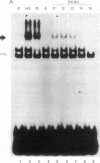
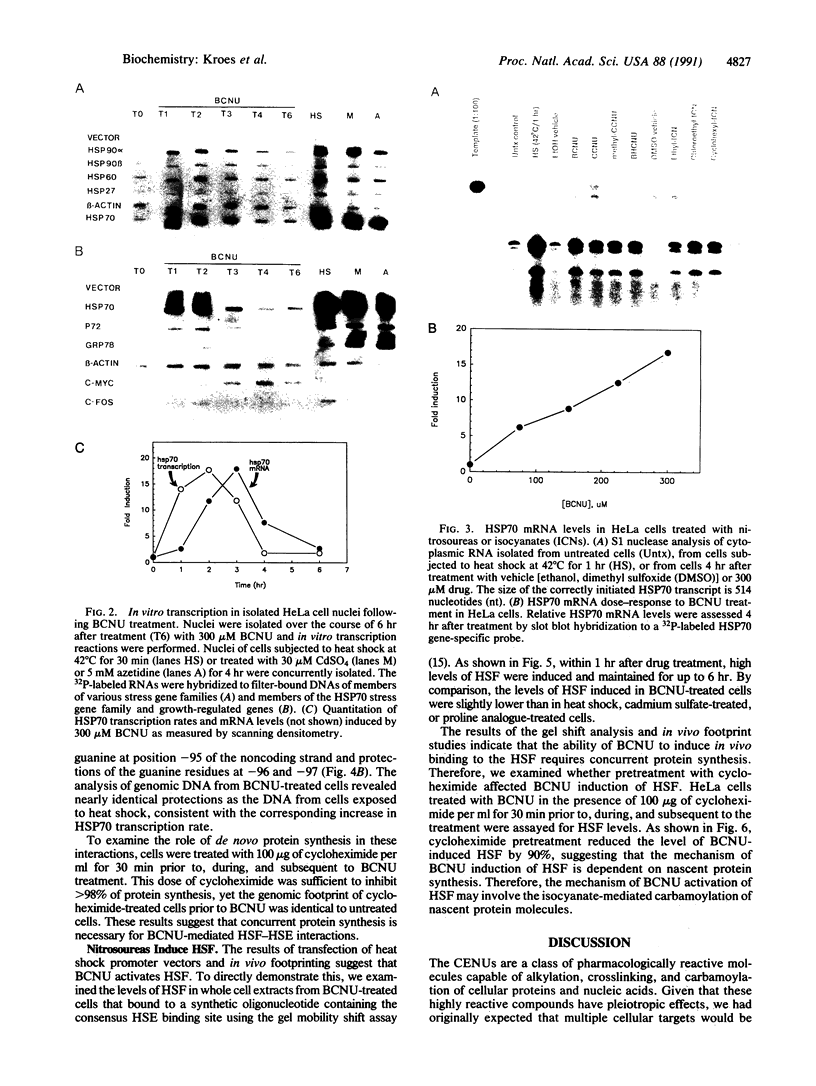
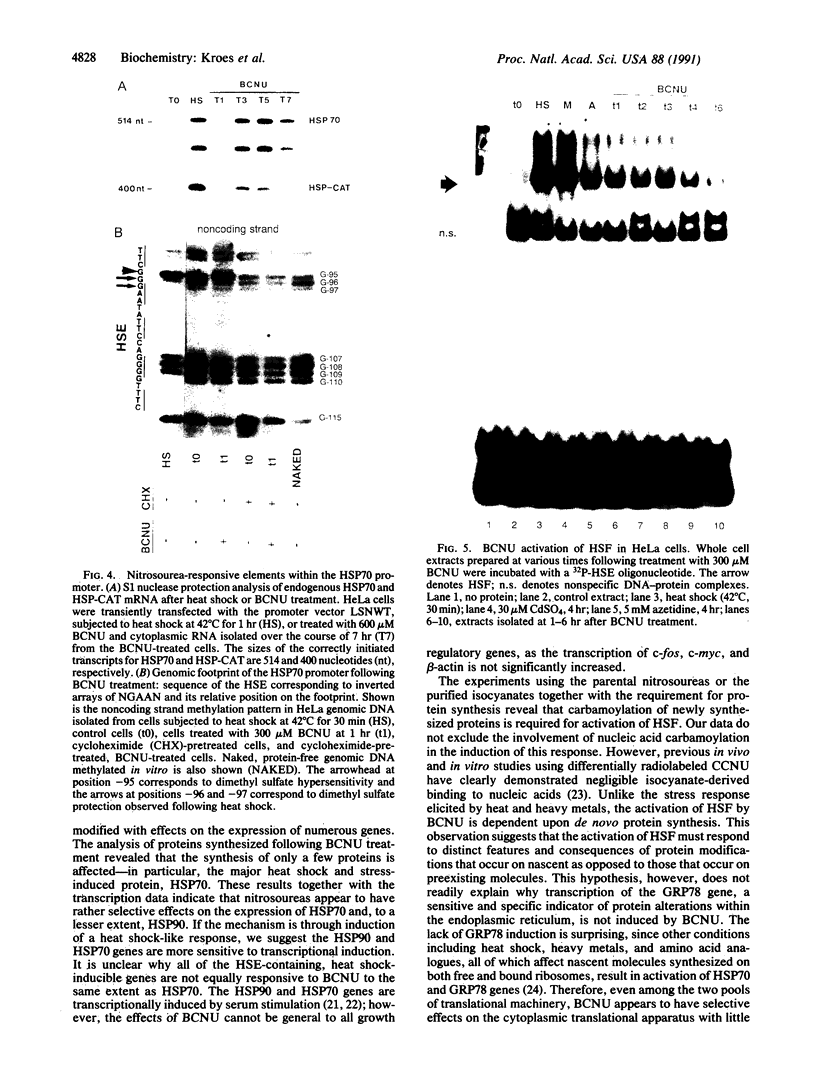
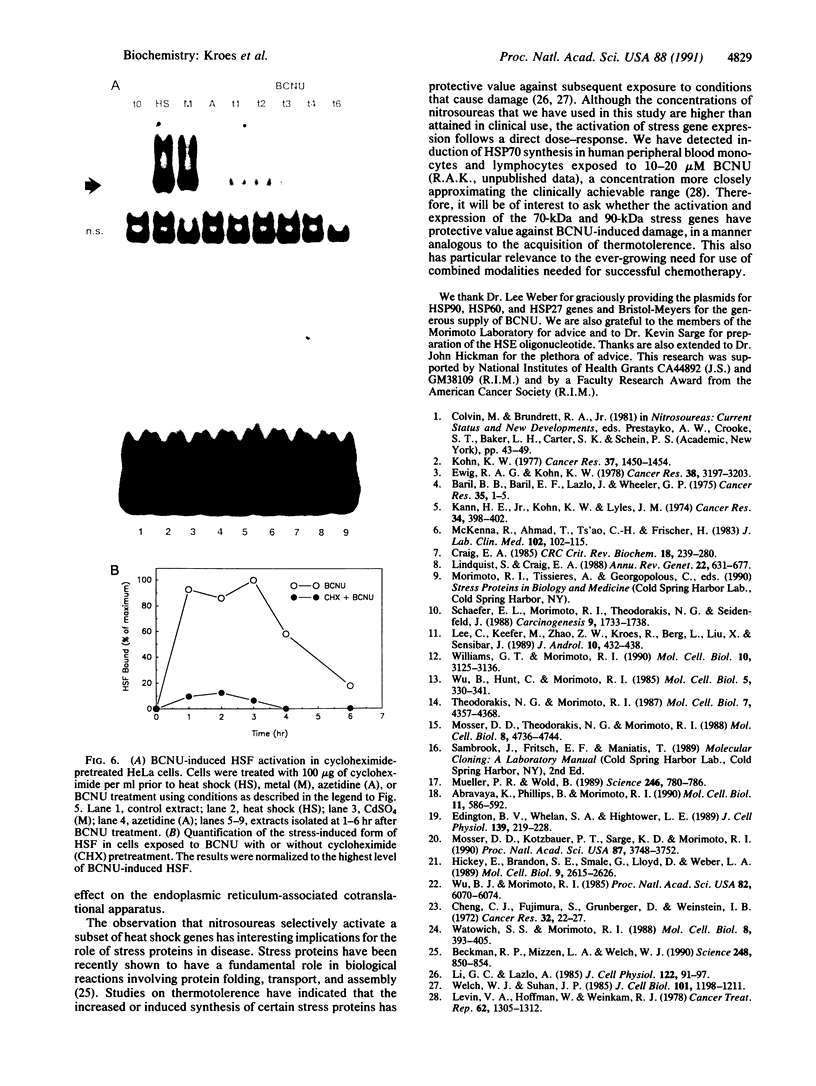
Abstract
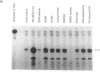
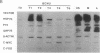
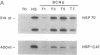
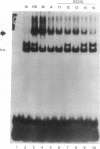
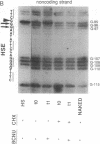
Treatment of cultured human tumor cells with the chloroethylnitrosourea antitumor drug 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) selectively induces transcription and protein synthesis of a subset of the human heat shock or stress-induced genes (HSP90 and HSP70) with little effect on other stress genes or on expression of the c-fos, c-myc, or beta-actin genes. The active component of BCNU and related compounds appears to be the isocyanate moiety that causes carbamoylation of proteins and nucleic acids. Transcriptional activation of the human HSP70 gene by BCNU is dependent on the heat shock element and correlates with the level of heat shock transcription factor and its binding to the heat shock element in vivo. Unlike activation by heat or heavy metals, BCNU-mediated activation is strongly dependent upon new protein synthesis. This suggests that BCNU-induced, isocyanate-mediated damage to newly synthesized protein(s) may be responsible for activation of the heat shock transcription factor and increased transcription of the HSP90 and HSP70 genes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abravaya K., Phillips B., Morimoto R. I. Heat shock-induced interactions of heat shock transcription factor and the human hsp70 promoter examined by in vivo footprinting. Mol Cell Biol. 1991 Jan;11(1):586–592. doi: 10.1128/mcb.11.1.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril B. B., Baril E. F., Laszlo J., Wheeler G. P. Inhibition of rat liver DNA polymerase by nitrosoureas and isocyanates. Cancer Res. 1975 Jan;35(1):1–5. [PubMed] [Google Scholar]
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Cheng C. J., Fujimura S., Grunberger D., Weinstein I. B. Interaction of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (NSC 79037) with nucleic acids and proteins in vivo and in vitro. Cancer Res. 1972 Jan;32(1):22–27. [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Edington B. V., Whelan S. A., Hightower L. E. Inhibition of heat shock (stress) protein induction by deuterium oxide and glycerol: additional support for the abnormal protein hypothesis of induction. J Cell Physiol. 1989 May;139(2):219–228. doi: 10.1002/jcp.1041390202. [DOI] [PubMed] [Google Scholar]
- Ewig R. A., Kohn K. W. DNA-protein cross-linking and DNA interstrand cross-linking by haloethylnitrosoureas in L1210 cells. Cancer Res. 1978 Oct;38(10):3197–3203. [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Smale G., Lloyd D., Weber L. A. Sequence and regulation of a gene encoding a human 89-kilodalton heat shock protein. Mol Cell Biol. 1989 Jun;9(6):2615–2626. doi: 10.1128/mcb.9.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann H. E., Jr, Kohn K. W., Lyles J. M. Inhibition of DNA repair by the 1,3-bis(2-chloroethyl)-1-nitrosourea breakdown product, 2-chloroethyl isocyanate. Cancer Res. 1974 Feb;34(2):398–402. [PubMed] [Google Scholar]
- Kohn K. W. Interstrand cross-linking of DNA by 1,3-bis(2-chloroethyl)-1-nitrosourea and other 1-(2-haloethyl)-1-nitrosoureas. Cancer Res. 1977 May;37(5):1450–1454. [PubMed] [Google Scholar]
- Lee C., Keefer M., Zhao Z. W., Kroes R., Berg L., Liu X. X., Sensibar J. Demonstration of the role of prostate-specific antigen in semen liquefaction by two-dimensional electrophoresis. J Androl. 1989 Nov-Dec;10(6):432–438. doi: 10.1002/j.1939-4640.1989.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Levin V. A., Hoffman W., Weinkam R. J. Pharmacokinetics of BCNU in man: a preliminary study of 20 patients. Cancer Treat Rep. 1978 Sep;62(9):1305–1312. [PubMed] [Google Scholar]
- Li G. C., Laszlo A. Amino acid analogs while inducing heat shock proteins sensitize CHO cells to thermal damage. J Cell Physiol. 1985 Jan;122(1):91–97. doi: 10.1002/jcp.1041220114. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- McKenna R., Ahmad T., Ts'ao C. H., Frischer H. Glutathione reductase deficiency and platelet dysfunction induced by 1,3-bis(2-chloroethyl)-1-nitrosourea. J Lab Clin Med. 1983 Jul;102(1):102–115. [PubMed] [Google Scholar]
- Mosser D. D., Kotzbauer P. T., Sarge K. D., Morimoto R. I. In vitro activation of heat shock transcription factor DNA-binding by calcium and biochemical conditions that affect protein conformation. Proc Natl Acad Sci U S A. 1990 May;87(10):3748–3752. doi: 10.1073/pnas.87.10.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D. D., Theodorakis N. G., Morimoto R. I. Coordinate changes in heat shock element-binding activity and HSP70 gene transcription rates in human cells. Mol Cell Biol. 1988 Nov;8(11):4736–4744. doi: 10.1128/mcb.8.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Schaefer E. L., Morimoto R. I., Theodorakis N. G., Seidenfeld J. Chemical specificity for induction of stress response genes by DNA-damaging drugs in human adenocarcinoma cells. Carcinogenesis. 1988 Oct;9(10):1733–1738. doi: 10.1093/carcin/9.10.1733. [DOI] [PubMed] [Google Scholar]
- Theodorakis N. G., Morimoto R. I. Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich S. S., Morimoto R. I. Complex regulation of heat shock- and glucose-responsive genes in human cells. Mol Cell Biol. 1988 Jan;8(1):393–405. doi: 10.1128/mcb.8.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Suhan J. P. Morphological study of the mammalian stress response: characterization of changes in cytoplasmic organelles, cytoskeleton, and nucleoli, and appearance of intranuclear actin filaments in rat fibroblasts after heat-shock treatment. J Cell Biol. 1985 Oct;101(4):1198–1211. doi: 10.1083/jcb.101.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Morimoto R. I. Maximal stress-induced transcription from the human HSP70 promoter requires interactions with the basal promoter elements independent of rotational alignment. Mol Cell Biol. 1990 Jun;10(6):3125–3136. doi: 10.1128/mcb.10.6.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B. J., Morimoto R. I. Transcription of the human hsp70 gene is induced by serum stimulation. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6070–6074. doi: 10.1073/pnas.82.18.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Hunt C., Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985 Feb;5(2):330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]