Abstract
The 42- and 19-kDa C-terminal fragments of merozoite surface protein 1 (MSP-142 and MSP-119, respectively) are both promising blood-stage vaccine candidate antigens. At present, it is not clear which of the two antigens will be more suitable for inclusion in a cocktail malaria vaccine. In the present study, we expressed the two C-terminal fragments of Plasmodium vivax MSP-1 (PvMSP-1) in an Escherichia coli expression system and purified them by using a rapid two-step protocol. Both of the products were recognized by monoclonal antibodies against PvMSP-1 as well as by immune sera from several individuals exposed to P. vivax. We analyzed and compared the immunological responses to recombinant PvMSP-119 and PvMSP-142 in mice by using six different adjuvant formulations. Moderate to high antibody responses were observed with both of the antigens in different adjuvant formulations. Surprisingly, alum, which is generally considered to be a poor adjuvant for recombinant malaria antigens, was found to be as good an adjuvant as Montanide ISA 720, ASO2A, and other adjuvant formulations. Most adjuvant formulations induced high levels of immunoglobulin G1 (IgG1), followed by IgG3 and IgG2. Lymphocytes from animals in the PvMSP-142- and PvMSP-119-immunized groups showed proliferative responses upon stimulation with the respective antigens, and high levels of interleukin-4 (IL-4), IL-5, and gamma interferon were detected in the culture supernatants. Immunodepletion studies with sera from mice immunized with these two antigens showed that while immunization with PvMSP-142 does produce a PvMSP-119-specific response, a substantial portion is also focused on structures in PvMSP-142 not represented by the epidermal growth factor-like domains of PvMSP-119. These findings may have implications for the design of MSP-1-based vaccine constructs.
The proteins expressed on the surface of invasive merozoites of Plasmodium are important targets for the development of an effective malaria vaccine. Among these, merozoite surface protein 1 (MSP-1) is a leading malaria vaccine candidate antigen (32). For Plasmodium falciparum, it has been shown that MSP-1 is synthesized as a 195-kDa protein that is sequentially processed into a series of fragments (22). Of particular interest among these fragments are a cysteine-rich 19-kDa fragment (MSP-119) and a C-terminal 42-kDa fragment (MSP-142); the 19-kDa fragment forms part of the 42-kDa protein. Several in vitro and in vivo studies have provided evidence suggesting that these two fragments of MSP-1 are the targets of protective immunity against asexual blood stages of malaria parasites. Monoclonal and polyclonal antibodies directed against the C-terminal 42-kDa fragment inhibited the invasion of erythrocytes by P. falciparum merozoites (5, 7). Recombinant MSP-119 of Plasmodium yoelii, P. falciparum, and Plasmodium cynomolgi resulted in protection in mice (P. yoelii) and in monkeys (P. falciparum and P. cynomolgi) (6, 36, 33). The presence of antibodies against P. falciparum MSP-1 (PfMSP-1) correlates with the development of clinical immunity against P. falciparum malaria (17). MSP-1-mediated protective immune responses have been shown to be largely antibody dependent, with high antibody titers being essential for protection (11).
Plasmodium vivax is the second most prevalent human malaria parasite; it is present mostly in South America, Asia, and Oceania (28). P. vivax merozoites express a surface protein equivalent to PfMSP-1, referred as PvMSP-1. Relatively little is known about PvMSP-1 with respect to its processing, the fate of putative processing fragments, and protective immune responses (9). However, two monkey immunization studies with recombinant PvMSP-1- and P. cynomolgi MSP-1 (PcMSP-1)-based antigens have highlighted the protective potential of these antigens. Rhesus monkeys immunized with baculovirus-expressed PcMSP-119 or PcMSP-142 in Freund's adjuvant were protected against the homologous challenge (33), and Saimiri monkeys immunized with a PvMSP-119-based immunogen were partially protected (39).
In contrast to the large number of studies that have been performed with PfMSP-1 (26) and P. yoelii MSP-1 (PyMSP-1) (12), relatively little is known about the immunogenicities of PvMSP-142 and PvMSP-119 formulated in different adjuvant systems (40), although immunization studies with these antigens expressed in baculovirus (31) or in yeast cells (39) have been reported. We describe here the expression and purification of both PvMSP-142 and PvMSP-119 from Escherichia coli and the immune responses in mice against these two antigens in six different adjuvant formulations. We found that both PvMSP-119 and PvMSP-142 were immunogenic in most adjuvant formulations, including alum, and that, although immunization with PvMSP-142 produced PvMSP-119-specific antibodies, a significant portion of the immune response was focused on the rest of the structure.
MATERIALS AND METHODS
Cloning of the PvMSP-119 and PvMSP-142 genes.
Genomic DNA of P. vivax was prepared from an Indian isolate by following the procedure described by Tungpradabkul and Panyim (38). This DNA was used as a template for the amplification of PvMSP-1 fragments corresponding to PvMSP-119 (1607 to 1704 bp; 98 amino acids) and PvMSP-142 (1275 to 1704 bp; 430 amino acids) with the following primers: F 1, 5′-CGC GGA TCC AAT GTG CAA ACT CAG TTA-3′, and R 1, 5′-T CGA ATC CTT CGA CCT CCT CGA TGT GTT TTG CGG GAG-3′; F 2, 5′-CGC GGA TCC GAC CAA GTA ACA ACG GGAGA-3′, and R 2, 5′-T CGA ATC CTT CGA CCT CCT CGA TGT GTT TTG CGG GAG-3′.
The PCR products were cloned into vector pGEM-T, and the cloned fragments were sequenced. Fragments corresponding to the PvMSP-119 and PvMSP-142 sequences were excised with restriction enzymes BamHI and SalI and ligated to the BamHI-SalI sites of vector pQE-30 (Qiagen), which provides six His residues at the N terminus of the expressed protein. The ligation mixtures were transformed into competent E. coli DH5α cells, and the recombinant clones were selected on ampicillin plates. The cloned inserts were sequenced and transferred to expression host strain E. coli M15(pREP4) (Qiagen).
Expression and purification of PvMSP-119 and PvMSP-142.
E. coli M15 cells containing recombinant plasmids pQE30-PvMSP-119 and pQE30-PvMSP-142 were grown in Luria broth containing ampicillin (100 μg/ml) and kanamycin (25 μg/ml) at 37°C with shaking until an optical density (OD) at 600 nm of 0.6 to 0.7 was reached. The expression of PvMSP-119 and PvMSP-142 was induced with 0.5 and 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Sigma Chemical Co.), respectively. The cultures were further grown at 37°C for 3 to 4 h, and the E. coli cells were harvested by centrifugation.
For the purification of PvMSP-119, the E. coli cell pellet was thawed in chilled sonication buffer (20 mM Tris, 500 mM NaCl [pH 8.0], 1 mM phenylmethylsulfonyl fluoride, 1% Tween 20). The bacteria were lysed on ice by sonication (Torbeo Ultrasonic Processor 36800; Cole Parmer) with five sonication cycles, each consisting of 10-s pulses at 10-s intervals. The bacterial lysate was centrifuged at 15,000 × g for 30 min at 4°C. The supernatant was incubated with Ni-nitrilotriacetic acid (NTA)-agarose resin (Qiagen) at 4°C for 1 h and applied to a column; the next step was a 10-column-volume wash with 20 mM Tris-500 mM NaCl (pH 8.0) buffer containing 10 mM imidazole. The bound protein was eluted with a linear gradient of 20 to 500 mM imidazole in 20 mM Tris-500 mM NaCl (pH 8.0) buffer. The fractions containing PvMSP-119 were pooled and extensively dialyzed against chilled 20 mM Tris (pH 8.0) buffer. The recombinant protein was further purified by ion-exchange chromatography with a Q-Sepharose column equilibrated with 20 mM Tris-HCl (pH 8.0) and eluted with a linear 0 to 500 mM NaCl gradient in Tris-HCl buffer. The eluates were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), the fractions containing a clear single protein band were pooled and dialyzed against chilled phosphate-buffered saline (PBS), and the protein concentrations were determined.
PvMSP-142 was expressed in inclusion bodies, and the cell pellet was dissolved in denaturation buffer (20 mM Tris, 6 M guanidine-HCl, 500 mM NaCl [pH 8.0]). After sonication and centrifugation, the supernatant was incubated with Ni-NTA-agarose for 1 h at room temperature, and the resin was packed into a column. The column was washed with 10 column volumes of wash buffer (8 M urea, 20 mM Tris-HCl [pH 8.0], 500 mM NaCl), and renaturation was carried out by applying to the column a decreasing gradient of 8 to 0 M urea dissolved in wash buffer. The column was washed with 10 column volumes of 20 mM Tris-HCl (pH 8.0), and the protein was eluted with a linear gradient of 5 to 150 mM imidazole in Tris-HCl buffer (pH 8.0) containing 500 mM NaCl. Recombinant PvMSP-142 was further purified by ion-exchange chromatography with a Q-Sepharose column by the same procedure as that described for PvMSP-119. Endotoxin levels in the protein samples were analyzed with a Limulus amebocyte lysate assay (Charles River Endosafe). The protein samples were also analyzed for host cell protein contamination by both Western blotting and an enzyme-linked immunosorbent assay (ELISA) with anti-E. coli antibodies (Cygnus Technologies).
The homogeneity of purified PvMSP-119 and PvMSP-142 was confirmed by reducing SDS-PAGE. Immunoblotting was carried out by standard protocols, and both PvMSP-119 and PvMSP-142 were probed with monoclonal as well as polyclonal antibodies under reducing and nonreducing conditions. Rabbit polyclonal anti-p19 antibody and mouse monoclonal antibodies E9.4 and F10.3 (MSP-119 specific), D14 and F20.14 (MSP-142 specific), and 5.14 were kind gifts from Shirley Longacre, Pasteur Institute. These antibodies were previously shown to react with recombinant PvMSP-142 (14).
Immunization of mice with recombinant PvMSP-119 and PvMSP-142 and different adjuvants.
The adjuvants used in the immunization study were Freund's complete adjuvant (CFA) and Freund's incomplete adjuvant (both from Sigma), alum (Alhydrogel; Superfos, Denmark), ASO2A (Glaxo Smith Kline Biologicals, Rixensart, Belgium), Montanide ISA 720 (Seppic Inc., Paris, France), MF59 (Chiron SPA, Siena, Italy), and QS21 (Antigenics, Inc., Boston, Mass.). The vaccine formulations were prepared according to the manufacturers' instructions.
The animals used in the study were housed and used strictly in accordance with the guidelines set by the National Institutes of Health in 1985. Groups of five BALB/c mice 4 to 6 weeks old were immunized intramuscularly with 25 μg of either PvMSP-119 or PvMSP-142 antigen in various adjuvant formulations. Control mice received only PBS mixed with any of the six adjuvants. The animals were boosted on days 21 and 42. The animals were bled on days 0 (preimmune), 14, 28, 35, 42, 49, 64, 70, and 120, and the sera thus obtained were used for immunoassays.
Collection of sera from P. vivax-infected patients.
A total of 10 serum samples were collected from P. vivax-infected human patients admitted to local hospitals in and around New Delhi, India. All of the patients presented with characteristic symptoms of high fever and chills. Thick and thin blood smears were prepared for the identification of parasites. Consent from these patients and approval from the Human Volunteers Research Ethical Committee of the International Centre for Genetic Engineering and Biotechnology were obtained prior to the study. After collection, blood samples were allowed to clot at room temperature, and serum samples were collected by centrifugation at 2,000 rpm (Herqeus biocentrifuge) for 10 min at 4°C and saved at −20°C until used. Serum samples were also collected and pooled from healthy individuals who had no known past history of malaria and who were slide negative at the time of blood sample collection.
ELISA.
Antibody responses in mice as well as in humans were evaluated by an ELISA. Briefly, 96-well microplates (Dynatech) were coated with 50 ng of either PvMSP-119 or PvMSP-142 in 0.06 M carbonate-bicarbonate buffer (pH 9.6) per well. The plates were kept overnight at 4°C, and the wells were blocked with 5% low-fat milk in PBS (pH 7.2) for 1 h at room temperature. The antigen-coated wells were sequentially incubated with serial dilutions of test sera and optimally diluted enzyme-labeled secondary antibody (horseradish peroxidase-labeled anti-mouse or anti-human immunoglobulin [IgG]). In between these incubations, the plates were washed with a 0.05% solution of Tween 20 in PBS. The enzyme reaction was developed with o-phenylediamine dihydrochloride-H2O2 in citrate phosphate buffer (pH 5.0), stopped with 8 N H2SO4, and recorded at 490 nm by use of a microplate reader (Molecular Devices). On the basis of preimmune sera giving an OD (mean and standard deviation [SD]) of 0.06 ± 0.014 at a dilution of 1:200, an OD cutoff of 0.1 (mean and 2 SDs) was selected for antibody titer determinations regardless of the adjuvant and antigen used.
To detect subclasses of mouse and human IgG among anti-PvMSP-119 and anti-PvMSP-142 antibodies, an ELISA was performed as described above but with secondary antibodies specific for mouse and human IgG1, IgG2a, IgG2b, and IgG3 (Sigma) at dilutions of 1:1,000.
IFA.
Antisera to recombinant proteins were also tested in an immunofluorescence assay (IFA) for their reactivity with the native proteins. The assay was performed essentially as described earlier (36). Briefly, multispot parasite slides were made from P. vivax-infected patients. The slides were air dried and fixed with an acetone-methanol (9:1) mixture at −20°C for 40 min. Polyclonal antibodies raised against either of the two proteins were diluted (1:500) with 0.5% bovine serum albumin in PBS-Tween 20, and the slides were incubated with the diluted antibodies in a sealed, moist box for 2 h at room temperature. The slides were washed with PBS-Tween 20 and incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Sigma) at a 1:200 dilution in 0.5% bovine serum albumin in PBS for 1 h at room temperature in the dark. The slides were rewashed, air dried, and mounted with an antifade solution to retard photobleaching (Bio-Rad). Fluorescence was examined by use of a Nikon SE300 microscope with a ×100 oil immersion objective.
Lymphoproliferative cellular responses.
BALB/c mice were immunized as described above with recombinant proteins formulated in different adjuvants. Fourteen days later, spleens were isolated, and single cell suspensions were prepared. Cells were plated at 5 × 106 cells/ml in a final volume of 200 μl per well in 96-well flat-bottom plates containing RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 55 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 100 U of penicillin-streptomycin/ml. Cells were cultured in the presence or absence of graded concentrations of homologous antigen (4 days) and concanavalin A (2 days). Splenocytes were pulse-labeled with 1 μCi of tritiated thymidine (Amersham Pharmacia Biotech) per well and harvested 16 h later onto glass fiber filters. [3H]Thymidine incorporation was determined by β-emission liquid scintillation spectroscopy (Betaplate; Pharmacia, Uppsala, Sweden). The geometric mean of the counts per minute for each set of quadruplicate wells was calculated, and the stimulation indices were calculated as the counts per minute for the test antigen divided by the counts per minute for the control. The statistical significance of the results was determined by use of Student's t test.
Cytokine production.
For determination of cytokine production, 5 × 106 splenocytes/ml were cultured in a final volume of 200 μl in 96-well flat-bottom plates in the presence or absence of recombinant proteins. Culture supernatants were collected after 48 h for interlukin-2 (IL-2) and IL-4 analyses and after 60 h for IL-5 and gamma interferon (IFN-γ) analyses. All of these cytokines were measured by using a murine cytokine immunoassay kit (Duo Set ELISA Developmental System; R & D Systems, Minneapolis, Minn.) by following the procedure recommended by the manufacturer.
Antibody depletion assay.
To determine the relative abundances of antibodies specific for PvMSP-119 and PvMSP-142 in P. vivax-infected human sera and in PvMSP-119- and PvMSP-142-immunized mouse sera, an antibody depletion assay was used. Initially, this depletion assay was standardized in the following manner. The wells of flat-bottom Immunolon-2 plates were coated with 100 μl of either PvMSP-119 or PvMSP-142 recombinant antigen (10 μg/ml). The wells were blocked with 5% low-fat milk in PBS (pH 7.2) for 1 h at room temperature. After all of the wells were washed with 0.05% Tween 20 in PBS and then with PBS (pH 7.2), the first two wells in the first column were incubated for 0.5 h with P. vivax-infected human sera or immunized mouse sera at a dilution of 1:500, while the remaining wells contained only wash buffer. Next, the sera from these two wells were transferred to the next respective wells in the second column and incubated for 0.5 h; once again, the remaining wells, including the first two wells in the first column, were filled with wash buffer. These serial incubations were carried out until all of the antibodies with respect to a particular antigen were depleted, as determined by color development in the wells by a standard ELISA. It was observed that for both PvMSP-119 and PvMSP-142, antigen-specific antibodies were completely removed from the respective sera after nine serial transfers. These antibody-depleted sera were subsequently analyzed for their reactivity with either PvMSP-119 or PvMSP-142 antigen by an ELISA. The reactivities of the two proteins with the depleted and undepleted sera were compared to analyze the relative contribution of each antigen.
RESULTS
Expression, purification, and immunological characterization of E. coli-expressed PvMSP-119 and PvMSP-142.
The PvMSP-1 gene fragments encoding PvMSP-119 and PvMSP-142 were cloned into vector pGEM-T and sequenced on both DNA strands. The nucleotide sequences and therefore the amino acid sequences of the inserts were found to be identical to the P. vivax SalI strain published sequence (GenBank accession number M75674) (13, 18). For the expression of PvMSP-119 and PvMSP-142, the inserts were cloned into vector pQE-30, and expression was induced with IPTG.
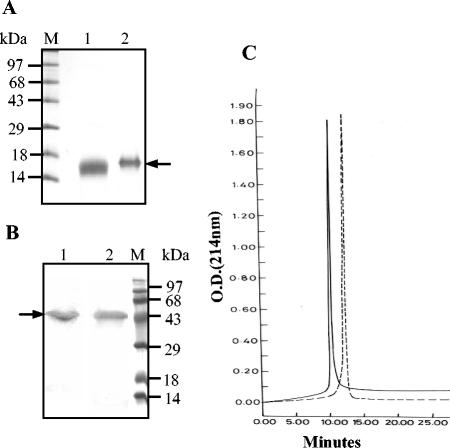
PvMSP-119 was expressed in both soluble and insoluble fractions in an approximate ratio of 1:0.5. The protein was purified from the soluble fraction by a two-step chromatographic procedure. It moved as a single band on SDS-PAGE under reducing conditions and had an apparent molecular mass of about ∼17 kDa (Fig. 1A, lane 2). Reverse-phase high-pressure liquid chromatography (HPLC) analysis of the protein with a C8 column revealed a single sharp peak (Fig. 1C).
FIG. 1.
(A and B) Coomassie blue-stained SDS-polyacrylamide gel of purified PvMSP-119 (A) and PvMSP-142 (B). Lanes: 1, nonreduced; 2, reduced. Arrows indicate the positions of the proteins. (C) Reverse-phase HPLC profiles of PvMSP-142 (broken line) and PvMSP-119 (solid line).
Unlike PvMSP-119, PvMSP-142 was expressed in inclusion bodies in E. coli. The inclusion bodies were solubilized under denaturing conditions; the protein was refolded and purified by Ni-NTA column chromatography followed by Q-Sepharose chromatography. SDS-PAGE analysis confirmed the purity of recombinant PvMSP-142, which showed an apparent molecular mass of ∼50 kDa on the gel (Fig. 1B, lane 2). Reverse-phase HPLC revealed a single symmetrical peak for the final PvMSP-142 preparation (Fig. 1C).
Both PvMSP-142 and PvMSP-119 migrated as a single homogeneous band on SDS-PAGE under nonreducing conditions (Fig. 1A, lane 1, and Fig. 1B, lane 1), indicating that they are largely composed of a single conformer. The final preparations of PvMSP-119 and PvMSP-142 contained 20 and 25 endotoxin units per 50 μg of protein, respectively. Host cell protein contamination was not observed in the protein samples, as determined by an ELISA and Western blot analysis.
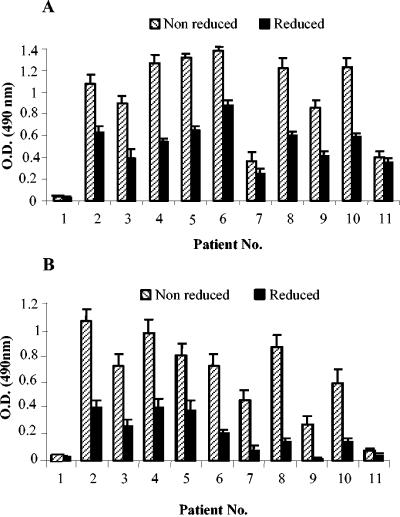
E. coli-expressed PvMSP-142 was recognized by all of the previously characterized monoclonal antibodies specific for both PvMSP-142 and PvMSP-119 (D14, F20.14, E9.4, F10.3, and 5.14) and a polyclonal antibody. PvMSP-119 was strongly recognized by a polyclonal antibody and monoclonal antibodies E9.4 and F10.3 (data not shown). Both of the recombinant proteins showed reactivity with all of the sera collected from P. vivax-infected patients in an ELISA under nonreducing conditions, and the reactivity decreased considerably under reducing conditions (Fig. 2).
FIG. 2.
Reactivity of PvMSP-119 (A) and PvMSP-142 (B) antigens in an ELISA with sera collected from P. vivax-infected patients and used at a dilution of 1:200. Error bars indicate SDs.
PvMSP-119 and PvMSP-142 induce specific antibody responses with different adjuvants in immunized mice.
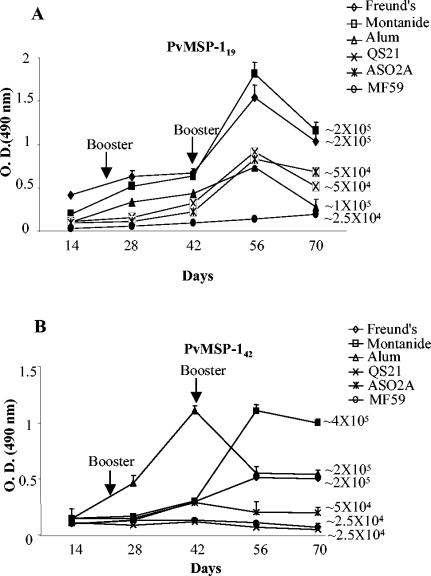
In order to find a suitable adjuvant for human immunization with PvMSP-119 or PvMSP-142, five different adjuvants—Montanide ISA 720, alum, ASO2A, QS21, and MF59—were examined by evaluating serum IgG antibodies produced in mice by an ELISA. In addition, serum IgG antibodies produced in mice immunized with antigens formulated in CFA and in incomplete Freund's adjuvant and with adjuvants alone were examined. The time course of the antibody responses in experimental animals is shown in Fig. 3. Sera from mice immunized with adjuvants alone were used as a negative control in all of the assays. PvMSP-119- and PvMSP-142-specific antibody titers were detected after primary immunization, and antibody levels were boosted with each subsequent dose of proteins. In most cases, antibody titers reached a peak after the second boost and gradually decreased thereafter. The control mice showed antibody titers similar to the preimmunization titers. However, antibody responses to the proteins varied somewhat, depending on the adjuvants used. For both of the antigens, the CFA, alum, and Montanide ISA 720 formulations produced high antibody titers; the responses obtained with the other formulations, although still high, were two- to fourfold lower than those obtained with the above-listed three adjuvants (Fig. 3). Antibodies raised against PvMSP-119 in different adjuvant formulations reacted with PvMSP-142 as effectively as with PvMSP-119 and vice versa (data not shown).
FIG. 3.
Immune responses in BALB/c mice immunized with PvMSP-119 (A) and PvMSP-142 (B) formulated in CFA and incomplete Freund's adjuvant, Montanide ISA 720, alum, ASO2A, QS21, and MF59 at a dilution of 1:25,000. Endpoint titers for each adjuvant at day 56 are shown next to each curve. Error bars indicate SDs.
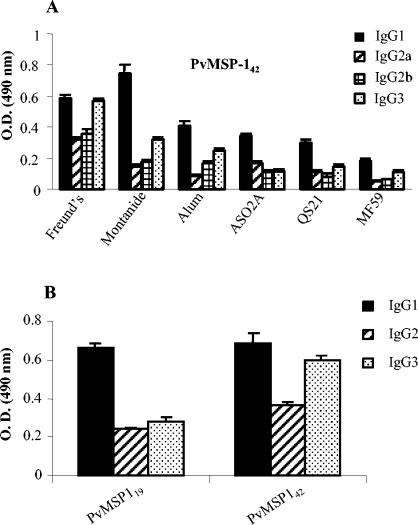
Isotype distributions of antibodies to PvMSP-119 and PvMSP-142.
Sera obtained from groups of mice immunized with PvMSP-142 in various adjuvant formulations were analyzed for anti-PvMSP-142 IgG1, IgG2a, IgG2b, and IgG3 by an ELISA. Animals in all of the groups showed high IgG titers, with IgG1 being the predominant isotype, followed by IgG3 and IgG2 (Fig. 4A). A similar isotype distribution pattern was observed for mice immunized with PvMSP-119 (data not shown). We also evaluated the specific subclasses of PvMSP-119- and PvMSP-142-specific IgG in the pooled sera collected from individuals with P. vivax infection. As shown in Fig. 4B, IgG1 and IgG3 were the predominant isotypes in these sera, as in the mouse sera.
FIG. 4.
(A) IgG isotype-specific antibody levels in mice immunized with PvMSP-142 antigen formulated in different adjuvants. Similar patterns were observed for PvMSP-119-immunized mice (data not shown). (B) IgG subclass responses to PvMSP-119 and PvMSP-142 in pooled sera from P. vivax-infected sera. Error bars indicate SDs.
Antibodies to PvMSP-119 and PvMSP-142 recognize the native parasite proteins.
Affinity-purified antibodies to PvMSP-119 and PvMSP-142 were also tested for their ability to recognize the native protein. Antibodies to both proteins tested positive in an IFA against blood-stage P. vivax from an Indian isolate. Bright fluorescence was observed in P. vivax schizonts with polyclonal antibodies raised against PvMSP-119 and PvMSP-142 in an IFA (data not shown). These antibodies also recognized an ∼200-kDa native protein in blood-stage P. cynomolgi lysates (data not shown).
PvMSP-119 and PvMSP-142 induce T-cell responses in immunized mice.
We also determined the T-cell responses in mice immunized with both antigens. Table 1 summarizes the cellular responses induced upon immunization with PvMSP-119 and PvMSP-142 in BALB/c mice. Compared to the adjuvant control, both PvMSP-119 and PvMSP-142, formulated in most of the above-mentioned adjuvants, induced significant proliferation (P < 0.05 to 0.005). However, there were differences in the proliferative responses among the various immunized groups (Table 1). Interestingly, both PvMSP-119 and PvMSP-142 in alum resulted in high proliferative responses (P < 0.005 and 0.01, respectively).
TABLE 1.
Lymphoproliferative and cytokine responses in mice immunized with PvMSP-119 and PvMSP-142
| Adjuvant | Antigen | Stimulation index | Concn (pg/ml of supernatant) ofa:
|
||
|---|---|---|---|---|---|
| IL-4 | IL-5 | INF-γ | |||
| CFA | PvMSP-119 | 12 ± 1.75 | 39.06 ± 7 | 148.43 ± 17 | 2000 ± 186 |
| PvMSP-142 | 6.2 ± 0.42 | 46.81 ± 4 | 93.7 ± 12 | 1,400 ± 91 | |
| Montanide ISA 720 | PvMSP-119 | 9 ± 1.52 | 41.66 ± 8 | 406.20 ± 22 | 750 ± 89 |
| PvMSP-142 | 2.68 ± 0.35 | 62.50 ± 8 | 46.3 ± 8 | 126 ± 11 | |
| Alum | PvMSP-119 | 19 ± 2.42 | 40.12 ± 6 | 410.6 ± 19 | 300 ± 24 |
| PvMSP-142 | 11.61 ± 0.64 | 45.1 ± 5 | 48.2 ± 9 | 1,450 ± 86 | |
| ASO2A | PvMSP-119 | 2 ± 0.20 | 88.01 ± 14 | 321.45 ± 14 | 260 ± 17 |
| PvMSP-142 | 13.12 ± 0.85 | 25.0 ± 1.5 | 77.2 ± 14 | 250 ± 28 | |
| QS21 | PvMSP-119 | 24 ± 2.85 | 46.87 ± 9 | 149.20 ± 12 | 238 ± 11 |
| PvMSP-142 | 2.43 ± 0.24 | 48.29 ± 5.5 | 184 ± 17 | ND | |
| MF59 | PvMSP-119 | 5 ± 0.35 | 27.34 ± 4 | 281 ± 15 | ND |
| PvMSP-142 | ND | ND | ND | ND | |
| Control | 1 ± 0.5 | 18 ± 5 | 42 ± 4 | 27 ± 9 | |
ND, not detectable. IL-2 levels were negligible.
We found that both of the PvMSP-1 fragments produced high titers of antigen-specific IgG1 but low levels of IgG2a (P < 0.001), a pattern indicative of a prolonged Th2 response. In order to examine whether this IgG isotype response in vivo was also reflected by the cytokine response, we analyzed the pattern of cytokine production. As shown in Table 1, both of the antigens, formulated in various adjuvants, stimulated quantitatively different levels of cytokine production. PvMSP-142- and PvMSP-119-immunized mice showed significant IFN-γ production with all of the adjuvants (P < 0.005). PvMSP-119 formulated in CFA and alum elicited a higher IFN-γ response than did this protein with the other formulations (P < 0.001), whereas PvMSP-142 formulated in CFA elicited the highest IFN-γ response (P < 0.001). The induction of a Th2 response was suggested by the production of IL-4 and IL-5 by both of the antigens; levels of IL-4 were highest with PvMSP-142 formulated in Montanide ISA 720 and with PvMSP-119 formulated in ASO2A (P < 0.001). The levels of IL-2 were found to be negligible in all groups of immunized mice.
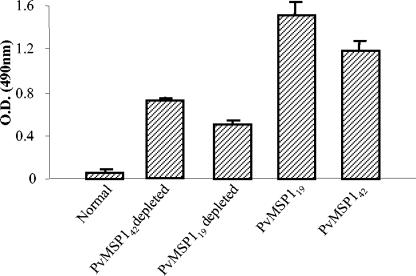
Immunodepletion assay to characterize the relative contributions of PvMSP-142- and PvMSP-119-specific epitopes in the human anti-PvMSP-1 response.
An ELISA depletion assay with pooled human sera from P. vivax-infected patients was used to determine the contributions of PvMSP-142-specific epitopes in the generation of an immune response during a natural infection. For the ELISA depletion assay, pooled patient sera were absorbed with PvMSP-119 antigen in such a way that all of the PvMSP-119 specific antibodies were lost. The reactivity of the depleted sera with PvMSP-142 antigen was compared with that of the undepleted sera at similar dilutions. As shown in Fig. 5, the patient sera depleted of PvMSP-119-specific antibodies (which showed no reactivity with PvMSP-119 after depletion) showed only about 40% reactivity with PvMSP-142 compared to the reactivity with the undepleted sera. Similarly, sera depleted of PvMSP-142-specific antibodies showed about 60% reactivity with PvMSP-119 (Fig. 5). These results suggested that the patient sera contained significant amounts of PvMSP-142-specific antibodies directed against epitopes other than those represented by the PvMSP-119 structure.
FIG. 5.
Immunodepletion assay showing relative contributions of PvMSP-142- and PvMSP-119-specific epitopes in pooled sera from P. vivax-infected patients. Error bars indicate SDs.
Similar results were obtained when sera from PvMSP-119- and PvMSP-142-immunized mice were used instead of human sera (data not shown). These data suggested that certain PvMSP-119-specific epitopes remain cryptic in the structure of PvMSP-142 and are exposed to the immune system only after secondary processing has taken place in the PvMSP-142 fragment to yield the PvMSP-119 fragment.
DISCUSSION
C-terminal fragments MSP-119 and MSP-142 are well characterized in several Plasmodium spp., including P. falciparum, P. yoelii, P. cynomolgi, and Plasmodium berghei, and have been shown to be the targets of protective immunity. In the present study, we assessed the immunogenicity in mice of E. coli-expressed PvMSP-119 and PvMSP-142 in different adjuvant formulations.
Purified recombinant PvMSP-119 and PvMSP-142 showed reactivity with their specific monoclonal as well as polyclonal antibodies. Since it has been suggested that the native conformation of the C-terminal region of MSP-1 of Plasmodium is crucial for the protective immune response (29), the conformational integrities of both proteins were established on the basis of their reactivities with P. vivax-infected patient sera. We found that the reduction of the disulfide bonds in the recombinant proteins resulted in a considerable loss of reactivity with the patient sera, suggesting that patient sera contain mostly conformation-dependent antibodies but, at the same time, that some antibodies against linear sequences of the C-terminal fragment are also present. Similar observations have been made with PfMSP-119, PfMSP-142, and sera from individuals infected with P. falciparum (16).
Several studies have demonstrated that both humoral and cellular immune responses are involved in protective immunity against malaria (32, 35, 21). One of the ways to influence the immune response to an antigen is through the use of an appropriate adjuvant (19, 39). However, in most animal studies, protective immunity with MSP-119 and MSP-142 was achieved with Freund's adjuvant, which is not acceptable for prophylactic vaccine use in humans (8, 12, 25, 37). For many years, the only adjuvant available for general use in human vaccines was alum; for many recombinant and synthetic subunit vaccines, particularly those for malaria, alum has proven to be a weak adjuvant and a poor inducer of Th1 and cytotoxic T-cell responses (1). These findings led to the development of several new adjuvant formulations, which are currently being considered for human use (1).
We tested the immunogenicities of the two PvMSP-1 fragments with six different adjuvants in BALB/c mice. We found that although both antigens produced significant immune responses in all of the adjuvants used, considerably higher antibody titers were observed with CFA, alum, and Montanide ISA 720. The high antibody response in mice to these antigens in alum came as a surprise, since homologous recombinant PfMSP-119 was found to be poorly immunogenic in alum (26). IgG isotype analysis revealed a predominance of IgG1, followed by IgG3 and IgG2. Similar observations were made in previous studies when mice were immunized with PyMSP-119 (2, 3). Although the results for IgG isotyping in a single strain of mice (BALB/c) may not be extrapolated to the response in humans, it is comforting to note that in at least two human-compatible adjuvants other than alum, an appropriate IgG isotype response was obtained. Interestingly, we found a similar IgG isotype pattern in P. vivax-infected patient sera. Passive immunity against malaria in humans has been shown to be dependent on high levels of IgG1 and IgG3 subtypes (4, 20, 35).
Both antigens showed T-cell stimulation in an in vitro assay, with stimulation indices varying depending on the adjuvant used. Cytokines play a critical role in determining the IgG subclass. IFN-γ is a product of Th1 cells, while IL-4 and IL-5 are secreted by Th2 cells. IL-4 is associated with an IgG1 response, which is an indicator of an antibody-mediated response, while IL-2 and IFN-γ are associated with the production of IgG2a, an indicator of cell-mediated immunity. The results of cytokine production (IL-4, IL-5, and IFN-γ) and IgG isotype (IgG1 > IgG3 > IgG2b > IgG2a) analyses indicated that both Th1 and Th2 subsets of T-helper cells are elicited by the recombinant antigens with most adjuvants. Similar results were reported in earlier studies with different malarial antigens (10, 14). This type of response, i.e., the activation of both Th1 and Th2 cells, is considered ideal for a blood-stage vaccine candidate antigen (27, 30, 34). In naive human volunteers, limited immunization studies with recombinant PfMSP-119 formulated in alum have shown it to induce high levels of antigen-specific Th1 (IFN-γ) and Th2 (IL-4 and IL-10) responses (24, 35).
Although both MSP-119 and MSP-142 are being developed as malaria vaccine candidate antigens, it has been argued that MSP-142, which contains all of MSP-119 at its C terminus, may be a better antigen, because it may contain additional T-helper cell epitopes in its N-terminal region (36). On the other hand, not only may MSP-119 have a smaller number of T-helper cell epitopes, but also there may be problems with their processing and presentation due to the large number of disulfide linkages that make up the two epidermal growth factor-like structures that MSP-119 represents (15). Which of these two antigens is more suitable on its own or as a component of a cocktail malaria vaccine remains a matter of debate (36). Our results showed that (i) both antigens were highly immunogenic in the various adjuvant formulations used in this study and that (ii) while PvMSP-142 immunization produced a significant amount of antibodies specific for the conformational structures represented by PvMSP-119, a certain amount of the antibody response was also focused on the structures in PvMSP-142 other than the epidermal growth factor-like domains at its own C terminus. However, it is not known whether the antibodies with the latter specificity contributed to the protective response. An antibody depletion assay with P. vivax-infected human sera, which showed a one-third reduction in the OD compared to that of the undepleted sera, may suggest that some of the epitopes are either absent or inaccessible to the immune system in the case of PvMSP-142. These results suggest that some PvMSP-119-specific epitopes may not be exposed or available when PvMSP-142 is used as an antigen. Similar results have been reported for baculovirus-expressed P. vivax and P. cynomolgi antigens based on the corresponding MSP-1 (23).
In conclusion, the results of the present study showed that E. coli-expressed PvMSP-119 and PvMSP-142 are immunogenic when formulated in various human-compatible adjuvants. More importantly, we found that these two antigens were highly immunogenic in an alum formulation, unlike the corresponding PfMSP-1 antigens, which are poorly immunogenic in this adjuvant (26). Finally, although PvMSP-142 immunization generated a substantial PvMSP-119-specific antibody response, PvMSP-119 may be a better vaccine candidate antigen, since there seems to be no compromise in the immune response, both qualitatively and quantitatively, when it is used alone. It will be useful to assess the protective efficacies of these antigens either alone or in combination with other vaccine candidate antigens and in formulations with alum or Montanide ISA 720.
Acknowledgments
We are pleased to acknowledge Shirley Longacre, Pasteur Institute, for providing monoclonal antibodies. We thank Joe Cohen, Glaxo Smith Kline Biologicals, for providing ASO2A; Charlotte Kensil, Antigenics Inc., Boston, Mass., for providing QS21; Rino Rappouli, Chiron SPA, for providing MF59; and Ghislaine Ionkoff, Seppic Inc., for providing Montanide ISA 720. We thank P. V. Lalitha and Asif Mohammed for help and Rakesh Singh for expert technical assistance in handling animals at the animal facility. We thank the World Health Organization and DBT, Government of India, for the infrastructural facility.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Aguado, T., H. Engers, T. Pang, and R. Pink. 1999. Novel adjuvants currently in clinical testing November 2-4, 1998, Fondation Merieux, Annecy, France: a meeting sponsored by the World Health Organization. Vaccine 17:2321-2328. [DOI] [PubMed] [Google Scholar]
- 2.Ahlborg, N., I. T. Ling, A. A. Holder, and E. M. Riley. 2000. Linkage of exogenous T-cell epitopes to the 19-kilodalton region of Plasmodium yoelii merozoite surface protein 1 (MSP-119) can enhance protective immunity against malaria and modulate the immunoglobulin subclass response to MSP-119. Infect. Immun. 68:2102-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahlborg, N., I. T. Ling, W. Howard, A. A. Holder, and E. M. Riley. 2002. Protective immune responses to the 42-kilodalton (kDa) region of Plasmodium yoelii merozoite surface protein 1 are induced by the C-terminal 19-kDa region but not by the adjacent 33-kDa region. Infect. Immun. 70:820-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck, H. P., I. Felger, B. Genton, N. Alexander, F. Al-Yaman, R. F. Anders, and M. Alpers. 1995. Humoral and cell-mediated immunity to the Plasmodium falciparum ring-infected erythrocyte surface antigen in an adult population exposed to highly endemic malaria. Infect. Immun. 63:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackman, M. J., H. G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion inhibiting antibodies. J. Exp. Med. 172:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns, J. M., Jr., J. M. Majarian, J. F. Young, T. M. Daly, and C. A. Long. 1989. A protective monoclonal antibody recognizes an epitope in the carboxyl-terminal cysteine rich domain in the precursor of the major merozoite surface antigen of the rodent malaria parasite Plasmodium yoelii. J. Immunol. 143:2670-2676. [PubMed] [Google Scholar]
- 7.Chang, S. P., H. L. Gibson, C. T. Lee-Ng, P. J. Barr, and G. S. N. Hui. 1992. A carboxyl-terminal fragment of Plasmodium falciparum gp 195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J. Immunol. 149:548-555. [PubMed] [Google Scholar]
- 8.Chang, S. P., S. E. Case, W. L. Gosnell, A. Hashimoto, K. Kramer, L. Tam, C. Q. Hashiro, C. M. Nikaido, and G. S. N. Hui. 1996. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect. Immun. 64:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper, J. A. 1993. Merozoite surface antigen 1 of Plasmodium. Parasitol. Today 9:50-54. [DOI] [PubMed] [Google Scholar]
- 10.Cunha, M. G., M. M. Rodrigues, and I. S. Soares. 2002. Comparison of the immunogenic properties of recombinant proteins representing the Plasmodium vivax vaccine candidate MSP-119 expressed in distinct bacterial vectors. Vaccine 20:385-396. [DOI] [PubMed] [Google Scholar]
- 11.Daly, T. M., and C. A. Long. 1995. Humoral response to a carboxyl-terminal region of the merozoite surface protein 1 plays a predominant role in controlling blood stage infection in rodent malaria. J. Immunol. 155:236-243. [PubMed] [Google Scholar]
- 12.Daly, T. M., and C. A. Long. 1996. Influence of adjuvants on protection induced by a recombinant fusion protein against malarial infection. Infect. Immun. 64:3032-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Portillo, H. A., S. Longacre, E. Khouri, and P. H. David. 1991. Primary structure of the merozoite surface antigen 1 of Plasmodium vivax reveals sequences conserved between different Plasmodium spp. Proc. Natl. Acad. Sci. USA 88:4030-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutta, S., L. A. Ware, A. Barbosa, C. F. Ockenhouse, and D. E. Lanar. 2001. Purification, characterization, and immunogenicity of a disulfide cross-linked Plasmodium vivax vaccine candidate antigen, merozoite surface protein 1, expressed in Escherichia coli. Infect. Immun. 69:5464-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egan, A., M. Waterfall, M. Pinder, A. Holder, and E. Riley. 1997. Characterization of Human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect. Immun. 65:3024-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egan, A. F., J. A. Chappel, P. A. Burghaus, J. S. Morris, J. S. McBride, A. A. Holder, D. C. Kaslow, and E. M. Riley. 1995. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP-119, the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect. Immun. 63:456-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan, A. F., J. Morris, G. Barnish, S. Allen, B. M. Greenwood, D. C. Kaslow, A. A. Holder, and E. M. Riley. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19 kDa C-terminal fragment of the merozoite surface antigen, PfMSP1. J. Infect. Dis. 173:765-769. [DOI] [PubMed] [Google Scholar]
- 18.Gibson, H. L., J. E. Tucker, D. C. Kaslow, A. U. Krettli, W. E. Collins, M. C. Kiefer, I. C. Bathurst, and P. J. Barr. 1992. Structure and expression of the gene for Pv200, a major blood stage surface antigen of Plasmodium vivax. Mol. Biochem. Parasitol. 50:325-334. [DOI] [PubMed] [Google Scholar]
- 19.Golding, B., M. Zaitseva, and H. Golding. 1994. The potential for recruiting immune responses toward type Th1 and Th2 cell help. Am. J. Trop. Med. Hyg. 50(Suppl.):33-40. [DOI] [PubMed] [Google Scholar]
- 20.Groux, H., and J. Gysin. 1990. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclasses. Res. Immunol. 141:529-542. [DOI] [PubMed] [Google Scholar]
- 21.Hirunpetcharat, C., J. H. Tian, D. C. Kaslow, N. V. Rooijen, S. Kumar, J. A. Berzofsky, L. H. Miller, and M. Good. 1997. Complete protective immunity induced in mice by immunization with 19 kDa C-terminal fragment of Plasmodium yoelii expressed in Saccharomyces cerevisiae. J. Immunol. 159:3400-3411. [PubMed] [Google Scholar]
- 22.Holder, A. A., M. J. Blackman, P. A. Burghaus, J. A. Chappel, I. T. Ling, N. McCallum-Deighton, and S. Shai. 1992. A malaria merozoite surface protein 1 (MSP-1) structure, processing and function. Mem. Inst. Oswaldo Cruz 87(Suppl. III):37-42. [DOI] [PubMed] [Google Scholar]
- 23.Holm, I., F. Nato, K. N. Mendis, and S. Longacre. 1997. Characterization of C-terminal merozoite surface protein 1 baculovirus recombinant proteins from Plasmodium vivax and Plasmodium cynomolgi as recognized by the natural anti-parasite immune response. Mol. Biochem. Parasitol. 89:313-319. [DOI] [PubMed] [Google Scholar]
- 24.Keitel, W. A., K. E. Kester, R. L. Atmar, A. C. White, N. H. Bond, C. A. Holland, U. Krzych, D. R. Palmer, A. Egan, C. Diggs, W. R. Ballou, B. F. Hall, and D. Kaslow. 2000. Phase I trial of two recombinant vaccines containing the 19 kDa carboxy terminal fragment of Plasmodium falciparum merozoite surface protein 1 (MSP-119) and T helper epitopes of tetanus toxoid. Vaccine 18:531-539. [DOI] [PubMed] [Google Scholar]
- 25.Kumar, S., A. Yadava, D. B. Keister, J. H. Tian, M. Ohl, P. Greenfield, L. H. Miller, and D. C. Kaslow. 1995. Immunogenicity and invivo efficacy of recombinant Plasmodium falciparum merozoite surface protein 1 in Aotus monkeys. Mol. Med. 1:325-332. [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar, S., W. E. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. G. Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in Aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 68:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, E. A. M., D. R. Palmer, K. L. Flanagan, W. H. Reece, K. Odhiambo, K. Marsh, M. Pinder, M. B. Gravenor, W. A. Keitel, K. E. Kester, C. Diggs, D. Kaslow, V. Apostolopoulos, W. R. Ballou, A. V. S. Hill, U. Krzych, and M. Plebanski. 2002. Induction of T helper type 1 and 2 responses to 19-kilodalton merozoite surface protein 1 in vaccinated healthy volunteers and adults naturally exposed to malaria. Infect. Immun. 70:1417-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levitus, G., and H. A. Del Portillo. 1994. Advances toward the development of an asexual blood stage MSP-1 vaccine of Plasmodium vivax. Mem. Inst. Oswaldo Cruz 89:81-84. [DOI] [PubMed] [Google Scholar]
- 29.Ling, I. T., S. A. Ogun, and A. A. Holder. 1994. Immunization against malaria with a recombinant protein. Parasite Immunol. 16:63-67. [DOI] [PubMed] [Google Scholar]
- 30.Long, C. A., T. M. Daly, P. Kima, and I. Srivastava. 1994. Immunity to erythrocytic stages of malaria parasites. Am. J. Trop. Med. Hyg. 50(Suppl.):27-32. [DOI] [PubMed] [Google Scholar]
- 31.Longacre, S., K. N. Mendis, and P. H. David. 1994. Plasmodium vivax merozoite surface protein 1 C terminal recombinant proteins in baculovirus. Mol. Biochem. Parasitol. 64:191-205. [DOI] [PubMed] [Google Scholar]
- 32.Miller, L. H., and S. L. Hoffman. 1998. Research toward vaccine against malaria. Nat. Med. 4:520-524. [DOI] [PubMed] [Google Scholar]
- 33.Perera, K. L., S. M. Hadunneti, I. Holm, S. Longacre, and K. Mendis. 1998. Baculovirus merozoite surface protein 1 C-terminal recombinant antigens are highly protective in a natural primate model for human Plasmodium vivax malaria. Infect. Immun. 66:1500-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson, A. W. T., R. S. Phillips, A. Severn, S. Moncada, and F. Y. Liew. 1993. The role of Th1 and Th2 cells in a rodent malaria infection. Science 260:1931-1934. [DOI] [PubMed] [Google Scholar]
- 35.Smith, N. C., A. Fell, and M. F. Good. 1998. The immune responses to asexual blood stages of malaria parasites. Immunology of intracellular parasitism. Chem. Immunol. 70:144-162. [DOI] [PubMed] [Google Scholar]
- 36.Stowers, A. W., V. Cioce, R. L. Shimp, M. Lawson, G. Hui, O. Muratowa, D. C. Kaslow, R. Robinson, C. A. Long, and L. H. Miller. 2001. Efficacy of two alternate vaccines based on Plasmodium falciparum merozoite surface protein 1 in an Aotus challenge trial. Infect. Immun. 69:1536-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian, J. H., S. Kumar, D. C. Kaslow, and L. H. Miller. 1997. Comparison of protection induced by immunization with recombinant proteins from different regions of merozoite surface protein 1 of Plasmodium yoelii. Infect. Immun. 65:3032-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tungpradabkul, S., and S. Panyim. 1985. Isolation of DNA and total RNA, p. 51-56. In S. Panyim, P. Wilariat, and Y. Yuthavong (ed.), Application of genetic engineering to research on tropical disease pathogens with special reference to plasmodia. World Health Organization, Geneva, Switzerland.
- 39.Yang, C., W. E. Collins, J. S. Sullivan, D. C. Kaslow, L. Xiao, and A. A. Lal. 1999. Partial protection against Plasmodium vivax blood-stage infection in Saimiri monkeys by immunization with a recombinant C-terminal fragment of merozoite surface protein 1 in block copolymer adjuvant. Infect. Immun. 67:342-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, C., W. E. Collins, L. Xiao, P. S. Patterson, R. C. Reed, R. L. Hunter, D. C. Kaslow, and A. A. Lal. 1996. Influence of adjuvants on murine immune responses against the C-terminal 19 kDa fragment of Plasmodium vivax merozoite surface protein 1 (MSP-1). Parasite Immunol. 18:547-558. [DOI] [PubMed] [Google Scholar]