Abstract
An apparent worldwide resurgence of invasive group A Streptococcus (GAS) infections remains unexplained. However, we recently demonstrated in mice that when an otherwise nonlethal intranasal GAS infection is preceded by a nonlethal influenza A virus (IAV) infection, induction of lethal invasive GAS infections is often the result. In the present study, we established several isogenic mutants from a GAS isolate and evaluated several virulence factors as candidates responsible for the induction of invasive GAS infections. Disruption of the synthesis of the capsule, Mga, streptolysin O, streptolysin S, or streptococcal pyrogenic exotoxin B of GAS significantly reduced mortality among mice superinfected with IAV and a mutant. In addition, the number of GAS organisms adhering to IAV-infected alveolar epithelial cells was markedly reduced with the capsule-depleted mutant, although this was not the case with the other mutants. Wild-type GAS was found to bind directly to IAV particles, whereas the nonencapsulated mutant showed much less ability to bind. These results suggest that the capsule plays a key role in the invasion of host tissues by GAS following superinfection with IAV and GAS.
Streptococcus pyogenes (group A Streptococcus [GAS]) is known to be the cause of such diseases as uncomplicated pharyngitis, impetigo, and acute rheumatic fever (10). In addition, severe invasive GAS infections, leading to a wide range of diverse diseases that include acute respiratory distress syndrome, renal failure, streptococcal toxic shock syndrome, sepsis, and cellulitis, have been reported in North America, Europe, and Japan (4, 23, 30, 35, 41). GAS invades the host via the upper respiratory tract or injured skin surfaces (10). Although many invasive GAS isolates are reportedly of the M1 or M3 serotype, which produce streptococcal pyrogenic exotoxin A (10), the reasons for the recent emergence, or reemergence, of invasive GAS infections remain unknown.
Statistically, the incidence of GAS infections is highest in winter, when influenza epidemics are common (5, 12, 28). In that regard, we recently used a mouse model to show that superinfection with influenza A virus (IAV) and GAS applied intranasally causes a lethal GAS infection that affects various tissues and organs of the host (32). In that study, GAS organisms adhered to the surface of IAV-infected alveolar epithelial cells of superinfected mice, although no GAS adhered to epithelial cells of mice infected with GAS alone. Moreover, our electron microscopic observations confirmed that GAS organisms bind directly to IAV particles on the cell surfaces. Taken together, these results suggest that IAV infection plays a key role in the enhanced internalization of GAS by alveolar epithelial cells, resulting in the emergence of sepsis and other invasive GAS diseases. Beyond this, however, little is known about the mechanisms via which invasive IAV-GAS superinfections occur.
Ashbaugh et al. (3) established a murine model of human necrotizing fasciitis and concluded that the GAS hyaluronic acid capsule and M protein, but not the cysteine protease streptococcal pyrogenic exotoxin B (SPEB), are critical for the development of tissue necrosis, secondary bacteremia, and eventually death. In addition, another GAS virulence factor, streptolysin O (SLO), which binds to cholesterol in red blood cell membranes, causing lysis, reportedly exerts various neurological effects, increases capillary permeability, and induces dermal necrosis in rabbits (1). On the other hand, direct studies of the role of SLO during infection recently revealed that the contribution of SLO to GAS virulence was limited in a murine dermonecrosis invasive infection model (26). Still another factor is streptolysin S (SLS), which like SLO is potently cytolytic (1). These findings raise questions as to the respective contributions made by the various GAS virulence factors to the induction of invasive infections with otherwise nonlethal doses of IAV and GAS. In the present study, genes encoding hyaluronic acid capsule, M protein, SLO, and SLS were inactivated by allelic replacement. We then examined the mortality among mice superinfected with IAV and wild-type strain SSI-9 or one of its isogenic mutant strains.
MATERIALS AND METHODS
Virus, bacteria, mice, and cell line.
IAV A/FM/1/47 (H1N1) (34) was suspended in phosphate-buffered saline (PBS) before use in all subsequent studies. The bacterial strains and plasmids used in this study are listed in Table 1. GAS strain SSI-9 (M1) was an isolate from a Japanese patient with severe invasive fasciitis and was provided by T. Murai (Toho University, Tokyo, Japan). For selection of GAS mutants, the organisms were grown in Todd-Hewitt broth supplemented with 0.2% yeast extract (THY) (Difco Laboratories, Detroit, Mich.) for 6 h at 37°C (31, 33), after which kanamycin (500 μg/ml) or spectinomycin (1 mg/ml) was added to the medium. Female BALB/c mice (H-2d, 8 weeks old, 18 to 20 g) were purchased from Charles River Inc. (Yokohama, Japan). A human alveolar epithelial cell line, A549, was grown in RPMI 1640 medium (Sigma, St. Louis, Mo.) supplemented with 10% fetal bovine serum (FBS) (Gibco-BRL, New York, N.Y.).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source | ||
|---|---|---|---|---|
|
S. pyogenes strains
|
||||
| SSI-9 | M type 1, isolated from patient with streptococcal toxic shock syndrome | 45 | ||
| TR-5 | Isogenic mutant of SSI-9; derivative of pYT1089; mga::aphA3 Kmr | 45 | ||
| TR-8 | Isogenic mutant of SSI-9; derivative of pSK141; hasA::aphA3 Kmr | This study | ||
| TR-10 | Isogenic mutant of SSI-9; derivative of pIN151; slo::aphA3 Kmr | This study | ||
| TR-11 | Isogenic mutant of SSI-9; derivative of pTM16; speB::aad9 Spr | This study | ||
| TR-17 | Isogenic mutant of SSI-9; derivative of pYT1111; sagA::aad9 Spr | This study | ||
| Plasmids | ||||
| pGEM-T Easy | Cloning vector; Apr | Promega | ||
| pIN151 | pSF151 with slo | This study | ||
| pSF151 | Suicide vector; for insertional mutagenesis; Kmr | 43 | ||
| pSF152 | Suicide vector; for insertional mutagenesis; Spr | 43 | ||
| pSK141 | pSF151 with hasA; Kmr | 24 | ||
| pTM16 | pSF152 with speB; Spr | This study | ||
| pYT1089 | pSF151 with mga; Kmr | 45 | ||
| pYT1111 | pSF152 with sagA; Spr | This study | ||
Mutagenesis using targeted plasmids and characteristic measurements of hasA, speB, slo, and sagA mutants.
PCR with specific primer sets (Table 2) was used to amplify gene fragments from the hasA, speB, slo, and sagA genes, encoding hyaluronate synthase, SPEB, SLO, and SLS, respectively. The PCR products were ligated into pGEM-T Easy vectors (Promega, Madison, Wis.) and digested with EcoRI, after which the fragments were cloned into vectors as described in Table 1. The resultant plasmids, pIN151, pTM16, and pYT1111, were transformed into GAS strain SSI-9 by electroporation, yielding the mutants TR-10 (slo), TR-11 (speB) and TR-17 (sagA), respectively (Fig. 1A and Table 1). The inactivated mutant strains were then selected by using kanamycin- or spectinomycin-containing agar plates. An hasA-inactivated mutant, TR-8, was constructed in the same way by first constructing E-1, as described previously (24); likewise, TR-5 was constructed as described previously (43, 45).
TABLE 2.
PCR primer sets used for targeted mutagenesis
| Gene | Primer designation | Primer sequence | Reference |
|---|---|---|---|
| hasA | hasA/1 | GAGGTGTAATTGTGCCTA | 24 |
| hasA/2 | TAGGGGCTGTAAGACAAAC | 24 | |
| slo | slo/1 | TAGGATCCAATACAGTCGCGTCGCTGGGC | This study |
| slo/2 | GAGAATTCCGGAGCTTCATTGCTCACACC | This study | |
| speB | speB/1 | CTCACCAGCGGATCATTTGACGC | This study |
| speB/2 | CAGCCATCAATTCTGAAATCGCC | This study | |
| sagA | sagA/1 | CTCAAGTTGCTCCTGGAGGCTG | This study |
| sagA/2 | ACTTCCAGTAGCAATTGAG | This study | |
| aad9 | aad9/F2 | CCACTCTCAACTCCTGATCC | This study |
| aad9/R2 | GTTAGCAGTTCGTAGTTATC | This study | |
| aphA3 | aphA3/F2 | TCCGTATCTTTTACGCAGCGG | 45 |
| aphA3/R2 | GGGATGAAGCCTGATTGGGAG | 45 |
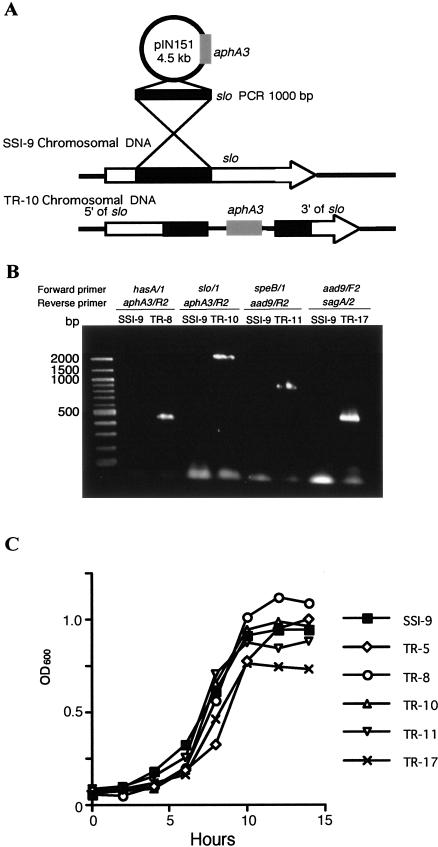
FIG. 1.
Targeted mutagenesis of slo in GAS strain SSI-9. (A) pIN151 contains an internal fragment of slo and a kanamycin resistance gene (aphA3). TR-10 was produced by a single-crossover recombination. The speB (TR-11) and sagA (TR-17) mutants were constructed in a similar fashion. (B) The hasA, slo, speB, and sagA mutants were subjected to PCR with a targeted gene-specific forward primer and an aphA3- or aad9-specific reverse primer. (C) Strain SSI-9 and its mutants (107 CFU per 50 μl) were inoculated into 5 ml of THY broth and incubated at 37°C. Growth was determined by measuring the optical density at 600 nm (OD600).
Detection of hyaluronic acid and sialic acid.
GAS-associated hyaluronic acid was removed from GAS organisms, and the uronic acid content was quantitated as described by Schranger et al. (39). Hyaluronic acid from S. zooepidemicus (Wako, Osaka, Japan) was used as the standard. Detection of capsular polysaccharide from GAS was performed as described previously (50), and the sialic acid content was estimated by using the thiobarbituric acid method (48).
Caseinolytic assay.
The proteolytic activity of SPEB was assessed by using a casein agar plate assay as described previously (7). Briefly, the test strain was inoculated onto THY agar plates containing 10% skim milk (Difco). The plates were then incubated for 48 h at 37°C in an anaerobic GasPak (Becton Dickinson, Mountain View, Calif.). Caseinolytic activity resulted in a zone of translucence surrounding the stab site.
Hemolytic assays.
SLO hemolytic assays were carried out essentially as described by Limbago et al. (26). Briefly, an overnight culture of test strains was adjusted to an absorbance of 1.0 at 600 nm, after which the supernatant was separated by centrifugation for 10 min at 7,500 × g and then filtered (pore size, 0.25 μm). The cleared supernatant was incubated for 10 min at room temperature with 10 mM dithiothreitol, after which two 0.5-ml aliquots of each sample were placed in 1.5-ml sterile tubes, and 25 μl of cholesterol (Sigma) was added to one of the aliquots. Following a 30-min incubation at 37°C, 0.25 ml of a 2% suspension of bovine erythrocytes in PBS was added to each aliquot, mixed by inversion, and incubated for 30 min at 37°C. Finally, 0.5 ml of PBS was added to each sample, and intact erythrocytes were collected by centrifugation for 1 min at 200 × g. Supernatants were then removed, and the release of hemoglobin was assessed by reading the absorbance at 541 nm.
SLS hemolytic assays were carried out as described by Sierig et al. (40). Test organisms were grown for 16 h in brain heart infusion broth supplemented with 2% Na2CO3 and 1% maltose. They were then washed in 100 mM potassium phosphate buffer (pH 7.0), after which 4 mg (dry weight) of the organism were suspended in 1 ml of 100 mM KPB (pH 7.0) containing 2 mM MgSO4 and 30 mM maltose. The suspension was incubated for 5 min at 37°C, induced by treatment with 0.5 mg of RNA core (Sigma) for 5 min, and centrifuged at 15,000 × g and 4°C for 20 min. The resultant supernatant was collected, and ammonium acetate (100 mM final concentration) was added to stabilize SLS. After preparation of a standardized suspension of sheep erythrocytes comprised of PBS (pH 6.5) containing 6% defibrinated sheep blood and 0.1% bovine serum albumin (BSA) (Sigma), 1 ml of the supernatant was incubated with 0.5 ml of the standardized erythrocyte suspension for 45 min at 37°C. Intact erythrocytes were removed by centrifugation, and release of hemoglobin was assessed by reading the absorbance at 541 nm.
Microbial infections and counting of microorganisms in internal organs.
To examine the lethality of superinfection with IAV and GAS, mice were intranasally administrated 25 μl of PBS with or without 100 focus-forming units (FFU) of IAV, followed 2 days later by intranasal administration with 25 μl of PBS containing 107 CFU of GAS. To determine the number of GAS organisms present in the lungs, liver, spleen, and kidneys, the organs were dissected and homogenized in PBS, after which 10-fold dilutions of each suspension were plated on THY blood agar plates, and colonies were counted after incubation overnight at 37°C (11).
Histopathology.
Tissue samples were fixed in 10% (vol/vol) neutral phosphate-buffered formalin and embedded in paraffin. Sections were cut to a thickness of 6 μm. For GAS and IAV hemagglutinin (HA)-expressed cell detection, sections were stained with rabbit GAS strain SSI-9 antiserum (29) and fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulin G and/or Alexa Fluor 568 (Molecular Probes, Eugene, Oreg.)-conjugated anti-IAV HA monoclonal antibody (MAb). Each section was assigned a number, which enabled unbiased examination with a model LSM 510 confocal microscope (Carl Zeiss, Oberkochem, Germany).
Assays for GAS adhesion and invasion.
Assays for GAS adhesion to or invasion of epithelial cells were carried out as described previously (32). Briefly, IAV (2 × 106 FFU) was inoculated onto semiconfluent A549 cell monolayers (105 cells) in 24-well culture plates and incubated with 200 μl of RPMI 1640 medium (pH 6.0) containing 0.2% BSA for 30 min at 37°C in 5% CO2 (34). The medium was then aspirated and replaced with fresh medium (1 ml/well), and the cells were incubated for 17 h at 37°C in 5% CO2. Thereafter, the cells were washed twice, inoculated with fresh GAS organisms (106 CFU) suspended in 200 μl of medium, and incubated for 2 h at 37°C in 5% CO2. Unattached bacteria were then removed by washing with PBS, and A549 cells were disrupted and serially diluted by addition of sterile distilled water and plated on THY agar plates to determine the total number of GAS organisms associated with the epithelial cells (24, 32).
For the invasion assay, GAS organisms (106 CFU/200 μl of conditioned medium) were incubated with cell monolayers for 2 h at 37°C in 5% CO2. Unattached bacteria were removed by washing with 1 ml of PBS, and the GAS organisms associated with the cell surface were killed by treatment with gentamicin (100 μg/ml) and penicillin (10 U/ml) for 1 h. The cell monolayers were then washed, disrupted with sterile distilled water, serially diluted in water, and plated on THY agar plates to determine the number of internalized GAS bacteria (24, 32).
Measurement of binding of GAS organisms to IAV particles.
IAV particles were suspended in PBS containing 0.2% BSA, after which an aliquot of IAV (0.5 ml) or PBS containing 0.2% BSA (0.5 ml) was added to the wells of a six-well multiplate (36, 49) and incubated overnight at 4°C. The wells were then washed three times with PBS, GAS organisms (5 × 106 CFU in 0.5 ml of PBS) were added, and the plate was incubated for an additional 1 h at 4°C. After washing six times with PBS, PBS containing 0.5% Triton X-100 (1 ml/well) was added to the wells and then incubated for 30 min at room temperature to remove adherent GAS. Tenfold dilutions of each suspension were plated on THY blood agar plates, and the colonies were counted after incubation overnight at 37°C. In parallel experiments, GAS organisms (109 CFU) were treated with neuraminidase (0.6 U per 0.5 ml of 50 mM sodium acetate supplemented with 0.9% NaCl and 0.1% CaCl2) for 3 h at 37°C to remove the sialic acid.
Statistical evaluations.
Fisher's exact test was carried out by using StatView software (SAS Institute Inc., Cary, N.C.) to evaluate differences between groups in the mortality experiments. To analyze data from other experiments, nonparametric Mann-Whitney U tests and Wilcoxon two-sample rank tests were used. P values of <0.05 were considered significant.
RESULTS
Targeted mutagenesis of hasA, slo, speB, and sagA.
Targeted inactivation of hasA, slo, speB, and sagA in GAS strain SSI-9 resulted in the generation of mutants TR-8, TR-10, TR-11, and TR-17, respectively. Each mutation was confirmed by PCR analysis of the chromosomal DNA with targeted gene-, aphA3-, and aad9-specific primers. Fragments spanning the region between the targeted gene and aphA3 or aad9 were amplified by using the appropriate primer pairs (Fig. 1B), and it was confirmed that only a single insert was present in the chromosome and that no transcript was made from the resultant gene (data not shown). Thus, all targeted genes were inactivated in these mutants. We assessed the growth rates of SSI-9 and its mutants and confirmed that the presence of an antibiotic cassette in the chromosome did not cause a lower growth rate (Fig. 1C).
Hyaluronic acid expression, caseinolysis, and hemolysis were measured to determine the degree to which mutation of hasA, speB, slo, and sagA had affected their activities. Quantitative chemical analysis revealed that TR-8, the hasA mutant, produced no detectable cell-associated hyaluronate. By contrast, the other strains synthesized almost 30 fg of hyaluronate per CFU (Table 3). We also confirmed that TR-11, the speB mutant, showed markedly reduced caseinolytic activity, while TR-10, the slo mutant, and TR-17, the sagA mutant, showed markedly reduced levels of SLO- or SLS-dependent hemolytic activity (Table 3).
TABLE 3.
Characteristics of wild-type and mutant strains of GAS used in this studya
| Strain | Hyaluronate in capsule (fg/CFU) | Caseinolytic activity (mm in diam) | SLO activity (% erythrocyte lysis) | SLS activity (% erythrocyte lysis) |
|---|---|---|---|---|
| SSI-9 | 38.5 ± 2.4 | 5.5 ± 0.1 | 43.3 ± 2.3 | 49.9 ± 6.3 |
| TR-5 (mga::aphA3) | 32.4 ± 3.3 | 4.9 ± 1.0 | 39.4 ± 4.6 | 53.3 ± 7.7 |
| TR-8 (hasA::aphA3) | <2 | 3.3 ± 1.8 | 41.1 ± 7.5 | 56.4 ± 2.5 |
| TR-10 (slo::aphA3) | 39.1 ± 4.4 | 5.7 ± 0.5 | 4.3 ± 0.2 | 48.5 ± 9.5 |
| TR-11 (speB::aad9) | 28.8 ± 1.9 | 0.4 ± 0.1 | 45.7 ± 1.9 | 55.8 ± 4.3 |
| TR-17 (sagA::aad9) | 31.5 ± 3.1 | 5.0 ± 0.2 | 41.8 ± 5.3 | 6.5 ± 1.7 |
All data are expressed as means ± standard errors of the means.
Effect of isogenic GAS mutagenesis on mortality among mice subjected to viral-bacterial superinfection.
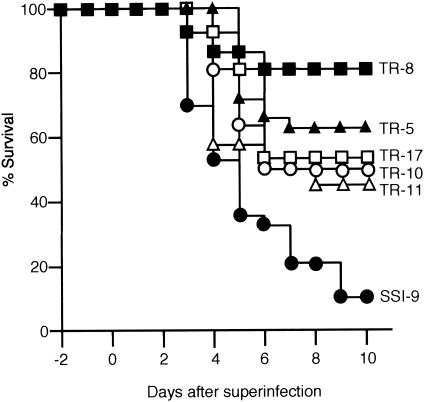
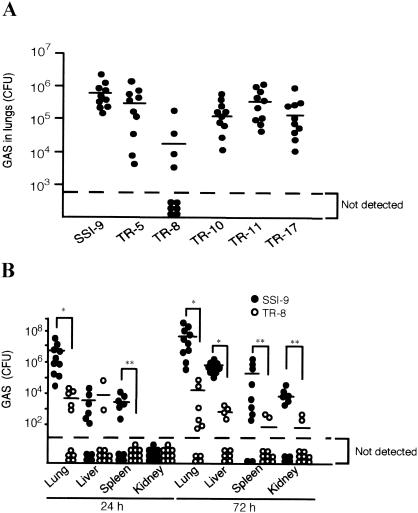
Alone, IAV at a dose of 100 FFU or GAS at a dose of 107 CFU did not induce a lethal infection in mice (32). On the other hand, prior infection with 100 FFU of IAV markedly increased mortality following inoculation with GAS (Fig. 2). Using the same experimental protocol, we then examined the effect on mouse mortality of the individual GAS virulence factors. The mortality among mice infected with SSI-9 mutants following IAV infection was significantly diminished, from 90% with wild-type strain SSI-9 to less than 60% for each of the mutants (Fig. 2). Notably, the most pronounced reduction in mortality rate (to only about 20%) was seen with the hasA-inactivated TR-8 mutant, which is consistent with the finding that among the six organisms tested, the TR-8 mutant exhibited the lowest levels in lung 24 h after infection (Fig. 3A). Moreover, levels of TR-8 were also significantly lower than those of wild-type SSI-9 in several other organs 72 h after superinfection (Fig. 3B).
FIG. 2.
Mortality among mice superinfected with IAV and the indicated GAS test strain. Groups of 30 mice were intranasally infected first with IAV (100 FFU) in 25 μl of PBS on day −2 and then with SSI-9 or one of its mutant strains (107 CFU) in 25 μl of PBS on day 0. Mortality was assessed daily for 10 days after GAS infection. Fisher's exact test was used to determine significant differences in mortality among the groups: P = 0.0012, P < 0.0001, P = 0.0127, P = 0.0470, and P = 0.0061, respectively, for TR-5, TR-8, TR-10, TR-11, and TR-17 versus SSI-9.
FIG. 3.
Effect of capsule removal on the number of GAS organisms in the internal organs of superinfected mice. (A) Ten mice were intranasally infected first with IAV (100 FFU) on day −2 and then with GAS strains (107 CFU) on day 0. The number of GAS organisms in the lungs was assessed on day 1. Circles under the broken line indicate that GAS was not detectable. Horizontal bars indicate the median values. Wilcoxon two-sample rank tests were used to evaluate the differences between groups: P = 0.1403, P = 0.0003, P = 0.1032, P = 0.2718, and P = 0.0880, respectively, for TR-5, TR-8, TR-10, TR-11, and or TR-17 versus SSI-9. (B) The numbers of GAS organisms in the lungs, livers, spleens, and kidneys of mice superinfected with IAV and SSI-9, or TR-8 were determined on days 1 and 3. Circles under the broken line indicate that GAS was undetectable. Horizontal bars indicate the median values. Mann-Whitney U tests were used to evaluate differences between groups *, P < 0.01; **, P < 0.05.
Adhesion and invasion of IAV-infected epithelial cells by isogenic GAS mutants.
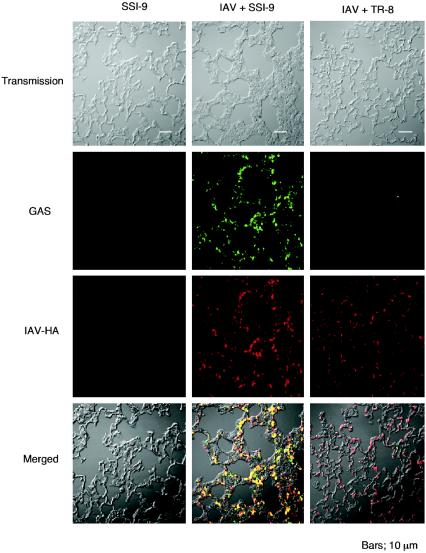
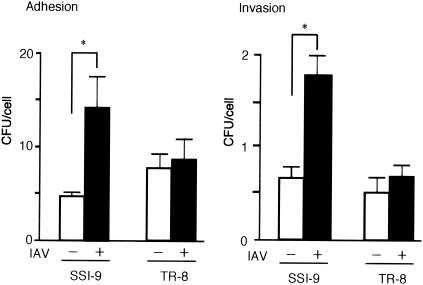
GAS organisms in the lungs of superinfected mice bind to IAV-infected alveolar epithelial cells (32). However, the fluorescence micrographs in Fig. 4 show that the numbers of GAS organisms associated with IAV-infected alveolar epithelial cells in the superinfected mice were diminished by inactivation of hasA. We also observed significantly less adhesion to and invasion of cultured A549 cells when they were infected with IAV and TR-8 than when they were infected with IAV and SSI-9 (Fig. 5). Collectively, then, the results presented so far strongly suggest that the GAS capsule is crucial for the enhanced adhesion to and invasion of IAV-infected epithelial cells by GAS.
FIG. 4.
Association of GAS with IAV-infected alveolar epithelial cells. Mice were infected with SSI-9 or superinfected with IAV and SSI-9 or TR-8 as described in the legend to Fig. 2. Lungs were harvested 48 h after GAS infection, and sections were stained with Alexa Fluor 568-conjugated anti-HA MAb or rabbit polyclonal anti-GAS antibody and fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulin G.
FIG. 5.
Expression of capsule by GAS is required for maximal adhesion to and invasion of cultured IAV-infected A549 epithelial cells. A549 cells were infected with IAV for 17 h. After subsequent infection with SSI-9 or TR-8 for 2 h, the number of GAS organisms adhering to or invading the epithelial cells was assessed. Data are shown as means ± standard errors of the means from 12 wells (four independent experiments performed in triplicate). Mann-Whitney U tests were used to evaluate the differences between groups. *, P < 0.01.
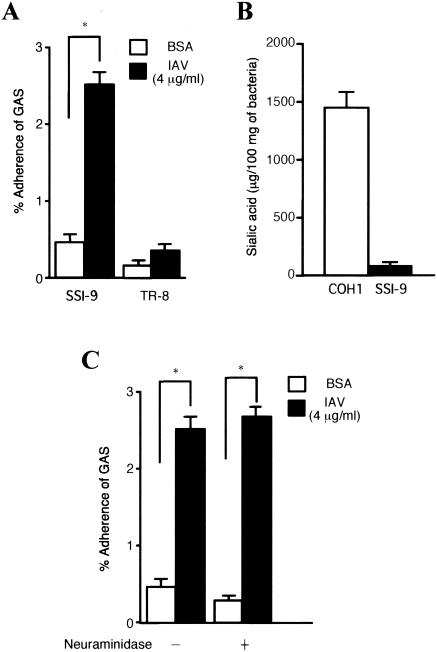
Our earlier electron microscopic observations revealed that GAS organisms bind directly to IAV particles exposed on the surface of epithelial cells (32). Here we confirmed that the binding of SSI-9 to IAV-coated plates was more than sixfold greater than that to plates without IAV (Fig. 6A). Furthermore, the nonencapsulated TR-8 mutant exhibited a significantly lower level of binding to IAV particles than wild-type SSI-9 (Fig. 6A).
FIG. 6.
Direct binding of GAS to IAV particles and effect of the GAS capsule and sialic acid on direct binding. (A) Wild-type SSI-9 or hasA-inactivated TR-8 mutant bacteria were added to IAV-coated wells (4 μg of IAV per ml, suspended in PBS containing 0.2% BSA), after which GAS binding to IAV particles was quantitated. Data are shown as means ± standard errors of the means from nine wells (three independent experiments performed in triplicate). Mann-Whitney U tests were used to evaluate differences between groups. *, P < 0.01 versus binding of SSI-9 to uncoated wells. (B) GAS (SSI-9) and GBS (COH1; type III) bacteria were cultured in THY broth, capsular polysaccharides from 100 mg (dry weight) of GAS and GBS were harvested, and the amount of sialic acid was estimated. (C) SSI-9 organisms were pretreated with or without neuraminidase, and the direct binding assay was performed.
Treatment of IAV-infected epithelial cells with anti-IAV HA MAb suppressed the binding of GAS to the epithelial cells (32). Since IAV-HA binds to sialic acid, we determined whether sialic acid was included in the GAS capsule. As shown in Fig. 6B, GAS possessed only trace amounts of sialic acid. Furthermore, the binding activity of GAS to IAV was not inhibited by pretreatment with neuraminidase (Fig. 6C).
DISCUSSION
Based on the hypothesis that microbial superinfections frequently accompanied a fatal outcome during the 1918 Spanish influenza epidemic, various attempts have been made to examine the interaction between influenza viruses and some bacteria in animal models (13, 16, 22, 25, 27, 32, 37). It is still unknown, however, which bacterial virulence factor(s) is important for the induction of invasive bacterial-viral superinfection. The present results indicate that the GAS capsule, SPEB, Mga, SLO, and SLS all participate in the induction of a lethal synergism.
We previously found that prior IAV infection enhances GAS internalization by alveolar epithelial cells (32). In the present study, therefore, we focused on which GAS virulence factor(s) is critical for that enhanced internalization. We found that depletion of the GAS capsule, but not Mga, SPEB, SLO, or SLS, reduced the number of GAS organisms in the lungs adhering to IAV-infected alveolar epithelial cells and regulated adhesion to and invasion of IAV-infected A549 cells by GAS. The wild-type GAS strain SSI-9 was found to bind directly to IAV particles, whereas the nonencapsulated TR-8 mutant showed much less ability to bind, suggesting that the GAS capsule plays a key role in the enhanced internalization of the organism by IAV-infected alveolar epithelial cells. On the other hand, nonencapsulation of GAS by transposon mutagenesis resulted in increased susceptibility to phagocytosis in human blood, even though the encapsulated wild-type strains survived (51). This leads us to hypothesize that the lower levels of nonencapsulated GAS present in the lungs may be due not only to diminished association with IAV-infected alveolar epithelial cells but also to higher levels of phagocytosis by alveolar phagocytes.
Insertion of an antibiotic cassette in the chromosome may play a role in decreased virulence. However, the growth rate of the mutants was nearly the same as that of SSI-9 (Fig. 1C), and the numbers of mutants, except TR-8, in lungs of the superinfected mice were the same as that of SSI-9 at 24 h after the superinfection (Fig. 3). Furthermore, hyaluronic acid expression in all of the mutant strains except TR-8, caseinolytic activity in all of the mutant strains except TR-11, and hemolytic activity in all of the mutant strains except TR-10 and TR-17 were completely conserved (Table 3). These results suggest that the simple presence of an antibiotic cassette in the chromosome of GAS does not lead to decreased virulence but rather that any changes in virulence are due to its insertion into key pathogenicity-determining genes.
Studies of the adhesion of GAS organisms to epithelial cells have implicated several bacterial surface molecules, including M protein (38), fibronectin-binding proteins (9, 31, 45, 47), and laminin-binding proteins (42, 46). In addition, some investigators have reported the capsule to be critical for pharyngeal colonization, resistance to phagocytosis, and virulence in experimental GAS infections (14, 20). For instance, inactivation of hasA blocked infection after intranasal inoculation in mice (19), and effective colonization and invasion of the pharynx by virulent GAS strains appear to require a specific interaction between the GAS capsule and CD44 (11). That the GAS capsule was also required for binding to IAV particles and IAV-infected alveolar cells suggests that the capsule may also play a novel pathogenic role in the induction of invasive GAS diseases following superinfection with otherwise nonlethal doses of IAV and GAS.
The mortality among mice superinfected with IAV and GAS was also reduced to various degrees by inactivation of mga, slo, speB, or sagA (Fig. 1). That these mutants produced capsular hyaluronic acid at levels similar to that seen in the parent strain SSI-9 (Table 3) suggests that the Mga regulon (which regulates expression of mrp/fcrA, emm, enn, scpA, and fbaA), SLO, SPEB, and SLS also act as virulence factors contributing to the induction of invasive GAS diseases. Notably, however, association of the organisms with IAV-infected epithelial cells (Fig. 3 and 4) and inflammation of the lungs (data not shown) were diminished only with the hasA mutant TR-8. We suggest that the binding of the GAS capsule to IAV surface molecules on IAV-infected epithelial cells is likely enhanced by the host inflammatory response to local tissue injury caused by SLO, SPEB, and SLS.
In the present study, nonencapsulation correlates with decreased invasion into A549 epithelial cells, though Jadoun et al. (20, 21) reported that a decreased capsule is associated with an increased uptake into epithelial cells in the presence of FBS. Depletion of the GAS capsule resulted in the exposure of several adhesins, especially fibronectin-binding proteins (45, 47), and it was suggested that the proteins induce enhanced internalization in the presence of fibronectin in FBS. Since FBS contains a component, termed β inhibitor, that inhibits the infectivity and hemagglutinating activity of IAV of the H1 and H3 subtypes (2), our experiments regarding internalization were performed in the absence of FBS. Therefore, we consider that the opposite effect was due to the lack of fibronectin in our assay system.
It remains unclear which molecules on the IAV surface bind the GAS capsule. One intriguing finding was that direct binding to IAV by sialic acid in the group B Streptococcus (GBS) capsule is mediated by fusion with IAV HA (18). HA, a 75-kDa protein expressed on the surface of the virus (6), participates in the fusion between virus envelopes and the endosomes of virus-infected cells prior to viral invasion of the cytoplasm (8, 17, 44). This does not appear to be the case with IAV and GAS, as GAS was found to possess only trace amounts of sialic acid and pretreating GAS with neuraminidase did not inhibit binding activity (Fig. 6B and C). Thus, the IAV protein that interacts with the GAS capsule remains to be identified.
Recently, McCullers and Rehg (27) found a lethal synergism between influenza viruses and Streptococcus pneumoniae in a mouse model. They speculated that influenza infection, which triggers elaboration of inflammatory cytokines, may indirectly up-regulate adhesion molecules on epithelial cells, providing a receptor for bacterial adhesion and invasion. For example, infection by adenovirus increases adhesion of pneumococci via a mechanism that is independent of the expression of adenovirus surface proteins, suggesting that a cell surface receptor was up-regulated (15).
In summary, we have shown that the GAS capsule, which appears to mediate the binding of the bacterium to IAV molecules exposed on the surface alveolar epithelial cells, is crucial for induction of lethal invasive GAS infections following superinfection with otherwise nonlethal doses of IAV and GAS.
Acknowledgments
We thank T. Uehira for technical assistance.
This study was supported by grants from the Japanese Ministry of Health, Welfare, and Labor; PRESTO, Japan Science and Technology Agency; and the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Editor: V. J. DiRita
REFERENCES
- 1.Alouf, J. 1980. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol. Ther. 11:661-717. [DOI] [PubMed] [Google Scholar]
- 2.Anders, E. M., C. A. Hartley, and D. C. Jackson. 1990. Bovine and mouse β inhibitor of influenza A viruses are mannose-binding lectins. Proc. Natl. Acad. Sci. USA 87:4485-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashbaugh, C. D., H. B. Warren, V. J. Carey, and M. R. Wessels. 1998. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J. Clin. Investig. 102:550-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxter, F., and J. McChesney. 2000. Severe group A streptococcal infection and streptococcal toxic shock syndrome. Can. J. Anaesth. 47:1129-1140. [DOI] [PubMed] [Google Scholar]
- 5.Brammer, T. L., H. S. Izurieta, K. Fukuda, L. M. Schmeltz, H. L. Regnery, H. E. Hall, and N. J. Cox. 2000. Surveillance for influenza—United States, 1994-95, 1995-96, and 1996-97 seasons. Morb. Mortal. Wkly. Rep. CDC Surveill. Summ. 49:13-28. [PubMed] [Google Scholar]
- 6.Bullough, P. A., F. M. Hughson, J. J. Skehel, and D. C. Wiley. 1994. Structure of influenza hemagglutinin at the pH of membrane fusion. Nature 371:37-43. [DOI] [PubMed] [Google Scholar]
- 7.Chaussee, M. S., D. Ajdic, and J. J. Ferretti. 1999. The rgg gene of Streptococcus pyogenes NZ131 positively influences extracellular SPE B production. Infect. Immun. 67:1715-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chernomordik, L. V., V. A. Frolov, E. Leikina, P Bronk, and J. Zimmerberg. 1998. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell Biol. 140:1369-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtney, H. S., J. B. Dale, and D. L. Hasty. 1996. Differential effects of streptococcal fibronectin-binding protein, FBP54, on adhesion of group A streptococci to human buccal cells and HEp-2 tissue culture cells. Infect. Immun. 64:2415-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cywes, C., I. Stamenkovis, and M. R. Wessels. 2000. CD44 as a receptor for colonization of pharynx by group A Streptococcus. J. Clin. Investig. 106:995-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demers, B., A. E. Simor, H. Vellend, P. M. Schlievert, S. Byrne, F. Jamieson, S. Walmsley, and D. E. Low. 1993. Severe invasive group A streptococcal infections in Ontario, Canada: 1987-1991. Clin. Infect. Dis. 16:792-800. [DOI] [PubMed] [Google Scholar]
- 13.Francis, T. E., and M. V. de Torregrosa. 1945. Combined infection of mice with H. influenzae and influenza virus by the intranasal route. J. Infect. Dis. 76:70-77. [Google Scholar]
- 14.Gryllos, I., C. Cywes, M. H. Shearer, M. Cary, R. C. Kennedy, and M. R. Wessels. 2001. Regulation of capsule gene expression by group A Streptococcus during pharyngeal colonization and invasive infection. Mol. Microbiol. 42:61-74. [DOI] [PubMed] [Google Scholar]
- 15.Hakansson, A., A. Kidd, G. Wadell, H. Sabharwal, and C. Svanborg. 1994. Adenovirus infection enhances in vitro adherence of Streptococcus pneumoniae. Infect. Immun. 62:2707-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harford, C. G., M. R. Smith, and W. B. Wood. 1946. Sulfonamide chemotherapy of combined infection with influenza virus and bacteria. J. Exp. Med. 83:505-518. [PubMed] [Google Scholar]
- 17.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell. Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 18.Hosaka, Y., A. Ikeura, Y. Harada, K. Kuroda, H. Hamayasu, T. Suzuki, K. Yamada Y. Kawase, and Y. Suzuki. 2000. Binding of influenza type A viruses to group B Streptococcus and haemagglutination by virus-bound bacteria. J. Electron Microsc. 49:765-773. [DOI] [PubMed] [Google Scholar]
- 19.Husmann, L. K., D.-L. Yung, S. K. Hollingshead, and J. R. Scott. 1997. Role of putative virulence factors of Streptococcus pyogenes in mouse model of long-term throat colonization and pneumonia. Infect. Immun. 65:1422-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jadoun, J., O. Eyal, and S. Sela. 2002. Role of CsrR, hyaluronic acid, and SpeB in the internalization of Streptococcus pyogenes M type 3 strain by epithelial cells. Infect. Immun. 70:462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadoun, J., and S. Sela. 2000. Mutation in csrR global regulator reduces Streptococcus pyogenes internalization. Microb. Pathog. 29:311-317. [DOI] [PubMed] [Google Scholar]
- 22.Jones, W. T., J. H. Menna, and D. E. Wennerstrom. 1983. Lethal synergism induced in mice by influenza type A virus and type Ia group B streptococci. Infect. Immun. 41:618-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaul, R., A. McGeer, D. E. Low, K. Green, and B. Schwartz. 1997. Population-based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indications, and microbiologic analysis of seventy-seven cases. Am. J. Med. 103:18-24. [DOI] [PubMed] [Google Scholar]
- 24.Kawabata, S., H. Kuwata, I. Nakagawa, S. Morimatsu, K. Sano, and S. Hamada. 1999. Capsular hyaluronic acid of group A streptococci hampers their invasion into human pharyngeal epithelial cells. Microb. Pathog. 27:71-80. [DOI] [PubMed] [Google Scholar]
- 25.LeVine, A. M., V. Koeningknecht, and J. M. Stark. 2001. Decreased pulmonary clearance of S. pneumoniae following influenza A infection in mice. J. Virol. Methods 94:173-186. [DOI] [PubMed] [Google Scholar]
- 26.Limbago, B., V. Penumalli, B. Weinrick, and J. R. Scott. 2000. Role of streptolysin O in a mouse model of invasive group A streptococcal diseases. Infect. Immun. 68:6384-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCullers, J. A., and J. E. Rehg. 2002. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis. 186:341-350. [DOI] [PubMed] [Google Scholar]
- 28.Musher, D. M., R. J. Hamill, C. E. Wright, J. E. Clarridge, and C. M. Ashton. 1996. Trends in bacteremic infection due to Streptococcus pyogenes (group A Streptococcus), 1986-1995. Emerg. Infect. Dis. 2:54-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa, I., M. Nakata, S. Kawabata, and S. Hamada. 2001. Cytochrome c-mediated caspase-9 activation triggers apoptosis in Streptococcus pyogenes infected epithelial cells. Cell. Microbiol. 3:395-405. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima, K., S. Ichiyama, Y. Iinuma, Y. Hasegawa, M. Ohta, K. Ooe, Y. Shimizu, H. Igarashi, T. Murai, and K. Shimokata. 1997. A clinical and bacteriologic investigation of invasive streptococcal infections in Japan on the basis of serotypes, toxin production and genomic DNA fingerprints. Clin. Infect. Dis. 25:260-266. [DOI] [PubMed] [Google Scholar]
- 31.Okada, N., A. P. Pentland, P. Falk, and M. G. Caparon. 1994. M protein and protein F act as important determinants of cell-specific tropism of Streptococcus pyogenes in skin tissue. J. Clin. Investig. 94:965-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto, S., S. Kawabata, I. Nakagawa, Y. Okuno, T. Goto, K. Sano, and S. Hamada. 2003. Influenza A virus-infected hosts boost invasive type of Streptococcus pyogenes infection in mice. J. Virol. 77:4104-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamoto, S., S. Kawabata, I. Nakagawa, and S. Hamada. 2001. Administration of superantigens protects mice from lethal Listeria monocytogenes infection by enhancing cytotoxic T cells. Infect. Immun. 69:6633-6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okuno, Y., Y. Isegawa, F. Sasao, and S. Ueda. 1993. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 67:2552-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid, S. D., N. P. Hoe, L. M. Smoot, and J. M Musser. 2001. Group A Streptococcus: allelic variation, population genetics, and host-pathogen interactions. J. Clin. Investig. 107:393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan, P. A., V. Pancholi, and V. A. Fischetti. 2001. Group A streptococci bind to mucin and human pharyngeal cells through sialic acid-containing receptors. Infect. Immun. 69:7402-7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanford, B. A., A. Shelokov, and M. A. Ramsay. 1978. Bacterial adherence to virus-infected cells: a cell culture model of bacterial superinfection. J. Infect. Dis. 137:176-181. [DOI] [PubMed] [Google Scholar]
- 38.Schranger, H. M., S. Alberti, C. Cywes, G. J. Dougherty, and M. R. Wessels. 1998. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. J. Clin. Investig. 101:1708-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schranger, H. M., J. G. Rheinwald, and M. R. Wessels. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J. Clin. Investig. 98:1954-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sierig, G., C. Cywes, M. R. Wessels, and C. D. Ashbaugh. 2003. Cytotoxic effects of streptolysin O and streptolysin S enhance the virulence of poorly encapsulated group A streptococci. Infect. Immun. 71:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Svensson, N., S. Oberg, B. Henriques, S. Holm, G. Kallenius, V. Romanus, and J. Giesecke. 2000. Invasive group A streptococcal infections in Sweden in 1994 and 1995: epidemiology and clinical spectrum. Scand. J. Infect. Dis. 32:609-614. [DOI] [PubMed] [Google Scholar]
- 42.Switalski, L. M., P. Speziale, M. Hook, T. Wadstrome, and R. Timpl. 1984. Binding of Streptococcus pyogenes to laminin. J. Biol. Chem. 259:3734-3738. [PubMed] [Google Scholar]
- 43.Tao, L., D. J. LeBlanc, and J. J. Ferretti. 1992. Novel streptococcal integration shuttle vectors for gene cloning and inactivation. Gene 120:105-110. [DOI] [PubMed] [Google Scholar]
- 44.Taubenberger, J. K., A. H. Reid, and T. G. Fanning. 2000. The 1918 influenza virus: a killer comes into view. Virology 274:241-245. [DOI] [PubMed] [Google Scholar]
- 45.Terao, Y., S. Kawabata, E. Kunitomo, J. Murakami, I. Nakagawa, and S. Hamada. 2001. Fba, a novel fibronectin-binding protein from Streptococcus pyogenes, promotes bacterial entry into epithelial cells, and the fba gene is positively transcribed under the Mga regulator. Mol. Microbiol. 42:75-86. [DOI] [PubMed] [Google Scholar]
- 46.Terao, Y., S. Kawabata, E. Kunitomo, I. Nakagawa, and S. Hamada. 2002. Novel laminin-binding protein of Streptococcus pyogenes, Lbp, is involved in adhesion to epithelial cells. Infect. Immun. 70:993-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terao, Y., S. Kawabata, M. Nakata, I. Nakagawa, and S. Hamada. 2002. Molecular characterization of a novel fibronectin-binding protein of Streptococcus pyogenes strains isolated from toxic shock-like syndrome patients. J. Biol. Chem. 277:47428-47435. [DOI] [PubMed] [Google Scholar]
- 48.Warren, L. 1959. The thiobarbituric acid assay of sialic acid. J. Biol. Chem. 234:1971-1975. [PubMed] [Google Scholar]
- 49.Watanabe, T., S. Watanabe, G. Neumann, H. Kida, and Y. Kawaoka. 2002. Immunogenicity and protective efficacy of replication-incompetent influenza virus-like particles. J. Virol. 76:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wessels, M. R., R. F. Haft, L. M. Heggen, and C. E. Rubens. 1992. Identification of a genetic locus essential for capsule sialylation in type III group B streptococci. Infect. Immun. 60:392-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wessels, M. R., A. E. Moses, J. B. Goldberg, and T. J. DiCesare. 1994. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc. Natl. Acad. Sci. USA 91:12238-12242. [DOI] [PMC free article] [PubMed] [Google Scholar]