Abstract
Inflammatory cytokines play an important role in human immune responses to malarial disease. However, the role of these mediators in disease pathogenesis, and the relationship between host protection and injury remains unclear. A total of 248 cases of severe Plasmodium falciparum malaria among children aged 3 months to 14 years residing in Bandiagara, Mali, were matched to cases of uncomplicated malaria and healthy controls. Using modified World Health Organization criteria for defining severe malaria, we identified 100 cases of cerebral malaria (coma, seizure, and obtundation), 17 cases of severe anemia (hemoglobin, <5 g/dl), 18 cases combined cerebral malaria with severe anemia, and 92 cases with hyperparasitemia (asexual trophozoites, >500,000/mm3). Significantly elevated levels (given as geometric mean concentrations in picograms/milliliter) of interleukin-6 (IL-6; 485.2 versus 54.1; P = <0.001), IL-10 (1,099.3 versus 14.1; P = <0.001), tumor necrosis factor alpha (10.1 versus 7.7; P = <0.001), and IL-12(p70) (48.9 versus 31.3; P = 0.004) in serum were found in severe cases versus healthy controls. Significantly elevated levels of IL-6 (485.2 versus 141.0; P = <0.001) and IL-10 (1,099.3 versus 133.9; P = <0.001) were seen in severe malaria cases versus uncomplicated malaria controls. Cerebral malaria was associated with significantly elevated levels of IL-6 (754.5 versus 311.4; P = <0.001) and IL-10 (1,405.6 versus 868.6; P = 0.006) compared to severe malaria cases without cerebral manifestations. Conversely, lower levels of IL-6 (199.2 versus 487.6; P = 0.03) and IL-10 (391.1 versus 1,160.9; P = 0.002) were noted in children with severe anemia compared to severe malaria cases with hemoglobin at >5 g/dl. Hyperparasitemia was associated with significantly lower levels of IL-6 (336.6 versus 602.1; P = 0.002). These results illustrate the complex relationships between inflammatory cytokines and disease in P. falciparum malaria.
Inflammatory cytokines play an important role in human immune responses to malaria disease, although the balance between pro- and anti-inflammatory cytokines and the pathogenic effects that can result from dysregulation are poorly understood. Malaria disease manifestations differ and appear to be regulated by age and the acquisition of immunity, host and parasite genetic polymorphisms, and regional variation. Variations in human cytokine responses and their link to malaria disease manifestations are the subject of much debate.
Differential activation of CD4+-T-cell populations and resultant cytokine activity in malaria has been well defined in murine models. Cytokine production (tumor necrosis factor alpha [TNF-α] and gamma interferon [IFN-γ]) appears necessary for the inhibition of parasitemia (6, 11, 25, 43) and stimulation of phagocytosis to enhance clearance of parasitized erythrocytes (35). Intraperitoneal injection of mice with recombinant interleukin-12 (IL-12) appears to induce IFN-γ-mediated protection against Plasmodium yoelii sporozoite challenge, and IL-12 injection before sporozoite challenge was found to protect monkeys against malaria (23, 44). Paradoxically, these cytokines are also implicated in the pathology of complicated malaria. T-cell-deficient nude mice do not develop cerebral manifestations upon parasite challenge (12). In addition, anti-TNF-α and anti-IFN-γ antibodies appear able to abolish the onset of cerebral malaria (17, 19). Of note, IL-10 has been proposed to downregulate Th1 cytokine production, resulting in a lower incidence of cerebral symptoms in mice coinfected with Plasmodium berghei and IL-10-stimulating LP-BM5 murine leukemia virus (10).
In humans, the role of inflammatory cytokines in malaria is less well defined. Levels of endogenous pyrogens, such as IL-6, IL-1β, and IL-8, are elevated in malaria disease (13, 34) and correlate with disease severity (8, 26, 28, 48, 50). TNF-α appears to be pivotal both in the early response to malaria and in late, pathological manifestations. Excess production of TNF-α is likely to be involved in the appearance of symptoms such as fever and headache associated with malaria disease (5, 30), and it has been linked to disease severity and complications (19, 28, 45). The mechanism may relate, in part, to increases in erythrocyte cytoadherence induced by proinflammatory cytokines via upregulation of adhesion molecules on vascular endothelium (22). Reverse transcriptase-PCR postmortem analysis of human brain tissue in patients who succumbed to cerebral malaria demonstrates expression of TNF-α and IL-1β (2, 41, 47). However, in contrast to observations in the murine model, monoclonal antibodies to TNF-α have been shown in humans to ameliorate fever but not the manifestations of cerebral malaria (29).
It is widely accepted that Th2 cytokines downregulate Th1-derived cytokines. Elevated levels of anti-inflammatory IL-10 have been reported in severe malaria (40, 42). Murine and in vitro studies have demonstrated IL-10's ability to inhibit TNF-α production in response to malarial antigens (21). Moreover, IL-10 has been shown in other models to prevent TNF-α-associated lethal endotoxemia (14, 24) and inhibit antigen-induced lymphoproliferation by downregulating major histocompatibility complex class II antigen expression on monocytes (9). Thus, IL-10 appears to play an important role in counteracting the potentially harmful host proinflammatory response to malaria antigens.
Most of the studies described above provide only fragmentary information in relatively small numbers of volunteers, precluding a comprehensive evaluation of the role of cytokines in the pathogenesis of the various malaria disease manifestations. Thus, in the present study we sought to further understand the complexities of cytokine responses and their role in the pathogenesis of clinical malaria by correlating cytokine responses to clinical disease, with an emphasis on patients with severe-malaria manifestations. To this end, we comprehensively examined the patterns of inflammatory cytokines [IL-1β, IL-6, IL-8, IL-10, IL-12(p70), and TNF-α] in serum by using a matched case-control format, involving a large population of African children with manifestations of severe malaria and uncomplicated malaria, as well as healthy controls.
MATERIALS AND METHODS
Study site and enrollment.
Serum was obtained from 776 Malian children (aged 3 months to 14 years) on enrollment into a case-control study evaluating risk and protective factors for severe malaria. The study site of Bandiagara (population, 13,600) is located in east central Mali in West Africa and has intense seasonal transmission (July to December) of P. falciparum malaria. The predominant ethnic group in the study area is Dogon (65%), with Peuhl (10%), Bambara (15%), and other ethnic groups (10%) also represented.
A total of 258 index cases of severe malaria from Bandiagara and surrounding areas were admitted to the Bandiagara Malaria Project ward from October 1999 to January 2003. Cases were classified as severe malaria based on modified criteria put forth by the World Health Organization (51) (Table 1). More than one clinical diagnosis for severe malaria was possible, but cerebral malaria and severe anemia were considered to be the primary defining features when they coexisted with other criteria. Each index case was age, residence, and ethnicity matched to a case of uncomplicated malaria and a healthy control.
TABLE 1.
Defining features of severe malaria (modified) as published by the World Health Organizationa
| Features of severe malaria |
|---|
| Coma (Blantyre coma scale [BCS] ≤ 2) |
| Seizure (one or more witnessed by the investigators)b |
| Obtundation (depressed consciousness with BCS >2) |
| Parasitemia, ≥500,000/mm3 |
| Lethargy or prostration (clinical judgment or child ≥7 months unable to sit unassisted) |
| Severe anemia (hemoglobin, ≤5 g/dl) |
| Respiratory distress (intercostal muscle retraction, deep breathing, grunting) |
| Hypoglycemia (glucose, ≤40 mg/dl) |
| Jaundice |
| Renal insufficiency as indicated by lack of urination for ≥1 day |
| Gross hematuria |
| State of shock (systolic blood pressure, ≤50 mm Hg; rapid pulse; cold extremities) |
| Inability to eat or drinkc |
| Repeated vomitingc |
Definition includes baseline parasitemia and one or more of the listed features.
Criteria are modified to allow enrollment for children with one witnessed seizure rather than two to err on the side of safety in this rural setting.
Definition included to avoid missing cases of impending severe illness, although these criteria were not used as the sole enrollment criteria.
The following definitions were used to match controls to the index case. Age categories were defined as 3 to 5 months, 6 to 11 months, 1 year, 2 years, 3 to 4 years, 5 to 6 years, 7 to 8 years, 9 to 10 years, 11 to 12 years, and 13 to 14 years. Residence was defined as one of eight distinct sectors of Bandiagara town or, in the case of children from villages nearby Bandiagara, that specific village. Uncomplicated malaria was defined as P. falciparum parasitemia, with an axillary temperature of ≥37.5°C, detected by active surveillance or parasitemia and symptoms leading to treatment-seeking behavior in the absence of other clear cause of fever on passive surveillance.
All controls were matched to the index case of severe malaria within 5 days of initial enrollment. Matched uncomplicated malaria controls were enrolled from the population of children presenting to the daily Bandiagara Malaria Project clinic. Healthy controls were enrolled after traveling to the home of the child with severe malaria and following a standardized routine of exiting the front entrance of a compound and making consecutive left turns until another compound with an eligible control was identified. Children were enrolled as healthy controls if they were asymptomatic for acute illness, had no evidence or history of chronic illness, and were found to be aparasitemic upon examination.
Study protocols were reviewed and approved by the local Malian Institutional Review Board, as well as the University of Maryland Institutional Review Board. Village assent was obtained from village chiefs, government officials, and traditional healers prior to study initiation. Individual informed consent was obtained from the legal guardian of each child prior to enrollment, although care for severe and uncomplicated malaria was offered regardless of study participation.
Clinical processing.
At enrollment, clinical information was taken and entered into standardized forms. Clinical and bedside physical exams were performed and diagnostic peripheral blood smears, hemoglobin, and glucose were obtained. An age- and weight-based aliquot of venous blood was obtained for study purposes. Index cases of severe malaria were classified based on modified World Health Organization criteria and treated for seizure activity, hypoglycemia, severe anemia, or additional complications accordingly. Weight-based intravenous quinine and intramuscular pyrimethamine-sulfadoxine therapy was used for the treatment of severe malaria, whereas a standard 3-day course of chloroquine was used for the treatment of uncomplicated malaria. Sulfadoxine-pyrimethamine (Fansidar) was used as second-line therapy in the event of treatment resistance upon follow-up.
Serum collection.
Patient whole blood (1 ml) was collected into sterile Eppendorf tubes on admission and prior to institution of antimalarial therapy. Blood was refrigerated at 4°C and allowed to coagulate for 4 to 6 h prior to processing via centrifugation. Sera were preserved at −70° C at the field site and transferred to the University of Maryland at Baltimore for processing by using liquid nitrogen storage containers. Samples remained frozen until inflammatory cytokine measurements were performed.
Circulating cytokine and cytokine receptor measurements.
Levels of IL-1β, IL-6, IL-8, IL-10, IL-12(p70), and TNF-α in serum were determined by utilizing cytometric bead array technology (BD Biosciences, San Diego, Calif.) and fluorescence detection by flow cytometry according to the manufacturer's recommendations with some modifications. This sensitive technique allows the detection by flow cytometry of multiple cytokines in small quantities of sample. Briefly, 40 μl of bead populations with discrete fluorescent intensities of Peridinin chlorophyll protein (PerCP)-Cy5.5 and coated with cytokine-specific capture antibodies were added to 40 μl of patient sera, and 40 μl of phycoerythrin-conjugated anti-human inflammatory cytokine antibodies. Simultaneously, standards for each cytokine (0 to 5,000 pg/ml) were likewise mixed with cytokine capture beads and phycoerythrin-conjugated reagent. The vortexed mixtures were allowed to incubate for 3 days, enhancing the lower limit of detection. Flow cytometric analysis was performed and analyzed by a single operator, and standard curves were derived from the cytokine standards. The lower limit of detection for the various cytokines evaluated ranged from 2.5 to 10 pg/ml. For results above the upper limit of detection, 1:4, 1:8, and 1:16 dilutional experiments were performed to accurately determine cytokine levels.
Statistical analysis.
Pooled analyses of differences in cytokine levels between clinical groups were performed by using two-sided Student t test for continuous variables with equal variance (SPSS 10.0; SPSS, Inc., Chicago, Ill.) and Mann-Whitney rank sum analysis for populations not normally distributed (SigmaStat 3.0; SigmaStat, Chicago, Ill.). To analyze differences in cytokine levels between matched pairs, the level for statistically significant differences (two-sided) was set at P < 0.01.
RESULTS
Patients.
Serum for cytokine measurements was available from 748 of 776 enrolled study children. Table 2 shows the characteristics at enrollment of these children; the study group consisted of 248 cases of severe malaria, 249 cases of uncomplicated malaria, and 251 healthy controls. The case fatality rate for severe malaria was 7.3% (18 of 248). Of the children who died, 7 had cerebral malaria, 5 had combined cerebral malaria and malaria associated severe anemia, 3 had severe anemia, and 3 had respiratory distress and/or symptoms of shock. The mean age of children with severe malaria was 40.5 months, although a significant difference was noted between those that survived versus those who died (41.4 months versus 29 months; P = 0.035). Of those with severe malaria, 92 (37%) were hyperparasitemic (as a sole criterion), 100 (40.3%) had cerebral malaria, 17 (6.9%) were severely anemic, 18 (7.2%) had combined cerebral malaria with severe anemia, and 21 (8.5%) met other criteria (i.e., respiratory distress, hypoglycemia, shock, prostration, etc.). Anemia was more common among those with a fatal outcome than in survivors (mean hemoglobin concentration of 6.12 g/dl versus 8.89 g/dl, respectively; P < 0.0001). The mean duration of symptoms until presentation was 2.8 days in the severe-malaria group.
TABLE 2.
Patient characteristics at enrollment in age, residence-, and ethnicity-matched severe malaria, mild malaria, and healthy control groups
| Characteristic | Severe malaria
|
Uncomplicated malaria | Healthy control | |
|---|---|---|---|---|
| Survived | Died | |||
| No. of subjects | 230 | 18 | 249 | 251 |
| Female (%) | 121 (53) | 10 (56) | 124 (50) | 117 (47) |
| Age in mo (range) | 41.1 (3-162) | 29 (8-99)a | 41.5 (6-168) | 40.6 (4.5-167) |
| Hemoglobin concn in g/dla (range) | 8.89 (2.6-13.5) | 6.12 (2.0-11.8)a | 9.5 (5.3-14.2)b | 10.6 (6.2-13.4)c |
| WBC/mm3 | 13,424 | 16,124 | 13,338 | 11,828c |
| Parasite densityd | 170,029 | 31,763a | 7,820b | 0c |
Denotes significance at the level of P < 0.05 performed between children that survived and those that died of severe disease. All analyses were performed with paired Student t test.
Paired t test significance (P < 0.05) determined between children with severe and uncomplicated malaria.
Paired t test significance (P < 0.05) determined between children with uncomplicated malaria and healthy. controls.
Data shown are the geometric means. After removal of hyperparasitemic individuals from children with severe disease, the geometric mean parasite density remained significantly elevated over children with uncomplicated disease with a mean parasite density of 34,054 asexual parasites/mm3.
Differences in cytokine levels between matched groups.
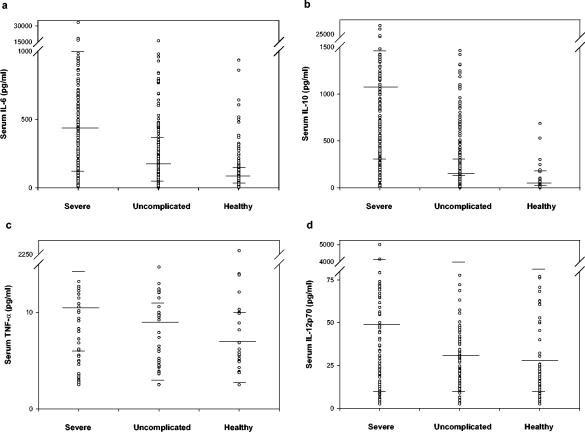
Significantly elevated levels (shown as geometric mean levels in picograms/milliliter) of proinflammatory IL-6 (485.2 versus 54.1; P < 0.001), TNF-α (10.1 versus 7.7; P < 0.001), and IL-12(p70) (48.9 versus 31.3; P = 0.004) were noted in severe cases versus healthy controls (Table 3 and Fig. 1). A borderline elevation in IL-8 (108 versus 90.2; P = 0.014) was likewise noted between these groups. An elevation in anti-inflammatory IL-10 (1,099.3 versus 14.1; P < 0.001) was seen between the severe malaria cases and healthy controls. Notably, 218 of 248 (88%) severe cases of malaria had detectable IL-6 levels in excess of 100 pg/ml, and 227 of 249 (91%) had >200 pg of IL-10/ml (data not shown). Significantly elevated levels of IL-6 (485.2 versus 141.0; P < 0.001) and IL-10 (1,099.3 versus 133.9; P < 0.001) were noted in severe malaria versus uncomplicated malaria controls (Fig. 1).
TABLE 3.
Inflammatory cytokine results between matched severe P. falciparum malaria, uncomplicated malaria, and healthy, aparasitemic controls from Bandiagara, Malia
| Cytokine | Severe malaria (n = 248)
|
Uncomplicated malaria (n = 249)
|
Healthy controls (n = 251)
|
|||
|---|---|---|---|---|---|---|
| Mean pg/ml (range) | Pb | Mean pg/ml (range) | Pc | Mean pg/ml (range) | Pd | |
| IL-1β | 15.8 (2.5-1,773) | 0.283 | 12.7 (2.5-636) | 0.401 | 14.7 (2.5-3,355) | 0.046 |
| IL-6 | 485.2 (10-33,100) | <0.001* | 141 (2.6-16,170) | <0.001* | 54.1 (2.5-11,111) | <0.001* |
| IL-8 | 108.4 (11.9-19,006) | 0.014 | 107.1 (5.0-29,071) | 0.22 | 90.2 (5.0-19,669) | 0.175 |
| IL-10 | 1,099.3 (10-27,677) | <0.001* | 133.9 (2.5-23,623) | <0.001* | 14.2 (3.3-529) | <0.001* |
| IL-12 (p70) | 48.9 (2.5-13,304) | 0.004* | 33.8 (2.5-3,798) | 0.026 | 31.3 (2.5-3,537) | 0.439 |
| TNF-α | 10.1 (2.5-150) | <0.001* | 8.8 (2.5-1,874) | 0.02 | 7.7 (2.5-2,296) | 0.047 |
*, Significant value as determined by Mann-Whitney rank sum analysis with a level of significance set at P < 0.01.
Severe versus healthy.
Severe versus uncomplicated.
Uncomplicated versus healthy.
FIG. 1.
Distribution of select serum inflammatory cytokine levels in children from Bandiagara, Mali with severe malaria and age-, residency-, and ethnicity-matched uncomplicated malaria and healthy controls. Geometric mean of cytokine levels (in pg/ml), as well as upper and lower quartiles, are indicated.
Cytokine levels in study participants with various severe-malaria manifestations.
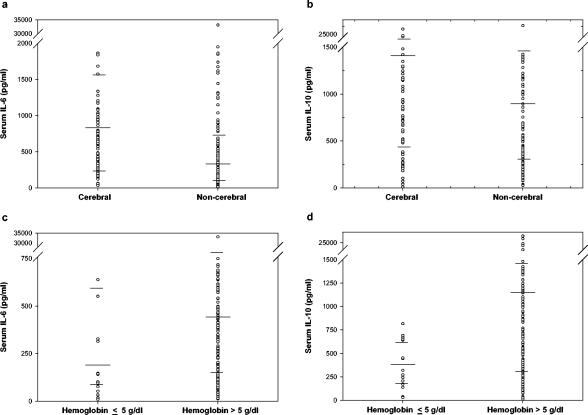
Subset analysis was performed on patients presenting with various manifestations of severe malaria (Table 4 and Fig. 2). Cerebral malaria was associated with significantly elevated levels (shown as geometric mean levels in picograms/milliliter) of IL-6 (754.5 versus 311.4; P < 0.001) and IL-10 (1,405.6 versus 868.6; P = 0.006) compared to severe malaria cases without cerebral manifestations. Likewise, similar results were found in cases of cerebral malaria combined with severe anemia with elevated levels of IL-6 (797.4 versus 311.4; P < 0.001) and IL-10 (1,430.8 versus 868.6; P = 0.003). Conversely, significantly lower levels of IL-10 (391.1 versus 1,160.9; P = 0.002) and borderline differences in IL-6 (199.2 versus 487.6; P = 0.03) were observed in children with malaria associated severe anemia compared to severe malaria cases with hemoglobin at >5 g/dl. Hyperparasitemia was associated with significantly lower levels of IL-6 (336.6 versus 602.1; P = 0.002). No significant differences were noted in cytokine levels between children that died (n = 18) and those who survived severe malaria (data not shown). Although the results did not reach statistical significance, a trend in reduced geometric mean IL-10 was noted in the children that died versus the children that survived (649.7 versus 1,145.5 pg/ml; P = 0.16). The IL-6/IL-10 ratio was not statistically different between children that died and those that survived (2.12 versus 1.98; P = 0.96).
TABLE 4.
Subset analysis of cytokine results based on criterion for enrollment as severe malaria
| Severe-malaria Criteriaa | No. of patients (n = 248) | Cytokine level (geometric mean pg/ml)c
|
|||||
|---|---|---|---|---|---|---|---|
| IL-1β | IL-6 | IL-8 | IL-10 | IL-12(p70) | TNF-α | ||
| Cerebral | 100 | 17.6 | 754.5 | 109.5 | 1,405.6 | 66.1 | 10.5 |
| Noncerebral | 130b | 14.4 | 311.4 | 102 | 868.8 | 43.6 | 9.5 |
| P | NSc | <0.001 | NS | 0.006 | NS | NS | |
| Severe anemia | 17b | 14.7 | 199.2 | 84.7 | 391.1 | 26.7 | 9.7 |
| Hemoglobin at >5 g/dl | 213 | 17.2 | 487.6 | 106.2 | 1,160.9 | 55.2 | 9.9 |
| P | NS | 0.03 | NS | 0.002 | NS | NS | |
| Cerebral/anemia | 18 | 18.8 | 797.5 | 116.2 | 1,430.8 | 55.6 | 10.9 |
| Neither | 213 | 14.4 | 311.4 | 102 | 868.8 | 43.6 | 9.5 |
| P | NS | <0.001 | NS | 0.003 | NS | NS | |
| Hyperparasitemia | 92 | 15.4 | 336.6 | 116.9 | 1,007.3 | 50.8 | 9.7 |
| <500,000/mm3 | 156 | 16.1 | 602.1 | 103.8 | 1,157.5 | 47.8 | 10.4 |
| P | NS | 0.002 | NS | NS | NS | NS | |
Significance levels were calculated by Mann-Whitney rank sum analysis.
Patients with concomitant cerebral malaria and severe anemia were eliminated from analysis.
NS, not significant.
FIG. 2.
Distribution of levels of IL-6 and IL-10 in the sera of children with subsets of severe malaria. (a and b) Cytokine levels in children with or without cerebral malaria; (c and d) cytokine levels in children with hemoglobin (hb) <5 g/dl or >5 g/dl. Geometric mean of cytokine levels (in pg/ml), as well as upper and lower quartiles, are indicated.
Age-specific cytokine levels in volunteers with various manifestations of severe malaria.
Age-specific cytokine levels in children with various manifestations of severe malaria were analyzed. Comparisons were made between cytokine levels in children ≤24 months old and children older than 24 months in the severe-malaria group as a whole, as well as by the criteria for severe disease. No significant differences were noted between median cytokine levels of any inflammatory cytokine (data not shown). The small numbers of children older than 72 months limited our ability to stratify children into other age groups.
DISCUSSION
Severe P. falciparum malaria is characterized by marked changes in cytokine production resulting from the immune response to the infection. Although the body of literature describing malaria as a systemic inflammatory illness is extensive, it is also heterogeneous and disperse, with comparisons of groups often involving few cytokines and small numbers of subjects of disparate ages, nationalities, and ethnic origins. Furthermore, the very definition of malaria is complicated with manifestations varying from mild clinical illness to coma, severe anemia, respiratory distress, and shock. Thus, we sought to comprehensively examine cytokine production in a large African cohort of age-, residence-, and ethnicity-matched children with severe or uncomplicated malaria and healthy controls and to stratify severe malaria into subsets to determine whether differences in cytokine levels in serum correlated with these varied disease manifestations. We found distinct differences in cytokine production correlating with disease severity in addition to discrete cytokine patterns in subsets of severe malaria cases. Our results suggest that cytokine production is a dynamic process. The limitations inherent in examining cytokine production at a single time point and in circulation rather than in the local microenvironments complicate the interpretation of these results. Nevertheless, insights could be gained from the cytokine differences observed between those with severe malaria and those with uncomplicated malaria and healthy controls, as well as from distinct cytokine production patterns that differed between cerebral malaria, severe anemia, and hyperparasitemia. A discussion of the significance of our observations follows.
We found significantly elevated levels of proinflammatory IL-6, IL-12(p70) and, to a lesser extent, TNF-α in the sera of the severe-malaria group compared to age-matched healthy children. In addition, anti-inflammatory IL-10 was elevated in the severe-malaria group. IL-6 is an important proinflammatory cytokine that is upregulated by TNF-α and acts in concert with other inflammatory mediators to control parasitemia (18, 48). IL-10 has an important role in immunoregulation, downregulating cytokine production (predominantly TNF-α, IL-6, and IL-12), inhibiting Th1 function, and promoting natural killer cell activity (3, 7, 39). Both IL-6 and IL-10 appear to correlate with disease severity since elevated levels were noted in the severe-malaria patients compared to the matched uncomplicated malaria cases and, similarly, in uncomplicated malaria cases compared to healthy controls (Table 3). IL-10 elevation also appears to be closely linked to IL-6 production, supporting the hypothesis that it acts in a counter-regulatory fashion (9, 21). Previous studies in which serial measurements of IL-6 and IL-10 were performed revealed an increase in the IL-6/IL-10 ratio (because of lower IL-10 levels) in samples drawn prior to death compared to paired survivors (8). Similarly, we noted a relative deficiency in IL-10, albeit not statistically significant, in children that died of severe malaria compared to children that survived, suggesting a loss of downregulatory function. However, we found no significant differences in IL-6/IL-10 ratios between these groups due to the fact that, in contrast to previous data, we found no significant elevation of IL-6 cytokine levels in children who died from severe malaria versus those who survived (data not shown), nor did we find a correlation of age to changes in cytokine levels. The small number of children that died may have prevented these findings from reaching statistical significance.
Although elevations were noted in the geometric mean values of TNF-α and IL-12(p70) in the severe-malaria group, both the absolute levels (in picograms/milliliter) and the differences between enrollment groups were small. Elevated TNF-α, produced mainly by blood monocytes and tissue macrophages, has been shown to correlate with disease severity in multiple studies (5, 8, 18, 19, 48). It is unclear whether the small differences in cytokine levels in serum noted in our study are biologically significant. To explore whether the low TNF-α levels observed in our subjects were the result of increased circulating soluble TNF-α receptors, we measured soluble TNF-α receptor I (p55/60) or TNF-α receptor II (p75/80) levels in a subset of subjects with various TNF-α levels. No significant correlations were observed between the levels of soluble TNF-α receptors and TNF-α (data not shown) in serum, indicating that high levels of TNF-α receptors were not the cause of the observed low levels of TNF-α in our study subjects.
IL-12 plays an important role in the adaptive immune response to malaria. Although a correlation with disease severity has been demonstrated, the levels of IL-12 have been found to be paradoxically lower in African children with severe malaria (31, 32, 38), possibly due to inhibition after phagocytosis of hemozoin or IL-10 induction (36). Although the differences were small, we found IL-12 to be elevated in cases of severe malaria, with little significant difference between subsets of severe malaria. The reasons for the lack of IL-1β elevation and the small TNF-α and IL-12 elevations may be the result of downregulation by IL-10. We hypothesize that an initial rise in TNF-α, IL-12, and possibly IL-1β resulted in enhanced IL-6 production, followed by increases in IL-10 production as a counter-regulatory mechanism, leading to blunting of the initial proinflammatory response. Furthermore, the delay in presentation for evaluation to clinic (mean, 2.8 days) may have contributed to the snapshot of cytokine patterns observed at the time of admission. It is also possible that the cytokine levels detected in circulation may not be a true representation of the levels present in the local microenvironments where the complex cytokine interactions leading to the induction of proinflammatory cytokines occurs.
To examine the association of proinflammatory cytokines to symptoms of severe malaria, children were stratified into those presenting with cerebral malaria (seizure, obtundation, or coma), severe anemia, or hyperparasitemia. Although cerebral malaria is believed to be at one end of the spectrum of severity, hyperparasitemia, at our site, appears to have less disease acuity, falling between that of uncomplicated malaria and severe disease. Children presenting with cerebral malaria were noted to have elevated levels of IL-6 and IL-10 compared to cases of severe malaria without cerebral manifestations. In addition, despite published reports of hyperparasitemia correlating with elevated IL-6 levels (8), we found significantly lower geometric mean levels of IL-6, substantiating our findings that this is a less acute form of severe malaria at our site and supporting our assumption that IL-6 correlates with disease severity.
Malaria-induced anemia is multifactorial, with hemolysis occurring more frequently in nonimmune children and dyserythropoiesis occurring more often in regions with frequent and recurrent infections. The role of cytokines in the development of anemia is not well understood. Murine studies have demonstrated that elevated TNF-α levels contribute to bone marrow suppression and red cell destruction (4, 46) whereas elevated IL-10 is thought to stimulate hematopoiesis (49). TNF-α elevation has been associated with anemia and high-density P. falciparum infection (45), whereas reduced IL-10 (27), and IL-10/TNF-α ratios have been demonstrated in African children with severe malaria-induced anemia (33, 37). After we stratified the severe malaria cases by enrollment criteria, those with severe anemia (without the manifestations of cerebral malaria) were noted to have lower levels of IL-10 compared to severe malaria cases with hemoglobin at >5 g/dl (P = 0.001). In addition, lower levels of IL-6 were noted in the severe-malaria group, although this observation did not meet our strict criteria of significance (P ≤ 0.01). Our result of low IL-10 levels in serum in the absence of significant elevations in TNF-α suggests a potential loss of downregulatory, anti-inflammatory function. IL-10 deficiency has been noted in patients who succumb to malaria (8). We hypothesize that the severity of disease associated with profound malaria-induced anemia is the result of a loss of host cytokine regulation. The absence of significant TNF-α elevation suggests that alternate counter-regulatory mechanisms aside from the established IL-10-TNF-α relationship may account for these findings.
Very little information is available concerning the role of IL-8 in the pathogenesis of malaria. IL-8 is a known neutrophil chemoattractant that has been shown to be elevated in a small group (n = 6) of severe, noncerebral P. falciparum adult malaria cases (13). In addition, expression of IL-8 mRNA has been demonstrated in placental malaria infection (1). We noted an increase in IL-8 levels that appeared to correlate with disease severity, although the level of IL-8 did not meet our strictly defined significance threshold (P ≤ 0.01). Thus, we conclude that it is unlikely that IL-8 plays a significant role in malaria pathogenesis.
The present study is limited by a number of factors. Cytokine levels have been demonstrated to vary based on circadian rhythm and within the time course of malaria illness. Longitudinal studies in sporozoite-challenged volunteers have demonstrated wide variation in the levels of TNF-α, IL-4, IL-6, and IFN-γ in serum (20). Although human immunodeficiency virus prevalence remains low (<2%) in this area, we were unable to rule out or diagnose concomitant bacterial infection, potentially altering the results. Although clinically apparent pneumonia or gastrointestinal infection could be diagnosed at the bedside, we did not possess culture capability to identify causative organisms of bacterial infection. Protean infections, such as nontyphoidal Salmonella spp., appear to correlate with P. falciparum infection (15, 16) and could have confounded our results, as could meningitis, the symptoms of which resemble cerebral malaria. Nevertheless, the majority of children recovered and were discharged after treatment with antimalarial medication alone, implicating malaria as the sole cause of symptoms. As mentioned above, it is unclear whether the level of cytokine detected systemically is reflective of biologically significant cytokine levels in defined microenvironments (e.g., brain, liver, and spleen) or whether a single serum measurement is reflective of a longitudinal process. Thus, additional studies are necessary to properly assess the impact of the marked differences in cytokines levels reported in this comprehensive analysis in malaria disease severity.
In summary, we have demonstrated in a large cohort of children that a complex milieu of cytokines is released with apparent feedback inhibition and cross-regulatory function. Furthermore, disease states (i.e., cerebral malaria and severe anemia) within the broadly defined severe-malaria category have unique and predictable cytokine patterns. Despite these findings, a deep understanding of the immune response to malaria remains elusive. Longitudinal studies of malaria disease states, in conjunction with studies of cytokine production by peripheral blood mononuclear cells collected from these children after in vitro incubation with malaria antigens, would add appreciably to our understanding of the role of cytokines in disease severity associated with P. falciparum infection.
Acknowledgments
This study was supported by a contract from the National Institutes of Allergy and Infectious Diseases (N01-AI-85346).
We thank the Bandiagara Malaria Project staff and the populace of Bandiagara, Mali, in West Africa, who have been very supportive of research efforts.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Abrams, E. T., H. Brown, S. W. Chensue, G. D. Turner, E. Tadesse, V. M. Lema, M. E. Molyneux, R. Rochford, S. R. Meshnick, and S. J. Rogerson. 2003. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated beta chemokine expression. J. Immunol. 170:2759-2764. [DOI] [PubMed] [Google Scholar]
- 2.Brown, H., G. Turner, S. Rogerson, M. Tembo, J. Mwenechanya, M. Molyneux, and T. Taylor. 1999. Cytokine expression in the brain in human cerebral malaria. J. Infect. Dis. 180:1742-1746. [DOI] [PubMed] [Google Scholar]
- 3.Cai, G., R. A. Kastelein, and C. A. Hunter. 1999. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-γ when combined with IL-18. Eur. J. Immunol. 29:2658-2665. [DOI] [PubMed] [Google Scholar]
- 4.Clark, I. A., and G. Chaudhri. 1988. Tumour necrosis factor may contribute to the anaemia of malaria by causing dyserythropoiesis and erythrophagocytosis. Br. J. Hematol. 70:99-103. [DOI] [PubMed] [Google Scholar]
- 5.Clark, I. A., W. B. Cowden, G. A. Butcher, and N. H. Hunt. 1987. Possible roles of tumor necrosis factor in the pathology of malaria. Am. J. Pathol. 129:192-199. [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, I. A., N. H. Hunt, G. A. Butcher, and W. B. Cowden. 1987. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J. Immunol. 139:3493-3496. [PubMed] [Google Scholar]
- 7.Conti, P., D. Kempuraj, K. Kandere, M. Di Gioacchino, R. C. Barbacane, M. L. Castellani, M. Felaco, W. Boucher, R. Letourneau, and T. C. Theoharides. 2003. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol. Lett. 86:123-129. [DOI] [PubMed] [Google Scholar]
- 8.Day, N. P., T. T. Hien, T. Schollaardt, P. P. Loc, L. V. Chuong, T. T. Chau, N. T. Mai, N. H. Phu, D. X. Sinh, N. J. White, and M. Ho. 1999. The prognostic and pathophysiologic role of pro- and anti-inflammatory cytokines in severe malaria. J. Infect. Dis. 180:1288-1297. [DOI] [PubMed] [Google Scholar]
- 9.de Waal, M. R., J. Haanen, H. Spits, M. G. Roncarolo, V. A. Te, C. Figdor, K. Johnson, R. Kastelein, H. Yssel, and J. E. de Vries. 1991. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T-cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 174:915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckwalanga, M., M. Marussig, M. D. Tavares, J. C. Bouanga, E. Hulier, J. H. Pavlovitch, P. Minoprio, D. Portnoi, L. Renia, and D. Mazier. 1994. Murine AIDS protects mice against experimental cerebral malaria: down-regulation by interleukin 10 of a T-helper type 1 CD4+-cell-mediated pathology. Proc. Natl. Acad. Sci. USA 91:8097-8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira, A., L. Schofield, V. Enea, H. Schellekens, M. P. van der, W. E. Collins, R. S. Nussenzweig, and V. Nussenzweig. 1986. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science 232:881-884. [DOI] [PubMed] [Google Scholar]
- 12.Finley, R. W., L. J. Mackey, and P. H. Lambert. 1982. Virulent Plasmodium berghei malaria: prolonged survival and decreased cerebral pathology in cell-dependent nude mice. J. Immunol. 129:2213-2218. [PubMed] [Google Scholar]
- 13.Friedland, J. S., M. Ho, D. G. Remick, D. Bunnag, N. J. White, and G. E. Griffin. 1993. Interleukin-8 and Plasmodium falciparum malaria in Thailand. Trans. R. Soc. Trop. Med. Hyg. 87:54-55. [DOI] [PubMed] [Google Scholar]
- 14.Gerard, C., C. Bruyns, A. Marchant, D. Abramowicz, P. Vandenabeele, A. Delvaux, W. Fiers, M. Goldman, and T. Velu. 1993. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J. Exp. Med. 177:547-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham, S. M., C. A. Hart, E. M. Molyneux, A. L. Walsh, and M. E. Molyneux. 2000. Malaria and Salmonella infections: cause or coincidence? Trans. R. Soc. Trop. Med. Hyg. 94:227. [DOI] [PubMed] [Google Scholar]
- 16.Graham, S. M., A. L. Walsh, E. M. Molyneux, A. J. Phiri, and M. E. Molyneux. 2000. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans. R. Soc. Trop. Med. Hyg. 94:310-314. [DOI] [PubMed] [Google Scholar]
- 17.Grau, G. E., H. Heremans, P. F. Piguet, P. Pointaire, P. H. Lambert, A. Billiau, and P. Vassalli. 1989. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc. Natl. Acad. Sci. USA 86:5572-5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grau, G. E., P. F. Piguet, P. Vassalli, and P. H. Lambert. 1989. Tumor-necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol. Rev. 112:49-70. [DOI] [PubMed] [Google Scholar]
- 19.Grau, G. E., T. E. Taylor, M. E. Molyneux, J. J. Wirima, P. Vassalli, M. Hommel, and P. H. Lambert. 1989. Tumor necrosis factor and disease severity in children with falciparum malaria. N. Engl. J. Med. 320:1586-1591. [DOI] [PubMed] [Google Scholar]
- 20.Harpaz, R., R. Edelman, S. S. Wasserman, M. M. Levine, J. R. Davis, and M. B. Sztein. 1992. Serum cytokine profiles in experimental human malaria: relationship to protection and disease course after challenge. J. Clin. Investig. 90:515-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho, M., M. M. Sexton, P. Tongtawe, S. Looareesuwan, P. Suntharasamai, and H. K. Webster. 1995. Interleukin-10 inhibits tumor necrosis factor production but not antigen-specific lymphoproliferation in acute Plasmodium falciparum malaria. J. Infect. Dis. 172:838-844. [DOI] [PubMed] [Google Scholar]
- 22.Ho, M., and N. J. White. 1999. Molecular mechanisms of cytoadherence in malaria. Am. J. Physiol. 276:C1231-C1242. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman, S. L., J. M. Crutcher, S. K. Puri, A. A. Ansari, F. Villinger, E. D. Franke, P. P. Singh, F. Finkelman, M. K. Gately, G. P. Dutta, and M. Sedegah. 1997. Sterile protection of monkeys against malaria after administration of interleukin-12. Nat. Med. 3:80-83. [DOI] [PubMed] [Google Scholar]
- 24.Howard, M., T. Muchamuel, S. Andrade, and S. Menon. 1993. Interleukin 10 protects mice from lethal endotoxemia. J. Exp. Med. 177:1205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jahiel, R. I., J. Vilcek, and R. S. Nussenzweig. 1970. Exogenous interferon protects mice against Plasmodium berghei malaria. Nature 227:1350-1351. [DOI] [PubMed] [Google Scholar]
- 26.Kern, P., C. J. Hemmer, J. Van Damme, H. J. Gruss, and M. Dietrich. 1989. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am. J. Med. 87:139-143. [DOI] [PubMed] [Google Scholar]
- 27.Kurtzhals, J. A., V. Adabayeri, B. Q. Goka, B. D. Akanmori, J. O. Oliver-Commey, F. K. Nkrumah, C. Behr, and L. Hviid. 1998. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351:1768-1772. [DOI] [PubMed] [Google Scholar]
- 28.Kwiatkowski, D., A. V. Hill, I. Sambou, P. Twumasi, J. Castracane, K. R. Manogue, A. Cerami, D. R. Brewster, and B. M. Greenwood. 1990. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336:1201-1204. [DOI] [PubMed] [Google Scholar]
- 29.Kwiatkowski, D., M. E. Molyneux, S. Stephens, N. Curtis, N. Klein, P. Pointaire, M. Smit, R. Allan, D. R. Brewster, G. E. Grau, et al. 1993. Anti-TNF therapy inhibits fever in cerebral malaria. Q. J. Med. 86:91-98. [PubMed] [Google Scholar]
- 30.Kwiatkowski, D., and M. Nowak. 1991. Periodic and chaotic host-parasite interactions in human malaria. Proc. Natl. Acad. Sci. USA 88:5111-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malaguarnera, L., and S. Musumeci. 2002. The immune response to Plasmodium falciparum malaria. Lancet Infect. Dis. 2:472-478. [DOI] [PubMed] [Google Scholar]
- 32.Malaguarnera, L., S. Pignatelli, J. Simpore, M. Malaguarnera, and S. Musumeci. 2002. Plasma levels of interleukin-12 (IL-12), interleukin-18 (IL-18) and transforming growth factor beta (TGF-β) in Plasmodium falciparum malaria. Eur. Cytokine Netw. 13:425-430. [PubMed] [Google Scholar]
- 33.May, J., B. Lell, A. J. Luty, C. G. Meyer, and P. G. Kremsner. 2000. Plasma interleukin-10:tumor necrosis factor (TNF)-alpha ratio is associated with TNF promoter variants and predicts malarial complications. J. Infect. Dis. 182:1570-1573. [DOI] [PubMed] [Google Scholar]
- 34.Mshana, R. N., J. Boulandi, N. M. Mshana, J. Mayombo, and G. Mendome. 1991. Cytokines in the pathogenesis of malaria: levels of IL-1β, IL-4, IL-6, TNF-α, and IFN-γ in plasma of healthy individuals and malaria patients in a holoendemic area. J. Clin. Lab. Immunol. 34:131-139. [PubMed] [Google Scholar]
- 35.Newsome, F. 1984. Increased phagocytosis of non-parasitized red cells in Plasmodium berghei malaria. Ann. Trop. Med. Parasitol. 78:323-325. [DOI] [PubMed] [Google Scholar]
- 36.O'Garra, A., and N. Arai. 2000. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 10:542-550. [DOI] [PubMed] [Google Scholar]
- 37.Othoro, C., A. A. Lal, B. Nahlen, D. Koech, A. S. Orago, and V. Udhayakumar. 1999. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J. Infect. Dis. 179:279-282. [DOI] [PubMed] [Google Scholar]
- 38.Perkins, D. J., J. B. Weinberg, and P. G. Kremsner. 2000. Reduced interleukin-12 and transforming growth factor-β1 in severe childhood malaria: relationship of cytokine balance with disease severity. J. Infect. Dis. 182:988-992. [DOI] [PubMed] [Google Scholar]
- 39.Pestka, S., C. D. Krause, D. Sarkar, M. R. Walter, Y. Shi, and P. B. Fisher. 2004. Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 22:929-979. [DOI] [PubMed] [Google Scholar]
- 40.Peyron, F., N. Burdin, P. Ringwald, J. P. Vuillez, F. Rousset, and J. Banchereau. 1994. High levels of circulating IL-10 in human malaria. Clin. Exp. Immunol. 95:300-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porta, J., A. Carota, G. P. Pizzolato, E. Wildi, M. C. Widmer, C. Margairaz, and G. E. Grau. 1993. Immunopathological changes in human cerebral malaria. Clin. Neuropathol. 12:142-146. [PubMed] [Google Scholar]
- 42.Sarthou, J. L., G. Angel, G. Aribot, C. Rogier, A. Dieye, B. A. Toure, B. Diatta, P. Seignot, and C. Roussilhon. 1997. Prognostic value of anti-Plasmodium falciparum-specific immunoglobulin G3, cytokines, and their soluble receptors in West African patients with severe malaria. Infect. Immun. 65:3271-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schofield, L., A. Ferreira, R. Altszuler, V. Nussenzweig, and R. S. Nussenzweig. 1987. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J. Immunol. 139:2020-2025. [PubMed] [Google Scholar]
- 44.Sedegah, M., F. Finkelman, and S. L. Hoffman. 1994. Interleukin 12 induction of interferon gamma-dependent protection against malaria. Proc. Natl. Acad. Sci. USA 91:10700-10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaffer, N., G. E. Grau, K. Hedberg, F. Davachi, B. Lyamba, A. W. Hightower, J. G. Breman, and N. D. Phuc. 1991. Tumor necrosis factor and severe malaria. J. Infect. Dis. 163:96-101. [DOI] [PubMed] [Google Scholar]
- 46.Taverne, J., N. Sheikh, J. B. De Souza, J. H. Playfair, L. Probert, and G. Kollias. 1994. Anaemia and resistance to malaria in transgenic mice expressing human tumour necrosis factor. Immunology 82:397-403. [PMC free article] [PubMed] [Google Scholar]
- 47.Udomsangpetch, R., S. Chivapat, P. Viriyavejakul, M. Riganti, P. Wilairatana, E. Pongponratn, and S. Looareesuwan. 1997. Involvement of cytokines in the histopathology of cerebral malaria. Am. J. Trop. Med. Hyg. 57:501-506. [DOI] [PubMed] [Google Scholar]
- 48.Urquhart, A. D. 1994. Putative pathophysiological interactions of cytokines and phagocytic cells in severe human falciparum malaria. Clin. Infect. Dis. 19:117-131. [DOI] [PubMed] [Google Scholar]
- 49.Van Vlasselaer, P., N. Falla, H. R. Van Den, J. Dasch, and M. R. de Waal. 1995. Interleukin-10 stimulates hematopoiesis in murine osteogenic stroma. Clin. Orthop. 313:103-114. [PubMed] [Google Scholar]
- 50.Wenisch, C., K. F. Linnau, S. Looareesuwan, and H. Rumpold. 1999. Plasma levels of the interleukin-6 cytokine family in persons with severe Plasmodium falciparum malaria. J. Infect. Dis. 179:747-750. [DOI] [PubMed] [Google Scholar]
- 51.World Health Organization. 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed] [Google Scholar]