Abstract
The ability of Enterococcus faecalis clinical isolates to adhere to immobilized extracellular matrixes (ECMs) coating the walls of microtiter plates was examined by microscopy. The ECMs consisted of fibronectin, laminin, collagen types I, II, IV, and V, fibrinogen, and lactoferrin. With the exception of fibrinogen, each isolate showed a different level of adherence to each of the ECMs. No significant level of adherence to fibrinogen was observed for any isolate. The tissue-specific adhesive strains AS11, AS12, AS14, AS15, HT11, and HT12, which showed highly efficient adherence to human bladder carcinoma T24 cells and human bladder epithelial cells, showed strong adherence to fibronectin, laminin, and collagen type I, II, IV, and V ECMs, and the levels were greater than 104 cells/mm2 of well surface coated by ECM. None of the isolates that showed little adherence to human bladder carcinoma T24 cells showed efficient adherence to all the ECMs. The levels of adherence of gelatinase-producing isolates to the collagens were lower than the levels of adherence of gelatinase-negative isolates. When tissue-specific adhesive strains that adhered strongly to each ECM were preincubated with fibronectin, the adherence of the strains to fibronectin was inhibited, but the adherence of the strains to collagen type IV was not inhibited. Likewise, preincubation with collagen type IV inhibited adherence to collagen type IV but not adherence to fibronectin. All of the E. faecalis isolates were shown to carry the ace gene by PCR analysis performed with specific primers for collagen binding domain A of ace. The ace gene encodes Ace (adhesin of collagen from enterococci). The prtF gene of group A streptococci, which encodes the fibronectin binding protein of group A streptococci, was not detected in the tissue-specific adhesive strains by Southern analysis performed with the prtF probe of the Streptococcus pyogenes JRS4 strain. Mutants with altered collagen binding were isolated by insertion of Tn916 into the chromosome of tissue-specific adhesive strain AS14. The number of mutant adhesive bacterial cells that adhered to collagen and also to laminin was 1 or 2 orders lower than the number observed for the wild-type strain, but the level of adherence to fibronectin remained the same as that of the wild-type strain.
Enterococci are commensal organisms found in humans and livestock, and they colonize the gastrointestinal tract, vaginal cavity, or oral cavity as part of the normal flora. Enterococci are opportunistic pathogens that cause infections in patients compromised by severe underlying disease. Enterococci are the major organisms that cause hospital-acquired infections, such as urinary tract infections, bloodstream infections, endocardium infections, wound infections, and infections related to the use of implanted devices (14, 15, 19, 21, 24, 25, 26, 43).
Enterococci are recognized as facultative pathogens which possess an intermediate level of virulence compared to streptococci and lactococci (1, 10). Secreted substances or substances (adhesins) in the bacterial cell surface of Enterococcus faecalis could act as virulence factors that undermine the commensal relationship in a compromised host. Substances encoded by the E. faecalis pheromone-responding plasmids are related to the virulence of E. faecalis (2, 3, 4, 6, 10, 17, 21). The pheromone-responsive plasmid pAD1 or pAD1-like plasmids encode a β-homolysin-bacteriocin (cytolysin [Cyl]) mediated by the same genetic determinant (11, 17, 20). The cytolysin is also encoded on the chromosome (16), and it contributes to the pathogenesis of E. faecalis infection in animal models (10, 19). In addition to cytolysin, the aggregation substance, which is encoded in pAD1 and is produced on the cell surface by induction of E. faecalis carrying pAD1 with pheromone (2, 6, 7, 41), has been shown to enhance adherence to renal tubular cells in a cell culture model, and it has also been shown to enhance internalization by cultured intestinal epithelial cells (23, 28).
Infecting microorganisms adhere to tissues of the host in the initial event of most infectious diseases. Many pathogenic bacteria adhere to extracellular matrixes (ECMs) via adhesins located on the bacterial cell surface (29). The ECMs of mammalian tissues are composed of a variety of macromolecules, including structural glycoproteins (i.e., collagen, laminin, fibronectin, fibrinogen, and lactoferrin) and proteoglycan (39). Collagen, laminin, and fibronectin are constituents of ECMs, and collagen is the major macromolecule and the single most abundant protein among these constituents (39). When present as a commensal organism, E. faecalis might possess many factors that are capable of interaction with the mucosal surfaces of host cells. It is highly probable that some of these factors may contribute to, or develop as, factors for adherence to the ECM layer of host cells during E. faecalis infections. Studies of the adherence of E. faecalis to ECMs are limited. In previous studies of the adherence of E. faecalis isolates to ECMs, workers have examined the binding of the isolates to soluble or immobilized ECMs (10). Reports of the adherence of E. faecalis isolates to each of the different ECMs have shown that there are a variety of phenotypes among the different strains (10). One of the E. faecalis adhesins that bind to ECMs, the collagen/laminin binding adhesin, has been identified, and the determinant (ace) has been cloned and characterized (27, 30, 40). The E. faecalis ace gene usually encodes a putative 31-amino-acid N-terminal signal sequence, followed by a 335-amino-acid nonrepetitive A domain, a B domain composed of various numbers of tandemly repeated 47-residue units with >90% identity, a cell wall-associated domain rich in proline residues that contains the cell wall-anchoring LPXTG consensus sequence, and a hydrophobic transmembrane region consisting of 18 amino acids, followed by a short cytoplasmic tail representing the carboxy-terminal end of the protein. The ace gene is present in all of the E. faecalis isolates that have been examined (30). The phenotypes for adherence of the isolates to immobilized collagen are different for the individual isolates (27, 40).
It is highly probable that the levels or phenotypes of adherence to the host tissue are different or variable for different E. faecalis isolates, and multiple factors might be necessary for tissue-specific adherence by E. faecalis. Scanning electron microscopy has been used to quantify the adherence of E. faecalis clinical strains, and strains capable of highly efficient adherence to human culture cells have been identified among clinical isolates (34). In this study, strains that show highly efficient adhesion to human culture cells and other clinical isolates were examined for the ability to bind to the ECMs.
MATERIALS AND METHODS
Bacteria and medium.
A total of 43 E. faecalis clinical strains were used in this study. Some of these 43 strains (strains AS11, AS12, AS14, AS15, HT11, and HT12) are tissue-specific adherent strains that show highly efficient adhesion to human bladder carcinoma T24 cells (34). The other strains are strains that exhibit inefficient adhesion to human bladder carcinoma T24 cells. The laboratory strains used were E. faecalis FA2-2 (Rifr Fusr) (4), E. faecalis OG1S (gelatinase positive) (6), E. faecalis OG1X, which is an isogenic gelatinase-negative derivative of OG1S (18), and Streptococcus pyogenes strain JRS4 (13, 32). The medium used in this study was Todd-Hewitt broth (Difco).
Preparation of bacterial cells for adherence assay.
An E. faecalis strain was cultured overnight in Todd-Hewitt broth at 37°C, and the cells were washed three times with phosphate-buffered saline (PBS) and suspended at concentrations of 3 × 108 to 6 × 108 cells per ml in PBS containing 0.1% Tween 80 and 0.1% bovine serum albumin (BSA).
Assay of adherence to ECM proteins.
ECMs were immobilized on the surfaces of microtiter dish wells as previously described (40). Each ECM solution contained 20 μg of ECM per ml of PBS. A 50-μl ECM solution was added to a microtiter well to coat the surface with ECM, and the microtiter dish was then incubated at 4°C overnight. The solution was decanted, blocked with 200 μl of 0.2% BSA in PBS at 4°C for 2 h, and washed three times with PBS. A 50-μl bacterial suspension was added to each well and incubated for 2 h at room temperature. The wells were washed three times with PBS containing 0.1% Tween 80 and 0.1% BSA. The number of bacterial cells that adhered to the surface of a well was determined with a microscope (Nikon, Tokyo, Japan). Five fields of vision were chosen at random for microscopic observation of each well surface, and the number of bacteria in each field was determined. Each experiment was performed more than three times.
Generation of Tn916 insertion mutants with altered adherence to collagen.
Derivatives produced by insertion of Tn916 into E. faecalis AS14 were generated as previously described (9). E. faecalis CG110 (9) was used as a donor in a 4-h filter mating with the recipient strain E. faecalis AS14SS, which is a streptomycin- and spectinomycin-resistant derivative of E. faecalis AS14 (34). Transconjugants were selected on Todd-Hewitt agar plates containing 6 μg of tetracycline per ml, 500 μg of streptomycin per ml, and 250 μg of spectinomycin per ml. To avoid selecting derivatives originating from the same mutant and to select mutants in which Tn916 was inserted into different sites, 6,200 independent mating experiments were performed, and one transconjugant was selected at random from each experiment, which resulted in 6,200 E. faecalis AS14SS::Tn916 derivatives. The adherence of each of the E. faecalis AS14SS::Tn916 derivatives to collagen type IV was examined.
Probe for prtF (determinant for fibronectin binding protein of S. pyogenes).
Plasmids pPTF5 (12) and pPTF54 (33) were kindly provided by M. Caparon and N. Okada. Plasmid pPTF5 contains the 2.9-kb EcoRV fragment of S. pyogenes JRS4 chromosomal DNA, which encodes the prtF determinant (12, 13). Plasmid pPTF54 contains a 0.6-kbp PCR product which encodes the fibronectin binding domain of prtF of S. pyogenes JRS4 (33).
Analysis of inhibition of adherence.
An E. faecalis strain was cultured overnight at 37°C, washed three times with PBS, and suspended in PBS at a concentration of 3 × 108 to 6 × 108 cells per ml. Then 100 μl of ECM protein (100 μg/ml) was added to 100 μl of bacterial cells and incubated for 1 h at 25°C (room temperature). After incubation, the cells were washed three times with PBS and then suspended in PBS containing Tween 80 (0.1%) and BSA (0.1%) at a concentration of 3 × 108 to 6 × 108 cells per ml. Then 50 μl of the bacterial cells was added to a well coated with an ECM and incubated for 2 h at 25°C (room temperature). The well was then washed three times with PBS containing Tween 80 (0.1%) and BSA (0.1%), and then the number of bacterial cells adhering to the well surface was determined with a microscope.
Chemicals.
Fibronectin, collagen types I, II, IV, and V, laminin, lactoferrin, and fibrinogen were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Southern hybridization and nucleotide sequence analysis of PCR product.
Southern hybridization was performed with the digoxigenin-based nonradioisotope system of Boehringer GmbH (Mannheim, Germany), and all procedures were based on the procedures described in the manufacturer's manual and standard protocols (30). Nucleotide sequence analysis was carried out as previously described (31, 37, 38). The nucleotide sequences of the pair of primers used for the collagen binding domain of the ace gene were as follows: ACEFP1, 5′-GAATTGAGCAAAAGTTCAATCGTT-3′; and ACERP1, 5′-GTCTGTCTTTTCACTTGTTTCTGT-3′. The following PCR primers corresponded to the nucleotide sequences of the external region of the ace gene; ACEFP2 (5′-TGGCCTGGGAAACAAAATGAGG-3′) and ACERP2 (5′-GCAGTACTTACCTTACGTTAGC-3′). These primers were used for analysis of the location of the Tn916 insertion into the ace gene.
Southern hybridization with the prtF gene probe, which encodes protein F of the fibronectin binding protein of group A streptococci, was performed under relatively low-stringency conditions. Under the lowest-stringency conditions, an extremely strong hybridization band that hybridized specifically to the prtF probe and several nonspecific bands were detected in the chromosomal DNA of S. pyogenes JRS4, which was used as the positive reference DNA.
RESULTS
Adherence of E. faecalis isolates to immobilized ECMs.
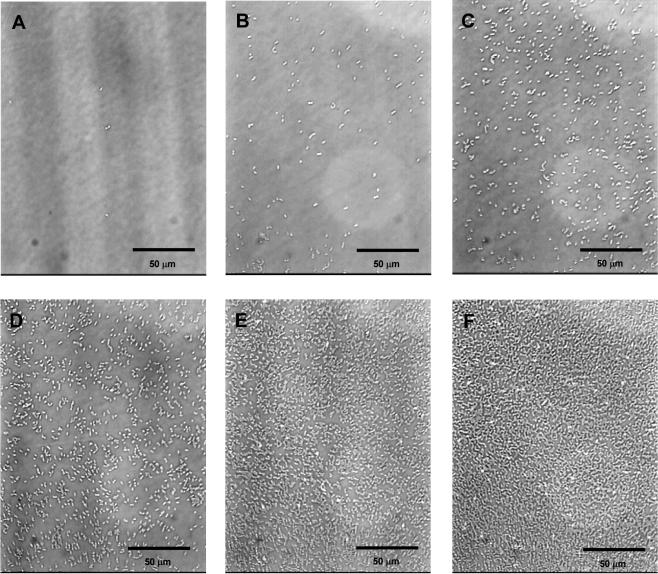
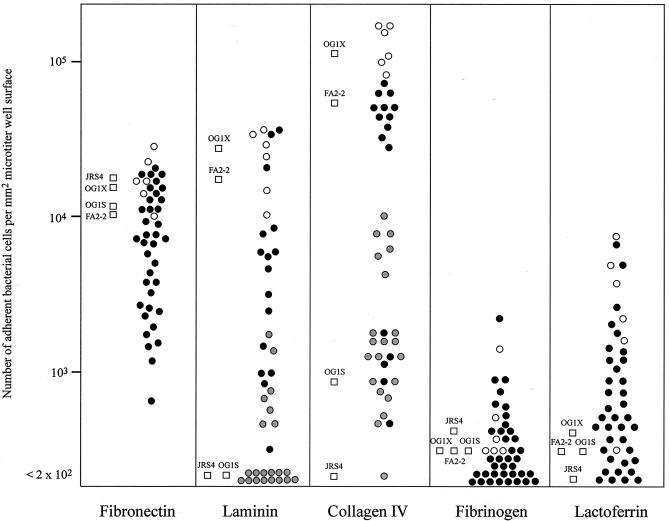
The adherence of E. faecalis clinical isolates to immobilized ECMs in plastic microtiter wells was examined by microscopy. Typical adherence results are shown in Fig. 1. The adherence of each of the isolates to each ECM and the adherence of each of the isolates to different ECMs are shown in Fig. 2 and Table, respectively. Figure 2 shows the adherence of the 43 clinical isolates examined, and Table 1 shows representative results for 30 of the 43 isolates. The levels of adherence to the ECMs for each isolate varied from no adherence to a high level of adherence (Fig. 2). By using a ×400 lens, the number of cells showing adherence to serum albumin in the absence of ECMs was determined. Either no adherence or a level of adherence that was less than 103 cells/mm2 was observed (Fig. 1A). In this assay, the values for no adherence or adherence of one or a few cells in a microscopic field were approximately 102 adherent cells/mm2 or less. Thus, the results indicated that a binding level of more than 103 cells/mm2 was significant with this assay method.
FIG. 1.
Typical results for adherence to collagen ECM. (A) No adherence or adherence of a few cells to serum albumin in the absence of ECM. The number of adherent cells was 1.5 × 102 cells/mm2 (<103 cells/mm2). (B to F) Different levels of adherence of the different strains to collagen type IV. The numbers of adherent cells were 4.4 × 103 cells/mm2 (B), 2.0 × 104 cells/mm2 (C), 4.5 × 104 cells/mm2 (D), 1.2 × 105 cells/mm2 (E), and 1.7 × 105 cells/mm2 (F).
FIG. 2.
Adherence of E. faecalis strains to each ECM. The open circles represent strains that adhere efficiently to human bladder carcinoma T24 cells. The other circles represent strains that adhere inefficiently to human bladder carcinoma T24 cells. The gray circles for laminin and collagen type IV adherence represent gelatinase-producing strains. The squares represent laboratory strains.
TABLE 1.
Adherence of E. faecalis isolates to ECMs
| Strain | Sourcea | Phenotype
|
Adherence to ECMsd
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gelatinaseb | Acec | Fibronectin | Laminin | Collagen type I | Collagen type II | Collagen type IV | Collagen type V | Fibrinogen | Lactoferrin | ||
| Strains with efficient adherence to T24 cells | |||||||||||
| AS11 | Urine | − | + | ++++ | ++ | +++ | +++ | ++++ | ++ | − | ++ |
| AS12 | Urine | − | + | +++ | ++ | +++ | +++ | ++++ | ++ | +/− | + |
| AS14 | Urine | − | + | +++ | +++ | ++++ | ++++ | ++++ | ++ | − | +/− |
| AS15 | Urine | − | + | +++ | +++ | ++++ | +++ | ++++ | ++ | + | ++ |
| HT11 | Urine | − | + | +++ | +++ | +++ | +++ | ++++ | ++ | +/− | + |
| HT12 | − | + | +++ | ++ | +++ | +++ | ++++ | ++ | ++ | ++ | |
| Strains with inefficient adherence to T24 cells | |||||||||||
| U09 | − | + | +++ | +++ | ++ | +++ | +++ | ++ | ++ | ++ | |
| U35 | − | + | +++ | ++ | ++ | +++ | +++ | + | +/− | +/− | |
| U66 | Urine | − | + | + | +++ | +++ | +++ | +++ | + | − | − |
| U54 | − | + | +/− | ++ | +++ | +++ | +++ | + | +/− | +/− | |
| U55 | − | + | + | +++ | +++ | +++ | +++ | +/− | + | + | |
| U52 | Urine | − | + | ++ | − | +/− | + | +/− | + | + | + |
| U19 | − | + | +++ | +/− | + | + | +/− | − | − | + | |
| U24 | − | + | +++ | +/− | + | + | + | − | +/− | +/− | |
| U58 | Urine | − | + | +/− | +/− | + | + | + | +/− | − | + |
| U02 | − | + | + | − | +/− | − | +/− | − | − | +/− | |
| U01 | Urine | + | + | ++ | − | +/− | +/− | ++ | +/− | +/− | ++ |
| U21 | Urine | + | + | ++ | − | + | +/− | ++ | +/− | − | +/− |
| U23 | Urine | + | + | +++ | − | + | + | ++ | +/− | − | − |
| U48 | Urine | + | + | ++ | − | ++ | + | ++ | + | − | +/− |
| U49 | Urine | + | + | +++ | − | +/− | ++ | +/− | +/− | +/− | + |
| U04 | + | + | ++ | − | + | − | + | +/− | +/− | +/− | |
| U14 | + | + | ++ | − | + | +/− | +/− | − | − | + | |
| U30 | Urine | + | + | + | − | − | − | + | − | − | − |
| U32 | Urine | + | + | +/− | − | ++ | + | + | + | +/− | +/− |
| U33 | Urine | + | + | − | − | + | +/− | + | + | − | +/− |
| U40 | Urine | + | + | + | − | ++ | + | +/− | + | + | + |
| U43 | Urine | + | + | + | − | + | +/− | + | + | − | +/− |
| U03 | + | + | +/− | − | +/− | +/− | + | +/− | +/− | +/− | |
| U11 | + | + | + | − | + | +/− | +/− | − | +/− | + | |
| Laboratory strains | |||||||||||
| FA2-2 | − | + | +++ | + | ++ | + | +++ | +/− | +/− | +/− | |
| OG1X | − | + | +++ | +++ | +++ | +++ | ++++ | ++ | +/− | + | |
| OG1S | + | + | +++ | − | + | − | +/− | +/− | +/− | +/− | |
| S. pyogenes JRS4 | − | − | +++ | − | − | +/− | +/− | − | + | − | |
No entry indicates a source other than urine.
−, gelatinase negative; +, gelatinase producing.
+, ace gene present as determined by PCR analysis; −, ace gene not present.
For the number of cells adhering to collagens, fibrinogen, and lactoferrin: − and +/−, less than 103 cells/mm2; +, 103 to 4.0 × 103 cells/mm2; ++, 4.0 × 103 to 2.0 × 104 cells/mm2; +++, 2.0 × 104 to 7.0 × 104 cells/mm2; ++++, more than 7.0 × 104 cells/mm2. For the number of cells adhering to fibronectin: − and +/−, less than 103 cells/mm2; +, 103 to 3.0 × 103 cells/mm2; ++, 3.0 × 103 to 1.0 × 104 cells/mm2; +++, 1.0 × 104 to 3.0 × 104 cells/mm2; ++++, more than 3.0 × 104 cells per mm2.
Strains AS11, AS12, AS14, AS15, HT11, and HT12, which showed strong adhesion to human carcinoma T24 cells, strongly adhered to fibronectin, laminin, and collagen types I, II, IV, and V (Table 1). The level of adherence of each of these strains to each of the ECMs was greater than 104 cells/mm2. These strains showed the highest levels of adherence to collagen type IV; for this compound the levels of adherence were greater than 7 × 104 cells/mm2.
In general, the strains showing inefficient adherence to human bladder carcinoma T24 cells did not adhere to fibronectin, laminin, and collagens; the only exception was strain U09 (Table 1). However, the level of adherence of U09 to the collagens was lower than the levels observed with the strains that adhered efficiently.
Almost all of the isolates adhered to fibronectin. The numbers of adherent bacterial cells were greater than 103 cells/mm2 (Fig. 2). Many of the isolates adhered to laminin or to collagen type I, II, IV, or V (Fig. 2). The levels of adherence of the gelatinase-producing isolates to the collagens were lower than the levels observed with the gelatinase-negative isolates (Fig. 2). More than one-half of the gelatinase-producing isolates (14 of the 21 gelatinase-producing isolates) did not adhere to laminin. Seven of the gelatinase-producing isolates adhered to collagen type IV at a level of 103 cells/mm2 or less (Fig. 2). The data for these isolates are not included in Table 1. The gelatinase-producing laboratory strain E. faecalis OG1S did not adhere efficiently to collagens or laminin. The gelatinase-negative strain E. faecalis OG1X derived from OG1S showed strong adherence to laminin or collagen at a level greater than 104 cells/mm2. These results suggested that gelatinase inhibited the adherence of gelatinase-producing strains to collagens or laminin.
Fifteen (32%) of the 43 isolates adhered to lactoferrin at a level of more than 103 cells/mm2, but the numbers of adherent cells observed for these isolates were less than 104 cells/mm2 (Fig. 2).
With the exception of two isolates, all the isolates showed levels of adherence to fibrinogen of less than 103 cells/mm2 (Fig. 2).
Adherence of E. faecalis strains harboring a pheromone-responsive plasmid to immobilized ECMs.
In a previous report (34), isolation of the tissue-specific adherent E. faecalis strains AS11, AS12, AS13, AS14, and AS15, which show highly efficient adhesion to human bladder carcinoma T24 cells, was described. Two of these strains, AS11 and AS13, harbor the pheromone-responsive plasmid pAS11 (60 kb, Cyl) and the constitutive aggregation plasmid pAS13 (61.4 kb, Tetr), which encode the aggregation substance for the mating aggregates of the pheromone-responsive plasmid (3, 6). Neither of these plasmids, when transferred into the laboratory strains E. faecalis FA2-2 and OG1X, conferred the efficiently adhesive phenotype to human bladder carcinoma T24 cells (34).
The E. faecalis FA2-2 or OG1X strain harboring pAS11 was also examined for its adherence to immobilized ECMs. The level of adherence of E. faecalis FA2-2(pAS11) or OG1X(pAS11) to each ECM was the same as the level of adherence of host strain E. faecalis FA2-2 or OG1X (data not shown). E. faecalis FA2-2 or OG1X harboring the constitutive aggregation plasmid pAS13 exhibited constitutive clumping and formed large aggregates in broth culture. Aggregates of each strain detached from the well surface when the well was washed with PBS after the strain had been incubated in a well coated with ECM. Thus, quantitative or reproducible analysis of adherence was difficult with the constitutive clumper (data not shown). These results suggested that the aggregation substance encoded on the plasmids plays little role in efficient adherence to immobilized ECMs.
Inhibition of adherence by fibronectin and collagen.
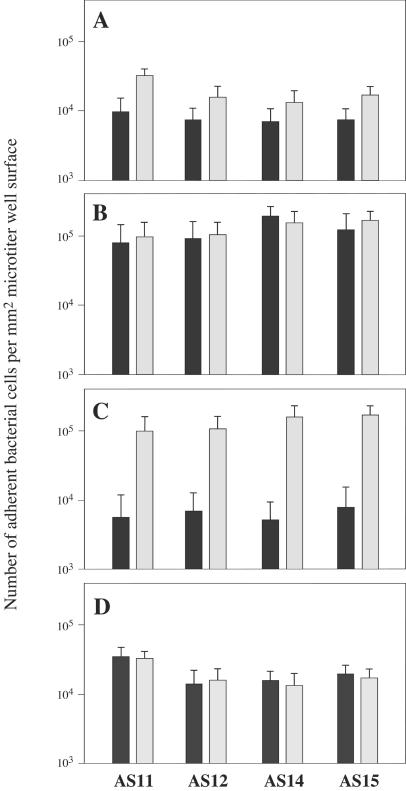
To examine whether fibronectin or collagen inhibits adherence of the strongly adhesive strains, adherence was examined after pretreatment of E. faecalis strains with fibronectin or collagen type IV. When E. faecalis AS11, AS12, AS14, or AS15 was preincubated with fibronectin, the adherence to fibronectin was inhibited and the number of bacterial cells adhering to fibronectin was about 2 × 10−1 to 4 × 10−1 times the number of bacterial cells that had not been pretreated that adhered to fibronectin, but the adherence to collagen type IV was not inhibited (Fig. 3).
FIG. 3.
Adherence of collagen type IV-treated or fibronectin-treated E. faecalis strains to ECMs. Solid bars, ECM-treated E. faecalis strains; gray bars, E. faecalis strains not treated with ECM. The experiments were carried out in triplicate. Each of the strains that adhered efficiently to bladder carcinoma T24 cells was treated with fibronectin (A and B) and then examined for adherence to fibronectin (A) and collagen type IV (B). In addition, each of the strains was treated with collagen (C and D) and then examined for adherence to collagen type IV (C) and fibronectin (D).
When E. faecalis AS11, AS12, AS14, or AS15 was preincubated with collagen type IV, the adherence to this collagen was inhibited, and the number of bacterial cells adhering to collagen type IV was about 10−1 times the number of bacterial cells that had not been pretreated that adhered to collagen type IV, but the adherence to fibronectin was not inhibited (Fig. 3).
Analysis of the determinant for collagen adhesin from enterococci (ace) and adhesin of the fibronectin binding protein (prtF).
The E. faecalis ace gene encodes Ace (adhesin of collagen from enterococci), which is a collagen binding MSCRAMM (microbial surface component recognizing adhesive matrix molecules) (29). The DNAs of the E. faecalis strains were screened by PCR for the presence of the Ace gene with primers specific for collagen binding domain A of ace (30). The DNAs of all the E. faecalis strains used in this study gave rise to the expected 864-bp product with the primer specific for the ace, indicating that E. faecalis strains have the ace gene (data not shown) (Table 1) (30).
The adhesins of the fibronectin binding proteins of group A streptococci are believed to mediate attachment to host cells (12, 13, 33). The prtF gene of group A streptococcus encodes protein F, which is a fibronectin binding adhesion protein (accession number L10919). Fibronectin binding proteins have been found in other streptococci, and the fibronectin binding domains of these proteins are highly homologous with the fibronectin binding domain of protein F (22). A fibronectin binding protein has not been found in E. faecalis. E. faecalis strains AS11, AS12, AS14, AS15, HT11, and HT12, which adhere efficiently to human bladder carcinoma T24 cells, fibronectin, and collagens, were studied to determine whether they encode a determinant homologous to prtF by using the pPTF5 probe and the pPTF54 probe, which contain the determinant of the fibronectin binding protein and the fibronectin binding domain of prtF, respectively (12). The prtF probe hybridized to a fragment of HindIII-digested S. pyogenes JRS4 DNA and did not hybridize to any HindIII-digested fragment of E. faecalis strains, indicating that there is no determinant homologous to prtF in the E. faecalis strains (data not shown).
Isolation of transposon insertion mutants with altered collagen binding.
Mutants with altered collagen binding were isolated by insertion of Tn916 into the chromosome of the E. faecalis AS14 strain as described in Materials and Methods. The AS14SS strain was a streptomycin- and spectinomycin-resistant derivative of the AS14 strain, and AS14SS showed the same adhesive properties with each ECM as the parent strain (data not shown). A total of 6,200 Tn916 insertion derivatives were obtained in independent experiments, and the derivatives were examined for adherence to collagen type IV. Thirty-eight mutants that did not adhere efficiently to collagen type IV were isolated in independent experiments. The Tn916 insertions of the mutants were studied by Southern hybridization with the Tn916 probe. As Tn916 contains one HindIII site, the Tn916 probe hybridizes to at least two fragments when DNA from an Tn916 insertion mutant is digested with HindIII (9). Tn916 hybridized to two unique HindIII fragments of the HindIII-digested chromosomal DNA of 14 of the 38 Tn916 insertion mutants, implying that Tn916 was inserted into a site in the chromosomal DNA of each of these mutants (data not shown). Thirteen of the 14 mutants produced the same hybridization pattern, implying that the transposon was inserted into the same site in all 13 of these mutants. One of the 14 mutants produced a hybridization pattern that was different from that of the other 13 mutants. In the remaining 24 mutants, the Tn916 probe hybridized to four or more fragments in the HindIII-digested chromosomal DNA, implying that Tn916 was inserted into several sites of the chromosomal DNA (data not shown). One pair of PCR primers was designed based on the previously published sequences of Tn916 (5, 8) and the ace gene (3a; data bank of The Institute for Genomic Research). The 14 mutants in which Tn916 hybridized to two unique HindIII fragments were analyzed by PCR performed with the PCR primers described above. The PCR products were detected in the 13 mutants that had the same hybridization pattern. The PCR products were sequenced, and computer analysis of the sequence revealed that Tn916 was inserted into the N terminus (start codon) of the ace gene (data not shown).
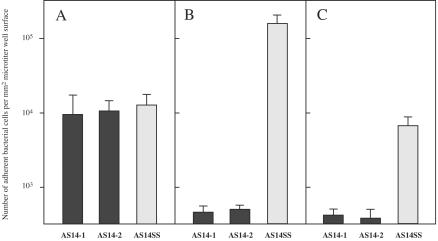
Compared with the number of AS14SS cells, the number of mutant AS14-1 cells that adhered to the collagens and laminin was about 1 or 2 orders of magnitude less (Fig. 4). The number of AS14-1 bacterial cells that showed adhesion to fibronectin was identical to the number of cells of parent strain AS14SS that showed adhesion to fibronectin. These results indicated that the adhesin encoded in the ace gene mediated adherence to collagens and also to laminin (27) but did not mediate adherence to fibronectin.
FIG. 4.
Adherence to ECMs of mutant E. faecalis AS14 strains with the Tn916 insertion in ace. Solid bars, ace mutant strains AS14-1 and AS14-2; gray bars, wild-type strain AS14SS. The experiments were carried out in triplicate. (A) Adherence to fibronectin. (B) Adherence to collagen type IV. (C) Adherence to laminin.
DISCUSSION
Microscopy was used in this study for quantitative analysis of the adherence of E. faecalis clinical isolates to immobilized ECMs coating the plastic wells of microtiter dishes. This method was sensitive enough to make it possible to quantify the adherence of E. faecalis isolates. It has been shown previously that tissue-specific enterococcal strains isolated from urinary tract infections also adhere very efficiently to human bladder carcinoma T24 cells. These strains also strongly adhere to human bladder epithelial cells. The adherence is inhibited by fibronectin, implying that adhesins on the cell surface mediate adherence to fibronectin. The strains that adhered efficiently to human bladder carcinoma T24 cells also adhered strongly to fibronectin, laminin, and collagen types I, II, IV, and V. They showed particularly strong adherence to collagen type IV, and the highest levels were greater than 7 × 104 cells/mm2. Many other strains adhered to between one and four of the fibronectin, laminin, and collagen type I, II, IV, and V ECMs. These results implied that the ability of E. faecalis to bind to fibronectin, laminin, and collagen types I, II, IV, and V plays a role in the efficient adherence to human tissue, although other factors may also play a role in the adherence.
The levels of adherence to each of the ECMs varied from 103 to more than 104 cells/mm2. Adherence of E. faecalis to a collagen(s) is mediated by the Ace protein, which is encoded by the ace gene (30). Ace also mediates the adherence to laminin (27). A polyclonal antibody analysis of domain A of Ace showed that the E. faecalis strains that adhere to immobilized collagen express Ace on the cell surface; on the other hand, strains that do not adhere to collagen do not express Ace on the cell surface, despite the presence of the ace gene (27, 30). In this study, we showed that the strains that adhered efficiently to immobilized collagen (i.e., strains AS11, AS12, AS14, and AS15) were specifically inhibited by pretreatment of the strains with collagen and that disruption of the ace gene of the efficient adhesive strain E. faecalis AS14 resulted in a specific decrease in the level of adherence of the mutant strain to immobilized collagen. These data implied that the adherence of these E. faecalis strains to collagens was mediated by Ace. The E. faecalis isolates used in this study had the ace gene, which was identified by PCR by using primers for domain A of ace (30). Despite the presence of the ace gene in the E. faecalis isolates, differences in the levels of adherence to the collagens were observed for each of the isolates, and this could have been due to differences in the expression of Ace on the E. faecalis cell surface (40). Although the determinant for adhesion to fibronectin has not been identified yet, the difference in the levels of adherence to fibronectin also might be due to differences in the expression a fibronectin adhesin.
The gelatinase-negative isolates adhered more efficiently to collagens and laminin than the gelatinase-producing isolates. The gelatinase-negative derivative E. faecalis OG1X also adhered more efficiently to collagens and laminin than the parent strain of the gelatinase-positive organism E. faecalis OG1S. These results implied that the gelatinase could degrade collagens and laminin in addition to gelatin. The gelatinase-positive isolates did not adhere efficiently to the wells coated with collagens or laminin since the ECMs on the surfaces of wells were degraded during incubation of the isolates in the wells used for the adherence assay.
Xiao et al. (40) showed that very few isolates exhibited adherence to collagen types I and IV and mouse laminin after growth of the isolates in standard in vitro physiological conditions (i.e., 37°C). This observation appears to conflict with our results. However, there is essentially no difference between the observations of Xiao et al. and our results. Xiao et al. also showed that two of the eight strains studied bound efficiently to collagen types I and II at 37°C. However, it seems that strains with intermediate levels of adherence to collagen may not be identified by the methods of these workers. This seemingly conflicting observation may be due to the use of direct observation of the adherence by microscopy to score the binding phenotype in our case, which may be a more sensitive method than the method used in binding studies that rely on determining the percentage of bacteria that have bound ECM protein.
There have been reports that E. faecalis isolates and E. faecalis laboratory strains bind to fibronectin (35). In other studies workers showed that there were low levels of binding or no binding of E. faecalis isolates to fibronectin (13, 36, 40, 42). It has been shown previously that the adherence of an E. faecalis strain to human bladder carcinoma T24 cells was inhibited by fibronectin, implying that the E. faecalis strain binds to fibronectin (34). S. pyogenes (group A streptococcus) encodes adhesin PrtF, which mediates adherence to fibronectin. In this study, S. pyogenes JR4 specifically adhered to fibronectin at a level of around 104 cells/mm2. Twenty-one of the 43 isolates and three E. faecalis laboratory strains adhered to fibronectin at levels that were the same as or greater than that of the JRS4 strain. The E. faecalis AS14 strain adhered to fibronectin, collagens, and laminin at levels that were greater than 104 cells/mm2. The Tn916 insertion mutant of the AS14 strain for collagen and laminin binding exhibited highly efficient binding to fibronectin, and fibronectin binding was unaffected. Similar results were obtained in the studies of inhibition of adherence by collagen and fibronectin. The adherence of E. faecalis AS14 to collagen was inhibited by collagen but was not inhibited by fibronectin, and the adherence of an E. faecalis strain to fibronectin was inhibited by fibronectin but was not inhibited by collagen. These results indicate that there was no relationship between the collagen binding adhesin Ace and the fibronectin binding adhesin. Although the specific substance that mediates adherence to fibronectin in E. faecalis has not been elucidated yet, these results imply that fibronectin binding could be mediated by a substance on the E. faecalis cell surface.
Acknowledgments
This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Sciences, and Technology (Tokuteiryoiki [C], Kiban [B]) and the Japanese Ministry of Health, Labor, and Welfare (H15-Shinko-9).
We thank Elizabeth Kamei for helpful advice.
Editor: V. J. DiRita
References
- 1.Andrewes, F. W., and T. J. Horder. 1906. A study of the streptococci pathogenic for man. Lancet ii:708-713. [Google Scholar]
- 2.Clewell, D. B. 1981. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol. Rev. 45:409-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clewell, D. B. 1993. Bacterial sex pheromone-induced plasmid transfer. Cell 73:9-12. [DOI] [PubMed] [Google Scholar]
- 4.Clewell, D. B., P. K. Tomich, M. C. Gawron-Burke, A. E. Franke, Y. Yagi, and F. Y. An. 1982. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J. Bacteriol. 152:1220-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clewell, D. B., S. E. Flannagan, Y. Ike, J. M. Jones, and C. Gawron-Burke. 1988. Sequence analysis of termini of conjugative transposon Tn916. J. Bacteriol. 170:3046-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunny, G. M., B. L. Brown, and D. B. Clewell. 1978. Induced cell aggregation and mating in Streptococcus faecalis, evidence for a bacterial sex pheromone. Proc. Natl. Acad. Sci. USA 75:3479-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunny, G. M., R. A. Craig, R. L. Carron, and D. B. Clewell. 1979. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid 2:454-465. [DOI] [PubMed] [Google Scholar]
- 8.Flannagan, S. E., L. A. Zitzow, Y. A. Su, and D. B. Clewell. 1994. Nucleotide sequence of the 18-kb conjugative transposon Tn916 from Enterococcus faecalis. Plasmid 32:350-354. [DOI] [PubMed] [Google Scholar]
- 9.Gawron-Burke, C., and D. B. Clewell. 1982. A transposon in Streptococcus faecalis with fertility properties. Nature 300:281-284. [DOI] [PubMed] [Google Scholar]
- 10.Gilmore, M. S., P. S. Coburn, S. R. Nallapareddy, and B. E. Murray. 2002. Enterococcal virulence, p. 301-354. In M. S. Gilmore, D. B. Clewell, P. M. Courvalin, G. M. Dunny, B. E. Murray, and L. B. Rice (ed.), The enterococci: pathogenesis, molecular biology, antibiotic resistance, and infection control. ASM Press, Washington, D.C.
- 11.Haas, W., B. D. Shepard, and M. S. Gilmore. 2002. Two-component regulator of Enterococcus faecalis cytolysin responds to quorum-sensing autoinduction. Nature 415:84-87. [DOI] [PubMed] [Google Scholar]
- 12.Hanski, E., and M. Caparon. 1992. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc. Natl. Acad. Sci USA 89:6172-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanski, E., P. A. Horwitz, and M. G. Caparon. 1992. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect. Immun. 60:5119-5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huycke, M. M., C. A. Spiegel, and M. S. Gilmore. 1991. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 35:1626-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huycke, M. M., D. F. Sahm, and M. S. Gilmore. 1998. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg. Infect. Dis. 4:239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ike, Y., and D. B. Clewell. 1992. Evidence that the hemolysin/bacteriocin phenotype of Enterococcus faecalis subsp. zymogenes can be determined by plasmids in different incompatibility groups as well as by the chromosome. J. Bacteriol. 174:8172-8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ike, Y., D. B. Clewell, R. A. Segarra, and M. S. Gilmore. 1990. Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis: Tn917 insertional mutagenesis and cloning. J. Bacteriol. 172:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ike, Y., R. A. Craig, B. A. White, Y. Yagi, and D. B. Clewell. 1983. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc. Natl. Acad. Sci. USA 80:5369-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ike, Y., H. Hashimoto, and D. B. Clewell. 1984. Hemolysin of Streptococcus faecalis subsp. zymogenes contributes to virulence in mice. Infect. Immun. 45:528-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ike, Y., H. Hashimoto, and D. B. Clewell. 1987. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infection. J. Clin. Microbiol. 25:1524-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jett, B. D., M. M. Huycke, and M. S. Gilmore. 1994. Virulence of enterococci. Clin. Microbiol. Rev. 7:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kline, J. B., S. Xu, A. L. Bisno, and C. M. Collins. 1996. Identification of a fibronectin-binding protein (GfbA) in pathogenic group G streptococci. Infect. Immun. 64:2122-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreft, B., R. Marre, U. Schramm, and R. Wirth. 1992. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect. Immun. 60:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maki, D. G., and W. A. Agger. 1988. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine 67:248-269. [PubMed] [Google Scholar]
- 25.Moellering, R. C., Jr. 1992. Emergence of Enterococcus as a significant nosocomial pathogen. Clin. Infect. Dis. 14:1173-1176. [DOI] [PubMed] [Google Scholar]
- 26.Murray, B. E. 1990. The life and times of the enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nallapareddy, S. R., X. Qin, G. M. Weinstock, M. Hook, and B. E. Murray. 2000. Enterococcus faecalis adhesin, Ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect. Immun. 68:5210-5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olmsted, S. B., G. M. Dunny, S. L. Erlandsen, and C. L. Wells. 1994. A plasmid-encoded surface protein on Enterococcus faecalis augments its internalization by cultured epithelial cells. J. Infect. Dis. 170:1549-1556. [DOI] [PubMed] [Google Scholar]
- 29.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 30.Rich, R. L., B. Kreikemeyer, R. T. Owens, S. LaBrenz, S. V. Narayana, G. M. Weinstock, B. E. Murray, and M. Hook. 1999. Ace is a collagen-binding MSCRAMM from Enterococcus faecalis. J. Biol. Chem. 274:26939-26945. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Scott, J. R., P. C. Guenthner, L. M. Malone, and V. A. Fischetti. 1986. Conversion of an M− group A streptococcus to M+ by transfer of a plasmid containing an M6 gene. J. Exp. Med. 164:1641-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sela, S., A. Aviv, A. Tovi, I. Burstein, M. G. Caparon, and E. Hanski. 1993. Protein F: an adhesin of Streptococcus pyogenes binds fibronectin via two distinct domains. Mol. Microbiol. 10:1049-1055. [DOI] [PubMed] [Google Scholar]
- 34.Shiono, A., and Y. Ike. 1999. Isolation of Enterococcus faecalis clinical isolates that efficiently adhere to human bladder carcinoma T24 cells and inhibition of adhesion by fibronectin and trypsin treatment. Infect. Immun. 67:1585-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shorrock, P. J., and P. A. Lambert. 1989. Binding of fibronectin and albumin to Enterococcus (Streptococcus) faecalis. Microb. Pathog. 6:61-67. [DOI] [PubMed] [Google Scholar]
- 36.Styriak, I., A. Laukova, C. Fallgren, and T. Wadstrom. 1999. Binding of selected extracellular matrix proteins to enterococci and Streptococcus bovis of animal origin. Curr. Microbiol. 39:327-335. [DOI] [PubMed] [Google Scholar]
- 37.Tomita, H., S. Fujimoto, K. Tanimoto, and Y. Ike. 1997. Cloning and genetic and sequence analyses of the bacteriocin 21 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pPD1. J. Bacteriol. 179:7843-7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomita, H., S. Fujimoto, K. Tanimoto, and Y. Ike. 1996. Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI17. J. Bacteriol. 178:3585-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westerlund, B., and T. K. Korhonen. 1993. Bacterial proteins binding to the mammalian extracellular matrix. Mol. Microbiol. 9:687-694. [DOI] [PubMed] [Google Scholar]
- 40.Xiao, J., M. Hook, G. M. Weinstock, and B. E. Murray. 1998. Conditional adherence of Enterococcus faecalis to extracellular matrix proteins. FEMS Immunol. Med. Microbiol. 21:287-295. [DOI] [PubMed] [Google Scholar]
- 41.Yagi, Y., R. E. Kessler, J. H. Show, D. E. Lopatin, F. Y. An, and D. B. Clewell. 1983. Plasmid content of Streptococcus faecalis strain 39-5 and identification of a pheromone (cPD1)-induced surface antigen. J. Gen. Microbiol. 129:1207-1215. [DOI] [PubMed] [Google Scholar]
- 42.Zareba, T. W., C. Pascu, W. Hryniewicz, and T. Wadstrom. 1997. Binding of extracellular matrix proteins by enterococci. Curr. Microbiol. 34:6-11. [DOI] [PubMed] [Google Scholar]
- 43.Zervos, M. J., S. Dembinski, T. Mikesell, and D. R. Schaberg. 1986. High-level resistance to gentamicin in Streptococcus faecalis: risk factors and evidence for exogenous acquisition of infection. J. Infect. Dis. 153:1075-1083. [DOI] [PubMed] [Google Scholar]