts of each cDNA pool and mean DNA copy numbers of flaB and actin genes of each DNA sample were automatically calculated from duplicate wells with use of the iCycler software. Tissue spirochete burdens were calculated as flaB DNA copy numbers per 106 actin DNA copies. Lipoprotein gene expression levels were presented as dbpA, ospC, BBF01, or vlsE mRNA copy numbers per 103 flaB mRNA transcripts.
TABLE 1.
qPCR primers and probes
| Gene | Primer | Probe |
|---|---|---|
| flaB | Forward 5′-GAGTTTCTGGTAAGATTAATGCTC-3′ | 5′-AGAGGTTTGTCACAAGCTTCTAGAAATACTTCAAAGGC-3′ |
| Reverse 5′-CATTTAAATTCCCTTCTGTTGTCTGA-3′ | ||
| dbpA | Forward 5′-CTTAAACTAACTATACTTGTTAAC-3′ | 5′-TTATATCATGTGGACTAACAGGAGCAAC-3′ |
| Reverse 5′-AATGTCTTTAGCGCTTCGTTC-3′ | ||
| ospC | Forward 5′-TACGGATTCTAATGCGGTTTTAC-3′ | 5′-TGAAGCGTTGCTGTCATCTATAGATGAAATTGCTGCT-3′ |
| Reverse 5′-GTGATTATTTTCGGTATCCAAACCA-3′ | ||
| BBF01 | Forward 5′-TAGCACAACATGCTCCAAACTC-3′ | 5′-ATCCGATGGAAAACCTGTTCCTGGGGAC-3′ |
| Reverse 5′-ACAATTTCCTCTTTTACTTCTGGGA-3′ | ||
| vlsE | Forward 5′-CTTATACTTTTCATTATAAGGAGACGATG-3′ | 5′-GCCAAGTTGCTGATAAGGACGACCCAAC-3′ |
| Reverse 5′-GCCTCTGCTACTAACCCAC-3′ | ||
| actin | Forward 5′-CATCATGAAGTGTGACGTTGAC-3′ | 5′-GTATGCCAATACAGTGCTGTCTGGTGGTACCAC-3′ |
| Reverse 5′-GCATCCTGTCAGCAATGCC-3′ |
Statistical analysis.
Data were analyzed using Microsoft Excel (Redmond, Wash.) software. A two-tailed Student t test was used to analyze qPCR and quantitative RT-PCR data. A P value of <0.05 was considered to be a significant difference.
RESULTS
The tissue microenvironment alters the antigenic profile of B. burgdorferi.
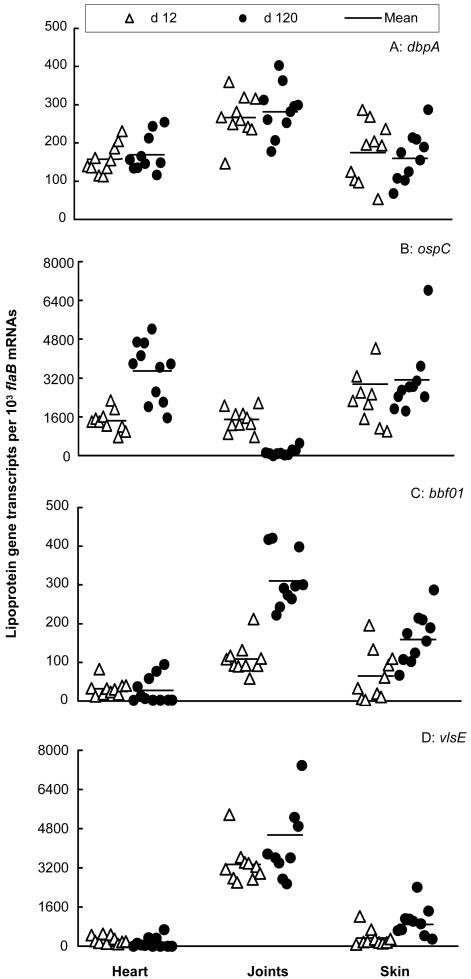
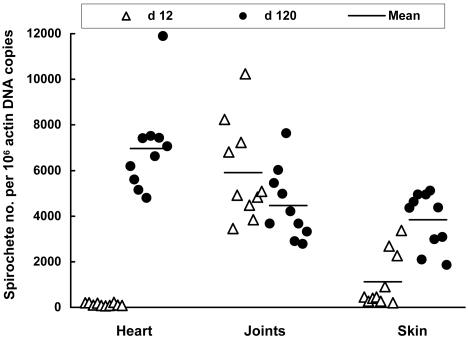
To investigate the influence of adaptive immune pressure on B. burgdorferi, an analysis of lipoprotein gene expression in selective tissues from spirochete-infected SCID mice was first performed. Mice were sacrificed at 12 and 120 days postinfection, time points representative of acute and persistent infection, respectively, and expression of four representative lipoproteins and the tissue spirochete burdens were investigated in the heart, joints, and skin. These lipoproteins, DbpA, OspC, BBF01, and VlsE, were investigated since they are considered to be expressed in vivo and surface exposed and may be involved in pathogenesis and immunity to infection (13, 15, 17, 18, 20, 25, 54). These selected tissues were investigated for different reasons: skin represents the tissue of initial entry and exit of B. burgdorferi, and both the heart and joints are major sites of inflammation in the murine model of Lyme disease (4). At 12 days postinfection, populations of spirochetes showed vastly different expression levels of dbpA, ospC, BBF01, and vlsE in tissues (Fig. 1). B. burgdorferi more actively transcribed dbpA in the joints than in the heart (P = 1.0 × 10−4) and skin (P < 0.008). ospC was more abundantly expressed in the skin than in the heart (P < 0.04) and joints (P < 0.05). BBF01 was expressed at much higher levels in the joints than in the heart (P = 4.2 × 10−5), and vlsE was transcribed more actively in the joints than in the heart (P = 4.9 × 10−10) and skin (P = 2.3 × 10−9). Within 12 days after infection, B. burgdorferi might not have fully adapted to the tissue microenvironments. Spirochetal gene expression was also examined at a later time point. At 4 months, the expression levels of three of these four genes dramatically changed. B. burgdorferi increased ospC transcription in the heart (P = 1.3 × 10−4) but reduced its expression in joints (P = 8.4 × 10−8). Spirochetes increased BBF01 expression in the joints (P = 5.1 × 10−7) and skin (P = 0.004) and vlsE expression in the skin (P = 0.007). The differences in transcription of dbpA, ospC, and vlsE have been reflected by previous differences in the levels of these proteins in these tissues (10). These data indicate that the tissue microenvironment influences spirochetal phenotypes in the absence of adaptive immune pressure. The B. burgdorferi load also differed in the tissues examined at 12 days postinfection (Fig. 2). The joints provided the best environment for B. burgdorferi replication since the spirochete numbers were similar in this tissue at 12 days and 4 months (P > 0.10). At the same time, the spirochetal burdens in the heart were only 1.9% (P = 2.4 × 10−9) and in the skin were 29.4% (P = 8.5 × 10−5) of those detected at 4 months (Fig. 2). Taken together, these data indicate that the tissue microenvironments affect the spirochete growth as well as influence the B. burgdorferi lipoprotein gene expression.
FIG. 1.
Tissue milieus modulate spirochetal antigenic profiles. Twenty SCID mice were infected with B. burgdorferi and euthanized at either 12 days (n = 10) or 4 months (n = 10). RNA samples were prepared from heart, joint, and skin tissues and converted to cDNA. flaB, dbpA, ospC, BBF01, and vlsE expression levels were analyzed by qPCR and presented as dbpA, ospC, BBF01, and vlsE mRNA copy numbers per 103 flaB mRNA copies.
FIG. 2.
Tissue spirochetal burdens in the absence of immune pressure. DNA samples were prepared from the heart, joints, and skin of the 20 SCID mice that were used to generate data for Fig. 1. Spirochetal flaB and mouse actin DNAs were quantified by qPCR. The data are expressed as flaB DNA copy numbers per 106 actin DNA copies.
Immune responses change spirochetal surface antigenic expression.
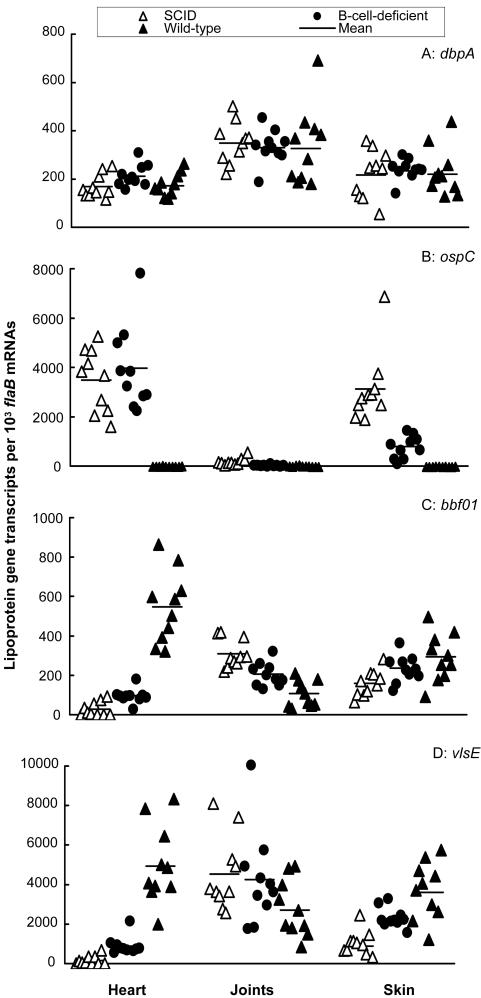
The immune system consists of numerous key components that directly interact with foreign invaders and protect the host from microbial infections. To persist in the immune environment, B. burgdorferi must respond to lethal attack by the immune system. We used SCID, B-cell-deficient, and immunocompetent mice to investigate how B. burgdorferi adapts to various immune environments. B-cell-deficient and wild-type mice were infected for 4 months to allow immune responses to fully develop and B. burgdorferi to adapt. As a control, SCID mice were infected for 4 months. Gene expression was investigated and is presented in Fig. 3. Of the four lipoprotein genes examined, only expression of dbpA was not affected by adaptive immune pressure. The presence of cellular immunity significantly reduced ospC expression in both the joints (P < 0.01) and skin (P = 1.2 × 10−4) and BBF01 expression in the joints (P = 2.7 × 10−3) and increased BBF01 and vlsE expression in both the heart (P = 4.1 × 10−4 and 2.7 × 10−4, respectively) and skin (P < 0.02 × 10−5 and 7.6 × 10−5, respectively). The most dramatic adaptation occurred under the influence of both humoral and cellular immune responses. B. burgdorferi decreased ospC expression 388, 9.3, and 446 times in the heart (P = 7.7 × 10−8), joint (P = 2.9 × 10−3), and skin (P = 1.9 × 10−6) tissues of wild-type mice compared to SCID mice, respectively. In contrast, adaptive immune pressure increased BBF01 expression 20 and 1.8 times in the heart (P = 5.8 × 10−8) and skin (P = 6.4 × 10−3), respectively, and vlsE expression 32 and 4.1 times in these two tissues (P = 5.3 × 10−8 and 0.02), respectively. B. burgdorferi significantly reduced both BBF01 (P = 2.6 × 10−3) and vlsE (P = 0.023) expression in the joints of wild-type mice. Overall, adaptive immune pressure substantially changed the surface antigenic expression of B. burgdorferi, given the fact that these four lipoproteins are surface-exposed antigens.
FIG. 3.
Immune responses change surface antigenic expression. A group of 10 SCID, B-cell-deficient, or wild-type mice were infected for 4 months. RNA samples were prepared from heart, joint, and skin tissues and converted to cDNA. flaB, dbpA, ospC, BBF01, and vlsE expression levels were analyzed by qPCR and presented as dbpA, ospC, BBF01, and vlsE mRNA copy numbers per 103 flaB mRNA copies.
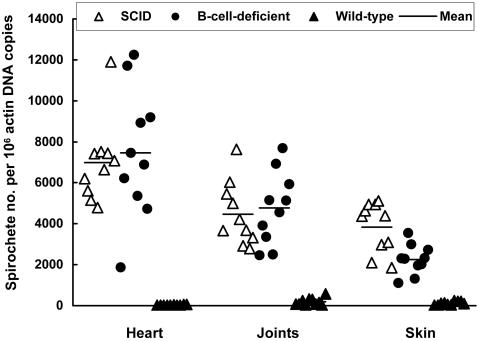
Although the presence of T cells significantly affected B. burgdorferi adaptation, cellular immunity was not able to control the tissue spirochetal burdens in the heart (P > 0.68) and joints (P > 0.70) (Fig. 4). T cells did reduce the bacterial loads of the skin tissue by 41% (P < 0.003). In contrast, humoral immunity played the dominant role in controlling tissue spirochetal burdens, leading to 258-, 24-, and 34-fold reductions in the heart (P = 1.9 × 10−9), joint (P = 7.4 × 10−8), and skin (P = 1.7 × 10−8) tissues, respectively, of wild-type mice compared to SCID mice.
FIG. 4.
Influence of immune pressure on tissue spirochetal burdens. DNA samples were prepared from the heart, joints, and skin of the SCID (n = 10), B-cell-deficient (n = 10), and wild-type (n = 10) mice that were used to generate data for Fig. 3. Spirochetal flaB and mouse actin DNAs were quantified by qPCR. The data are expressed as flaB DNA copy numbers per 106 actin DNA copies.
Anti-Borrelia antibodies change spirochetal surface antigenic expression.
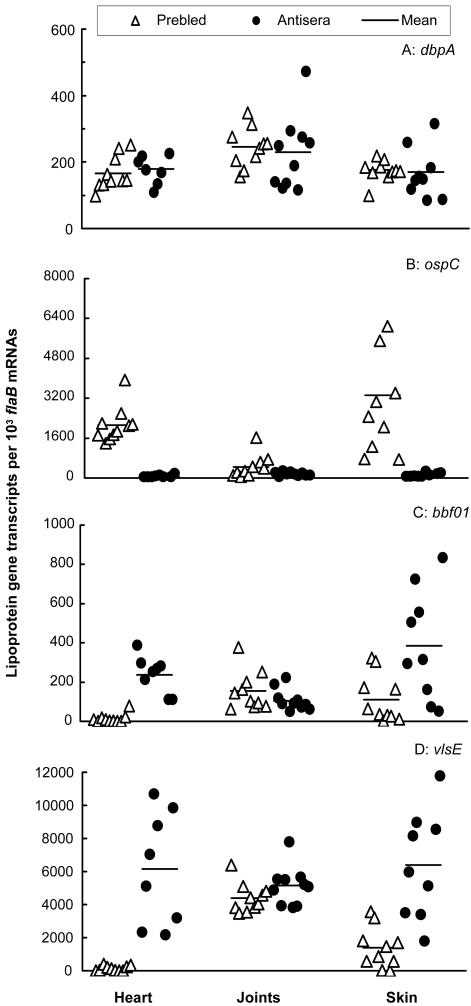
To examine whether specific antibodies in the absence of B and T cells are able to modify the surface antigenic expression and to reduce the tissue spirochetal burdens, antiserum transfer was conducted. SCID mice were infected with B. burgdorferi for 4 months, an interval in which the spirochetes had adapted to the host, and then passively immunized with either prebled mouse sera as a control or anti-Borrelia sera. Like the immune pressure generated in the immunocompetent mice, passive transfer did not influence dbpA expression (Fig. 5). The administration of specific antibodies altered the spirochetal surface antigenic expression similarly to what had been noted in immunocompetent mice. These included decreased ospC expression in the heart (76-fold, P = 4.5 × 10−7), joint (4-fold, P = 0.04), and skin (58-fold, P = 0.001) tissues and increased BBF01 and vlsE expression in both the heart (17- and 44-fold; P = 1.7 × 10−6 and 0.02, respectively) and skin (3.5- and 6-fold, respectively; P = 0.01 and 1.6 × 10−8, respectively) tissues. However, the transferred specific antiserum was not able to reduce the expression of either BBF01 (P = 0.20) or vlsE (P = 0.45) in the joint tissue.
FIG. 5.
Anti-Borrelia antibodies change surface antigenic expression. Twenty SCID mice were infected with B. burgdorferi for 4 months and then passively immunized with prebled mouse sera (n = 10) or murine anti-Borrelia antisera (n = 10). RNA samples were prepared from heart, joint, and skin tissues and converted to cDNA. flaB, dbpA, ospC, BBF01, and vlsE expression levels were analyzed by qPCR and presented as dbpA, ospC, BBF01, and vlsE mRNA copy numbers per 103 flaB mRNA copies. Spirochetal mRNA was not detectable in two heart samples and one skin sample of the passively immunized group, consistent with the results of no spirochetal DNA being detected in these tissues.
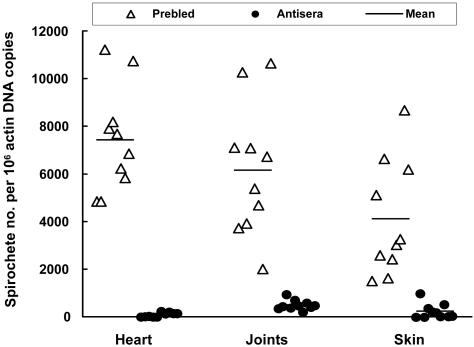
The specific antisera also significantly decreased the tissue spirochetal burdens (Fig. 6). These included the tissue spirochetal reductions of 81, 12, and 17 times in the heart (P = 4.2 × 10−9), joints (P = 5.0 × 10−6), and skin (P = 9.1 × 10−5), respectively. The passive transfer (Fig. 6) was not as effective as the immune pressure developing in the immunocompetent mice in controlling the tissue spirochetal burden, probably because the procedure could not provide an antibody titer as high as the immune response induced by infection.
FIG. 6.
Influence of anti-Borrelia sera on tissue spirochetal burdens. DNA samples were prepared from the heart, joints, and skin of the 20 SCID mice that were used to generate data for Fig. 5. Spirochetal flaB and mouse actin DNAs were quantified by qPCR. The data are expressed as flaB DNA copy numbers per 106 actin DNA copies.
OspC monoclonal antibody changes spirochetal surface antigenic expression.
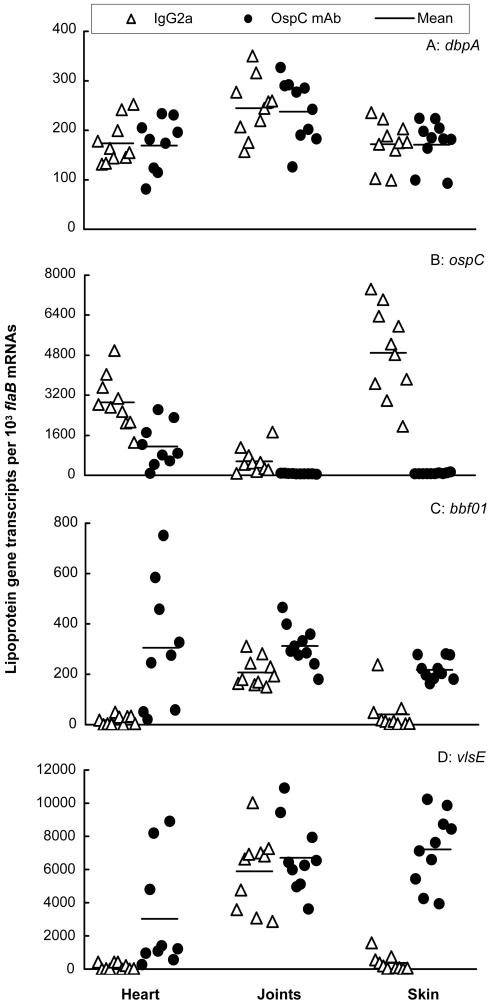
Our previous study has shown that OspC antibody selects against OspC-expressing spirochetes in the skin tissue (27). In the present study, we examined whether the specific antibody changed expression of other lipoprotein genes and tissue spirochetal burdens. SCID mice were infected with B. burgdorferi for 4 months and passively immunized with either mouse IgG2a as a control or OspC monoclonal antibody. The antibody decreased ospC expression 2.5, 26, and 158 times in the heart (P = 0.001), joints (P = 0.003), and skin (P = 9.5 × 10−8), respectively (Fig. 7). Although dbpA expression was not affected in the transfer study, the passive immunization significantly increased BBF01 transcription by 18, 1.5, and 5.5 times in the heart (P = 0.002), joints (P = 0.003), and skin (P = 3.0 × 10−6), respectively. To respond to the attack by OspC antibody, B. burgdorferi also increased vlsE expression 20 times both in the heart (P = 0.02) and in the skin (P = 1.6 × 10−8) but not in the joints (P > 0.42).
FIG. 7.
OspC monoclonal antibody changes surface antigenic expression. Twenty SCID mice were infected with B. burgdorferi for 4 months and then passively immunized with either mouse IgG2a (n = 10) or OspC monoclonal antibody (n = 10). RNA samples were prepared from heart, joint, and skin tissues and converted to cDNA. flaB, dbpA, ospC, BBF01, and vlsE expression levels were analyzed by qPCR and presented as dbpA, ospC, BBF01, and vlsE mRNA copy numbers per 103 flaB mRNA copies. Spirochetal mRNA was not detectable in one heart specimen of the passively immunized group, consistent with the result of no spirochetal DNA being detected in this tissue.
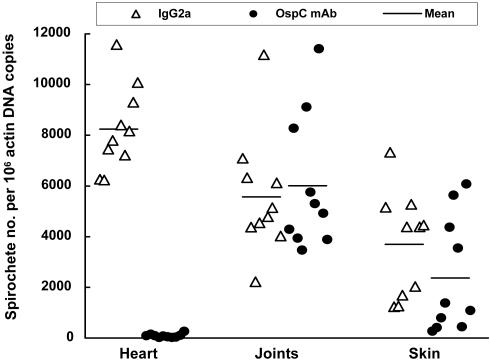
Although OspC antibody effectively reduced ospC expression in the heart, joints, and skin, it significantly reduced the tissue spirochetal burdens only in the heart (125-fold, P = 8.4 × 10−12). There were no significant differences in the spirochete number in the joints (P = 0.70) and skin (P = 0.19) between the two treatment groups (Fig. 8).
FIG. 8.
OspC monoclonal antibody selectively reduces tissue spirochetal burdens in the heart. DNA samples were prepared from the heart, joints, and skin of the 20 SCID mice that were used to generate data for Fig. 7. Spirochetal flaB and mouse actin DNAs were quantified by qPCR. The data are expressed as flaB DNA copy numbers per 106 actin DNA copies.
DISCUSSION
The present study examined the influence of various in vivo conditions on the expression of four prototypic surface lipoprotein antigens and showed that the tissue milieus shape the surface antigenic expression of B. burgdorferi, especially in the immune environment. In the absence of immune pressure, B. burgdorferi presented itself in multiple phenotypes depending on the tissues where it resided and the time courses over which infection persisted. For instance, B. burgdorferi increased ospC expression 2.4 times in the heart from 12 days to 4 months postinfection while it reduced ospC expression 8.0-fold in the joints. In contrast, spirochetes increased BBF01 expression 2.8 times in the joints during the same period. The immune response is an overwhelming force that may select for spirochetes with a modified surface antigenic expression in all the tissues, and these alterations may help B. burgdorferi evade immune attack. The study also examined the role of both humoral and cellular immune responses in controlling the tissue spirochetal burdens. Although cellular immunity does not directly kill extracellular bacteria, it triggers inflammatory responses that are harmful to foreign invaders and recruits and activates phagocytes to clear infection. Our study indicates that cellular immunity affects the tissue spirochetal burdens only in the skin. Humoral immunity, in contrast, is able to effectively reduce the tissue spirochetal burdens in all of the tissues examined.
Among the four lipoprotein genes examined, dbpA appeared to be the only one that was constantly expressed in all the tissues regardless of immune pressure, suggesting that DbpA may be important for mammalian infection and/or not amenable to host antibodies in vivo. Alternatively, B. burgdorferi may lack the ability to regulate this gene during murine infection, although evidence indicates that it is coregulated with ospC during in vitro cultivation (21). ospC was abundantly expressed in both heart and skin in the absence of adaptive immune pressure. Unlike ospC, both BBF01 and vlsE were more actively expressed in the joints than in the heart and skin. In fact, B. burgdorferi transcribed both BBF01 and vlsE below a detectable level in some of the heart and skin specimens, suggesting that these two genes are not essential for in vivo survival if adaptive immune pressure is absent. This is consistent with previous studies that show that plasmid lp28-1, which carries both BBF01 and vlsE (16), is required for persistent infection only of immunocompetent mice (24, 41, 50) and not of SCID mice (23, 40). These previous studies demonstrate that lp28-1 is unnecessary for in vivo survival of B. burgdorferi if adaptive immune pressure does not exist. Therefore, some or all of the genes on lp28-1 may play a critical role in immune evasion. Without them, B. burgdorferi would be cleared before a persistent infection could be established in immunocompetent mice. Although lp28-1 carries 32 open reading frames, BBF01 and vlsE may be the only two unique and functional genes, since the rest are either paralogues found on other borrelial genetic elements, frameshifted genes, or pseudogenes or encode less than 100 amino acids (16). Thus, these are the two most likely lipoprotein genes on lp28-1 that participate in the establishment of chronic infection in the immune environment. Interestingly, the present study showed that B. burgdorferi was able to increase expression of these two antigens by 32 times in both heart and skin tissues under adaptive immune selection pressure. Their expression levels were less affected in the presence of an immune response in the joints, probably because their transcriptional levels were already high in the absence of immune pressure.
B. burgdorferi VlsE undergoes vigorous antigenic variation during mammalian infection (55) and thus may not be easily targeted by protective antibodies. Not just the primary but the secondary and tertiary structures of VlsE are available (12). Its antigenic structure has also been extensively investigated (25, 26, 31). VlsE contains two invariable domains at the N and C termini that surround a central variable domain that consists of six variable regions and six invariable regions (25, 54). Both the invariable domains and regions are not exposed at the surface of the intact spirochete and thus cannot serve as targets of antibody when the bacterium is alive (12, 25, 28, 30). At least the C-terminal domain and two invariable regions are immunodominant and may serve as decoy epitopes to divert immune responses away from the variable regions (25, 28). Even so, a weak humoral response to the invariable regions is indeed detected during murine infection (35). This may be the reason why vlsE expression was somewhat reduced in the joints of infected immunocompetent mice compared with SCID mice. The transferred anti-Borrelia sera were not able to significantly reduce vlsE expression of the joint tissue, probably because the introduced specific antibodies had lower specificities for the variable regions of VlsE or contained an insufficient titer. OspC antibody did not affect vlsE expression at all in the joints since the preparation was free of other antibodies. However, it remains unaddressed whether antibodies to the variable regions of VlsE are bactericidal in the murine host.
The function of BBF01 is unknown. A previous study has shown that BBF01 antibody is able to reduce the severity of arthritis in infected SCID mice (13). However, the nature of BBF01 antibody activity remains to be addressed since the specific antibodies do not protect mice from infection nor affect the tissue spirochetal burdens (14). Although the present study cannot conclude if specific antibodies are able to target BBF01 in the heart and skin since the expression of this lipoprotein increased under immune pressure in these two tissues, our data indicate that the antigen may be targeted in the joints since the gene's transcription was significantly reduced by the immune response generated in immunocompetent mice. The transferred anti-Borrelia sera were not able to effectively reduce BBF01 expression, but OspC antibody significantly increased its expression in the joints, also suggesting that BBF01 may be targeted in this tissue. Anti-Borrelia sera did not significantly reduce BBF01 expression, probably because the passive transfer could not provide a sufficient anti-BBF01 titer, while OspC antibody increased BBF01 expression, probably because it could not confer immune selection pressure against BBF01-expressing spirochetes. BBF01 expression may be more protected against adaptive immune pressure in the skin and heart than in the joints, since this gene was more actively expressed in these two tissues than in the joints in the presence of adaptive immune pressure. There has been no evidence that BBF01 undergoes antigenic variation. The expression of this lipoprotein was elevated against adaptive immune pressure probably due to the existence of a host ligand that can interact with the antigen. Such interactions may interfere with specific antibody binding to the antigen. A differential expression of such ligands in different tissues may lead to varying resistance of BBF01 expression against immune-mediated selection. Extensive studies demonstrate that DbpA binds host ligands such as decorin (6, 18). This is consistent with the present study, which showed that dbpA expression was not significantly influenced by the immune response. In contrast, we have found that the interactions of DbpA with host decorin somewhat protect the lipoprotein's expression in the joint tissue of chronically infected wild-type mice compared to decorin-deficient mice (F. T. Liang et al., unpublished data).
In the absence of adaptive immune pressure, B. burgdorferi presents itself in different phenotypes depending on the tissues where it is nourished. For instance, OspC expressers dominate in the heart and skin while BBF01 and VlsE expressers flourish in the joints. In response to immune pressure, B. burgdorferi phenotypes that express antibody-targeted antigens such as OspC may be selected against, and spirochetes that express non-antibody-targeted antigens, exemplified by VlsE and BBF01, may be more likely to persist. This antigenic adaptation appears to be essential for the spirochetes to survive as the host immune response develops and to proceed to persistent infection. The spirochetal phenotype with a high BBF01 and VslE expression and a lower OspC expression dominates in all the tissues examined during chronic infection and thus may be the resistant form of B. burgdorferi in the hostile immune environment. It remains to be addressed whether this surface antigenic modification is solely due to spirochetal phenotypic changes.
ospC was more abundantly expressed in both the heart and skin than in the joints in the absence of immune pressure. OspC antibody virtually cleared OspC but was not able to significantly reduce the spirochetal burdens in the skin and joints, suggesting that this lipoprotein may not be required for the normal survival of B. burgdorferi in these tissues. In contrast, OspC antibody nearly cleared infection in the heart, suggesting that this antigen may be essential for spirochetal survival in this tissue. However, the present study cannot rule out the possibility that the unique niche of the heart tissue may persistently induce ospC expression and thus make B. burgdorferi more susceptible to the antibody, even though OspC is not required for the survival in this tissue.
The present study has clearly demonstrated that humoral immune pressure reduces ospC expression and increases BBF01 and vlsE expression to generate spirochetal phenotypes that are most likely to resist immune attack. To date, we can quantify only in vivo gene expression at the spirochetal population level in each selective tissue milieu and cannot examine gene expression by individual spirochetes within tissues. Immune selection of certain spirochetal phenotypes facilitates persistent infection, and the exact molecular mechanisms that govern the modification of the spirochetal surface antigenic expression in response to immune attack remain to be addressed.
Acknowledgments
We thank Steven Norris (University of Texas, Houston) for providing clonal isolate B31 5A11. We thank Debbie Beck for assistance with the murine studies.
This work was supported by NIH/NIAID and NIH/NIAMS grants. F.T.L. is a recipient of an Arthritis Foundation Investigator Award. E.F. is the recipient of a Burroughs Wellcome Clinical Scientist Award in Translational Research.
Editor: D. L. Burns
REFERENCES
- 1.Akins, D. R., K. W. Bourell, M. J. Caimano, M. V. Norgard, and J. D. Radolf. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Investig. 101:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878-884. [DOI] [PubMed] [Google Scholar]
- 3.Babb, K., N. El-Hage, J. C. Miller, J. A. Carroll, and B. Stevenson. 2001. Distinct regulatory pathways control expression of Borrelia burgdorferi infection-associated OspC and Erp surface proteins. Infect. Immun. 69:4146-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold, S. W., M. S. de Souza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:959-971. [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, E. L., B. P. Guo, P. O'Neal, and M. Hook. 1999. Adherence of Borrelia burgdorferi. Identification of critical lysine residues in DbpA required for decorin binding. J. Biol. Chem. 274:26272-26278. [DOI] [PubMed] [Google Scholar]
- 7.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67:3181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 9.Chen, J., M. Trounstine, F. W. Alt, F. Young, C. Kurahara, J. F. Loring, and D. Huszar. 1993. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int. Immunol. 5:647-656. [DOI] [PubMed] [Google Scholar]
- 10.Crother, T. R., C. I. Champion, X. Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2003. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect. Immun. 71:3419-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Silva, A. M., N. S. Zeidner, Y. Zhang, M. C. Dolan, J. Piesman, and E. Fikrig. 1999. Influence of outer surface protein A antibody on Borrelia burgdorferi within feeding ticks. Infect. Immun. 67:30-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eicken, C., V. Sharma, T. Klabunde, M. B. Lawrenz, J. M. Hardham, S. J. Norris, and J. C. Sacchettini. 2002. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J. Biol. Chem. 277:21691-21696. [DOI] [PubMed] [Google Scholar]
- 13.Feng, S., E. Hodzic, and S. W. Barthold. 2000. Lyme arthritis resolution with antiserum to a 37-kilodalton Borrelia burgdorferi protein. Infect. Immun. 68:4169-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng, S., E. Hodzic, K. Freet, and S. W. Barthold. 2003. Immunogenicity of Borrelia burgdorferi arthritis-related protein. Infect. Immun. 71:7211-7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fikrig, E., M. Chen, S. W. Barthold, J. Anguita, W. Feng, S. R. Telford III, and R. A. Flavell. 1999. Borrelia burgdorferi erpT expression in the arthropod vector and murine host. Mol. Microbiol. 31:281-290. [DOI] [PubMed] [Google Scholar]
- 16.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 17.Gilmore, R. D., Jr., K. J. Kappel, M. C. Dolan, T. R. Burkot, and B. J. Johnson. 1996. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect. Immun. 64:2234-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo, B. P., E. L. Brown, D. W. Dorward, L. C. Rosenberg, and M. Hook. 1998. Decorin-binding adhesins from Borrelia burgdorferi. Mol. Microbiol. 30:711-723. [DOI] [PubMed] [Google Scholar]
- 19.Haake, D. A. 2000. Spirochetal lipoproteins and pathogenesis. Microbiology 146:1491-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagman, K. E., P. Lahdenne, T. G. Popova, S. F. Porcella, D. R. Akins, J. D. Radolf, and M. V. Norgard. 1998. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect. Immun. 66:2674-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katona, L. I., S. Ayalew, J. L. Coleman, and J. L. Benach. 2000. A bactericidal monoclonal antibody elicits a change in its antigen, OspB of Borrelia burgdorferi, that can be detected by limited proteolysis. J. Immunol. 164:1425-1431. [DOI] [PubMed] [Google Scholar]
- 23.Labandeira-Rey, M., J. Seshu, and J. T. Skare. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 71:4608-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labandeira-Rey, M., and J. T. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang, F. T., A. L. Alvarez, Y. Gu, J. M. Nowling, R. Ramamoorthy, and M. T. Philipp. 1999. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J. Immunol. 163:5566-5573. [PubMed] [Google Scholar]
- 26.Liang, F. T., L. C. Bowers, and M. T. Philipp. 2001. C-terminal invariable domain of VlsE is immunodominant but its antigenicity is scarcely conserved among strains of Lyme disease spirochetes. Infect. Immun. 69:3224-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang, F. T., M. B. Jacobs, L. C. Bowers, and M. T. Philipp. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang, F. T., M. B. Jacobs, and M. T. Philipp. 2001. C-terminal invariable domain of VlsE may not serve as target for protective immune response against Borrelia burgdorferi. Infect. Immun. 69:1337-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang, F. T., J. M. Nowling, and M. T. Philipp. 2000. Cryptic and exposed invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi sl. J. Bacteriol. 182:3597-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang, F. T., and M. T. Philipp. 1999. Analysis of antibody response to invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi. Infect. Immun. 67:6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma, J., C. Gingrich-Baker, P. M. Franchi, P. Bulger, and R. T. Coughlin. 1995. Molecular analysis of neutralizing epitopes on outer surface proteins A and B of Borrelia burgdorferi. Infect. Immun. 63:2221-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marconi, R. T., M. E. Konkel, and C. F. Garon. 1993. Variability of osp genes and gene products among species of Lyme disease spirochetes. Infect. Immun. 61:2611-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mbow, M. L., R. D. Gilmore, Jr., and R. G. Titus. 1999. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect. Immun. 67:5470-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDowell, J. V., S. Y. Sung, L. T. Hu, and R. T. Marconi. 2002. Evidence that the variable regions of the central domain of VlsE are antigenic during infection with Lyme disease spirochetes. Infect. Immun. 70:4196-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narasimhan, S., M. J. Camaino, F. T. Liang, F. Santiago, M. Laskowski, M. T. Philipp, A. R. Pachner, J. D. Radolf, and E. Fikrig. 2003. Borrelia burgdorferi transcriptome in the central nervous system of non-human primates. Proc. Natl. Acad. Sci. USA 100:15953-15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nowling, J. M., and M. T. Philipp. 1999. Killing of Borrelia burgdorferi by antibody elicited by OspA vaccine is inefficient in the absence of complement. Infect. Immun. 67:443-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purser, J. E., M. B. Lawrenz, M. J. Caimano, J. K. Howell, J. D. Radolf, and S. J. Norris. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48:753-764. [DOI] [PubMed] [Google Scholar]
- 41.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramamoorthy, R., and D. Scholl-Meeker. 2001. Borrelia burgdorferi proteins whose expression is similarly affected by culture temperature and pH. Infect. Immun. 69:2739-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rathinavelu, S., A. Broadwater, and A. M. de Silva. 2003. Does host complement kill Borrelia burgdorferi within ticks? Infect. Immun. 71:822-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadziene, A., P. A. Thompson, and A. G. Barbour. 1993. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J. Infect. Dis. 167:165-172. [DOI] [PubMed] [Google Scholar]
- 46.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seiler, K. P., and J. J. Weis. 1996. Immunity to Lyme disease: protection, pathology and persistence. Curr. Opin. Immunol. 8:503-509. [DOI] [PubMed] [Google Scholar]
- 48.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 49.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas, V., J. Anguita, S. Samanta, P. A. Rosa, P. Stewart, S. W. Barthold, and E. Fikrig. 2001. Dissociation of infectivity and pathogenicity in Borrelia burgdorferi. Infect. Immun. 69:3507-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilske, B., V. Preac-Mursic, S. Jauris, A. Hofmann, I. Pradel, E. Soutschek, E. Schwab, G. Will, and G. Wanner. 1993. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect. Immun. 61:2182-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]
- 53.Yang, X., T. G. Popova, M. S. Goldberg, and M. V. Norgard. 2001. Influence of cultivation media on genetic regulatory patterns in Borrelia burgdorferi. Infect. Immun. 69:4159-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, J. R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, J. R., and S. J. Norris. 1998. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect. Immun. 66:3689-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]