Abstract
The stringent response is a mechanism by which bacteria adapt to nutritional deficiencies through the production of the guanine nucleotides ppGpp and pppGpp, produced by the RelA enzyme. We investigated the role of the relA gene in the ability of an extracellular pathogen, Pseudomonas aeruginosa, to cause infection. Strains lacking the relA gene were created from the prototypical laboratory strain PAO1 as well as the mucoid cystic fibrosis isolate 6106, which lacks functional quorum-sensing systems. The absence of relA abolished the production of ppGpp and pppGpp under conditions of amino acid starvation. We found that strains lacking relA exhibited reduced virulence in a D. melanogaster feeding assay. In conditions of low magnesium, the relA gene enhanced production of the cell-cell signal N-[3-oxododecanoyl]-l-homoserine lactone, whereas relA reduced the production of the 2-heptyl-3-hydroxy-4-quinolone signal during serine hydroxamate induction of the stringent response. In the relA mutant, alterations in the Pseudomonas quinolone system pathways seemed to increase the production of pyocyanin and decrease the production of elastase. Deletion of relA also resulted in reduced levels of the RpoS sigma factor. These results suggest that adjustment of cellular ppGpp and pppGpp levels could be an important regulatory mechanism in P. aeruginosa adaptation in pathogenic relationships.
Bacteria often face nutritional limitations and therefore have specialized mechanisms to adapt to these situations. The stringent response is one such mechanism that enables bacteria to cope with nutritional stress (14). During amino acid starvation, production of the nucleotides guanosine tetraphosphate and guanosine pentaphosphate (ppGpp and pppGpp, respectively) is enhanced via the ribosome-associated RelA protein (5). RelA activity is triggered through binding of uncharged tRNA at the ribosome. The spoT gene encodes a hydrolase that may also have ppGpp and pppGpp synthetase activity under conditions such as carbon starvation (33). In some bacterial species, both spoT and relA must be inactivated in order to completely eliminate the ppGpp and pppGpp synthetase activity (55).
Increased ppGpp and pppGpp levels result in changes in a number of physiological processes, including reduction of stable RNA synthesis, inhibition of growth, and enhancement of amino acid biosynthetic pathways. In Escherichia coli, high levels of ppGpp and pppGpp result in enhanced expression of the stationary-phase sigma factor RpoS and RpoS-regulated genes (16). It is believed that ppGpp and pppGpp direct transcription of genes under the control of alternative sigma factors such as RpoS, by enhancing the competitiveness of these sigma factors for the holoenzyme (29). In addition to their role in transcriptional regulation, high ppGpp and pppGpp levels can also affect the translational efficiency of proteins with high frequencies of rare codons (27). Thus, ppGpp and pppGpp exert their effects at multiple levels.
The stringent response is believed to be important for the ability of a number of intracellular human pathogens to cause disease. In Legionella pneumophila, ppGpp and pppGpp accumulation triggers a switch between an avirulent replicative phase and a virulent motile phase, in which the bacteria spread to neighboring cells (18). These changes are mediated through the GacA homologue LetA (19). In Mycobacterium tuberculosis, ppGpp and pppGpp are involved in processes important for entry into the latent phase of the disease (35, 40). In Listeria monocytogenes, the ability to adhere to surfaces and to infect mice is dependent on the stringent response (48). A mutation in relA of Vibrio cholera affects pathogenicity in the rabbit ileal loop and the suckling mouse models of infection (20). The V. cholerae relA mutation also reduced virulence factor production (cholera toxin and toxin-coregulated pilus) (20). Finally, a recent paper by Pizarro-Cerda and Tedin (39) reports that a relA spoT double mutant of Salmonella enterica serovar Typhimurium is highly attenuated in vivo and noninvasive in vitro. This mutant also has reduced expression of two virulence regulators, HilA and InvF (39).
P. aeruginosa has the ability to occupy a wide range of environmental niches with various nutritional abundances. It can also cause infection in a variety of hosts, including humans, plants, nematodes, and insects, during which it encounters different nutritional environments. There are several processes related to the virulence of P. aeruginosa that could be impacted by the stringent response. Perhaps the most notable is the potential for interaction between the stringent response and quorum sensing.
Quorum-sensing systems allow bacteria to control gene expression through the production of small-molecule signals (32). P. aeruginosa produces two acylhomoserine lactone signals, N-[3-oxododecanoyl]-l-homoserine lactone (3-oxo-C12-HSL), produced via LasI (36), and N-butyryl homoserine lactone (C4-HSL) (37, 54) as well as a quinolone signal, 2-heptyl-3-hydroxy-4-quinolone (PQS) (38). For the P. aeruginosa las and rhl system, the response regulators LasR (15) and RhlR (34) bind autoinducers and activate transcription of a number of target genes in a hierarchical system.
It is well established that quorum sensing is important in P. aeruginosa infections of several hosts, including humans (7, 10). A link between the stringent response and quorum sensing is suggested by several observations. For example, the stringent response and quorum sensing are known to be integrated in a marine Vibrio isolate (45) and in Myxococcus xanthus (8, 21). In Myxococcus xanthus, A-signaling is dependent upon ppGpp and pppGpp (21), which in turn leads to fruiting body formation. In Vibrio sp. strain S14, the relationship is reversed, with AHL signaling required for ppGpp and pppGpp production in response to carbon starvation (45). Recently, it was shown that a mutation in relA in Agrobacterium tumefaciens abolishes the stationary-phase-dependent expression of the acylhomoserine lactone degradation system, suggesting that this system is coupled to and regulated by the ppGpp and pppGpp stress response machinery (56). In P. aeruginosa, a potential link between quorum sensing and the stringent response might occur through polyphosphate kinase. Deficiencies in ppGpp and pppGpp could result in lower polyphosphate kinase activity (28). Polyphosphate kinase is necessary for swimming and swarming motility, virulence, and maximal autoinducer production by P. aeruginosa (41). Still another potential link to quorum sensing may occur through the RpoS sigma factor, which negatively regulates rhlI transcription (53). In this case, ppGpp and pppGpp could cause negative regulation of rhlI synthesis by enhancing RpoS production.
Van Delden et al. (51) recently reported a link between quorum sensing and the stringent response in P. aeruginosa. These researchers compared the levels of autoinducers and quorum-sensing-controlled genes following induction of the stringent response. They observed that overexpression of Escherichia coli relA in P. aeruginosa resulted in earlier 3-oxo-C12-HSL and C4-HSL production. They also found that addition of serine hydroxamate could elicit premature autoinducer production (51). In a separate study, Guina et al. (17) observed that, during growth in low-magnesium conditions, RelA protein levels were enhanced. They also observed that LasI but not RhlI protein levels are increased, as is the production of the PQS signal (17). It was not determined if the changes in LasI protein also result in increased autoinducer synthesis, nor is it known if RelA is required for increased LasI or 3-oxo-C12-HSL synthesis under these conditions.
The possibility of a link between quorum sensing and the stringent response prompted us to investigate whether the relA gene would affect the virulence of P. aeruginosa in a model infection system. In these studies, we used an insect model of infection to demonstrate that the stringent response does affect the virulence of P. aeruginosa. We also found that C4-HSL, 3-oxo-C12-HSL, and PQS were each affected differently by mutations of the relA gene and these effects were dependent on the growth conditions, suggesting that the relA gene contributes to the pathogenesis of P. aeruginosa through both quorum-sensing-dependent and -independent pathways.
MATERIALS AND METHODS
Creation of relA mutant.
The complete genome sequence of P. aeruginosa PAO1 (46) contains a predicted relA homologue with 65% similarity to E. coli relA. PCR primers were designed to amplify 2,276 bases corresponding to nucleotides 6 to 27 (5′-GGCGCAGTCATCGGTGGGCTAC-3′) and 2262 to 2281 (5′-CGCCGCGCCTCGATGATGTT-3′ ) of the relA open reading frame. This region was amplified and cloned in the pCR2.1 cloning vector (Invitrogen) to generate pCRrelA. A 900-bp gentamicin resistance cassette from pUCGm (43) was inserted as a SalI fragment into the SalI site that occurs within the relA sequence to generate pCRrelA::aacC1. This construct was then introduced as an EcoRI fragment into the suicide vector pEX18Ap (25) to create pEXrelA::aacC1. Biparental mating between E. coli SM10(pEXrelA::aacC1) and P. aeruginosa PAO1 was used to replace the wild-type allele with the mutant relA::aac1. Double crossovers were selected with agar containing 5% sucrose and gentamicin (50 μg/ml). The mutation was confirmed by PCR with the same primers, which gave a single band of approximately 3,200 bp, compared to 2,300 bp for the wild type. This procedure was also used to create a relA mutant of the mucoid cystic fibrosis isolate 6106.
To create a plasmid for overexpression of relA in P. aeruginosa, a 4.0-kb fragment containing relA was amplified via PCR and inserted into the cloning vector pCR2.1. This fragment was then isolated as a HindIII-PstI fragment and cloned into the corresponding sites in the pUCP18 shuttle vector (44). In all media tested, the growth rates of PAO1, PAO1 relA, and the complemented strains were similar.
ppGpp and pppGpp assays.
The method of Cashel (4) was used to determine the levels of ppGpp and pppGpp. Cells were grown overnight in morpholinepropanesulfonic acid (MOPS) minimal medium (4). The following morning, they were subcultured by diluting 1:100 into MOPS phosphate-free minimal medium containing 10 μCi of 32P per ml (3.7 × 105 Bq/ml), which was supplemented with 1 mg of Casamino Acids per ml. The cells were cultured to an A600 of 0.20, at which time dl-serine hydroxamate (Sigma) was added at a final concentration of 400 μg/ml to induce serine starvation conditions. After a 30-min incubation, nucleotides were extracted with an equal volume of cold formic acid and visualized following separation on polyethyleneimine-cellulose chromatography sheets and exposure on a phosphoimaging plate.
Drosophila infection assays.
Drosophila melanogaster flies were infected with a feeding assay modified from that of Chugani et al. (6). Each strain to be tested was grown overnight on Luria broth (LB) agar. The following morning, single colonies were inoculated into 2 ml of MOPS minimal medium supplemented with Casamino Acids and grown at 37°C with shaking for 8 h. In order to obtain sufficient numbers for strain 6106 infections, LB broth was used and the cultures were grown for 12 h instead of 8 h. Male Oregon R D. melanogaster flies, 3 to 5 days old, were separated into vials (10 to 12 per vial) without food or water for 5 h. An equal number of fly vials were prepared, containing 5 ml of 5% sucrose agar, and after solidification, a 2.3-cm Whatman filter disk was placed on the surface of the agar. After growth, 1.6 ml of each culture was centrifuged, and the cells were resuspended in 170 μl of sterile 5% sucrose. This suspension was added to the surface of the filter paper. Flies were then transferred to the vials containing the cell suspensions and incubated at 28°C. Ten vials for each PAO1 strain were used, as well as 10 vials for the control treatment (5% sucrose alone), while five vials were used for each 6106 strain. The bacteria were placed on the filter only once, but the flies were allowed to feed on the filter throughout the experiment.
Measurement of 3-oxo-C12-HSL, C4-HSL, and PQS production.
The levels of the autoinducers 3-oxo-C12-HSL and C4-HSL in 50 μl of spent culture supernatants were determined with the reporter plasmids pKDT17 (tacp-lasR lasB-lacZ) and pECP61.5 (tacp-rhlR rhlAB-lacZ), as described previously (10). Supernatants were extracted with an equal volume of acidified ethyl acetate and stored at −20°C until used in the bioassay. For analysis of PQS production, cultures were inoculated to an A600 of 0.05 in 200 ml of MOPS minimal medium supplemented with Casamino Acids to a final concentration of 100 μg/ml. When the cultures reached an A600 of 0.5, each culture was split, and serine hydroxamate was added to one half to a final concentration of 400 μM. Following growth at 37°C for 24 h, 10 ml of culture was extracted twice with 10 ml of acidified ethyl acetate as previously described (3). The extract was dried and resuspended in 100 μl of a 1:1 dilution of acidified ethyl acetate-acetonitrile; 20 μl of the concentrated extract was separated by thin-layer chromatography and photographed as described previously (3).
Measurement of pyocyanin production.
Pyocyanin was measured according to the method of Essar (12). Following growth in MOPS minimal medium supplemented with Casamino Acids for 12 h, 5 ml of culture supernatant was extracted with 3 ml of chloroform. The chloroform layer was then extracted with 1 ml of 0.2 M HCl, and the absorbance of the HCl layer was measured at 520 nm.
Measurement of elastase production.
Elastase in supernatant samples was measured with the elastin-Congo Red assay (42). Supernatants were obtained from bacteria grown in MOPS minimal medium at 37°C for 24 h. Assays are based on three separate growth curves.
Western immunoblotting.
Western immunoblots to measure RpoS levels were performed as described by Brown et al. (2). Protein samples were prepared from liquid cultures by removing 1.0-ml samples at specified intervals, measuring the A540, centrifuging for 5 min at maximum speed, and immediately resuspending in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample buffer. Cells were resuspended in a volume of sample buffer such that the concentration was 0.01 A540 units/μl and stored at −20°C. Prior to separation by SDS-PAGE, the protein samples were denatured in boiling water for 5 min. Following electrophoresis, the proteins were transferred to a Nytran membrane (Schleicher and Schuell). Polyclonal antiserum against RpoS was prepared previously in rabbits as described by Kojic and Venturi (26). Rabbit anti-RpoS antiserum was diluted 1:2,000 in blocking buffer and incubated with the membrane for 1 h at 37°C. The membrane was then washed three times with phosphate-buffered saline and then incubated with the secondary antibody solution, which consisted of goat anti-rabbit immunoglobulin G coupled to horseradish peroxidase and diluted 1:5,000 in blocking buffer for 1 h. The membrane was then rinsed three times with phosphate-buffered saline, and bound antibody was detected with a Bio-Rad peroxidase detection kit according to the manufacturer's instructions.
RESULTS
relA affects ppGpp and pppGpp production under conditions of amino acid limitation.
Our objective in these studies was to determine the effect of the stringent response on the virulence of P. aeruginosa. A defined relA mutant derivative of the prototypical laboratory strain PAO1 was created. We also created a plasmid for overexpressing relA on the shuttle vector pUCP18.
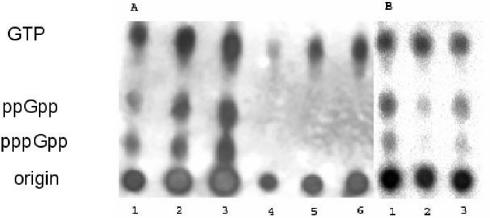
To test whether the production of the second-messenger nucleotides ppGpp and pppGpp were being affected by these mutations, we cultured these strains in MOPS minimal medium supplemented with amino acids, and labeled the cells with 32P-phosphate. Following the addition of serine hydroxamate to induce serine starvation, the nucleotides were extracted and separated via thin-layer chromatography. In these assays, ppGpp and pppGpp were undetectable in the relA mutant, whereas wild-type PAO1 produced high levels of ppGpp and pppGpp (Fig. 1A). The relA strain may still have the ability to produce some ppGpp under other conditions, such as carbon starvation, that we did not specifically test. We were also able to restore ppGpp and pppGpp production through overexpressing the relA gene on the multicopy vector pUCP18 (Fig. 1B). Even without serine hydroxamate, ppGpp and pppGpp were produced from this construct at levels comparable to those in strain PAO1 with the control plasmid pUCP18 induced with serine hydroxamate. These results indicate that the relA gene is necessary for ppGpp and pppGpp production in P. aeruginosa.
FIG. 1.
ppGpp and pppGpp analysis of wild-type and relA strains under amino acid starvation conditions. ppGpp and pppGpp levels in the PAO1 and relA strains in response to induction with serine hydroxamate (SHX) or expression of relA in multicopy. Cells were labeled with radioactive phosphate, and nucleotides were extracted and visualized by thin-layer chromatography followed by exposure to X-ray film (A) or to a phosphoimaging plate (B). (A) Serial dilution (1:125, 1:25, and 1:5) of strain PAO1 (lanes 1 to 3) and strain PAO1 relA (lanes 4 to 6) taken 30 min after induction with serine hydroxamate (500 μM). (B) Lanes 1 and 2 are both strain PAO1 samples with and without serine hydroxamate, respectively, compared to strain PAO1 relA complemented with pUCPrelA without serine hydroxamate induction (lane 3).
relA is required for full virulence of P. aeruginosa in D. melanogaster.
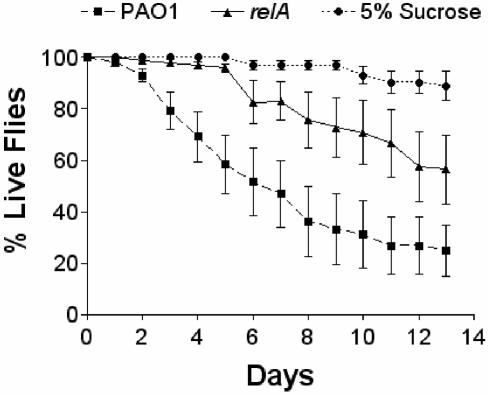
P. aeruginosa can infect a wide range of plants and animals. A very sensitive infection model is the D. melanogaster model of infection (9). We used this model in order to assess the impact of the relA gene and ppGpp and pppGpp production on the virulence of P. aeruginosa. Male Oregon R fruit flies, 2 to 3 days old, were starved for 5 h and then fed a solution of either wild-type or relA P. aeruginosa PAO1 in 5% sucrose. The flies were then monitored over a 2-week period. The flies that were fed the mutant strain died more slowly than those fed the wild-type strain. By day 14, approximately 25% of the flies fed the wild-type bacteria were alive, compared with 50% of the flies that were fed the relA mutant (Fig. 2). The number of viable bacteria added to each vial and the number of viable bacteria surviving after 7 days on the filters were the same for both groups. These results indicate that the P. aeruginosa relA mutant is attenuated in killing in the D. melanogaster model of infection.
FIG. 2.
D. melanogaster killing by wild-type and relA PAO1 strains. Bacteria suspended in sucrose were applied to sterile filter disks covering the surface of a vial. Male fruit flies were then transferred to each vial. The average number of viable cells applied to the filters was 2.22 × 109 for strain PAO1 and 2.32 × 109 for the relA mutant. After 7 days of incubation, the number of viable bacteria was 2.21 × 107 for PAO1 and 3.85 × 107 for the relA mutant.
Impact of a mutation in relA on quorum sensing.
As noted in the introduction, numerous organisms have an association between quorum sensing and the control of the stringent response by relA. It is possible that the decreased virulence of the P. aeruginosa relA mutant could be partially explained by decreased production of autoinducers and the subsequent expression of virulence factors during these infections. In order to test this possibility, we measured the production of 3-oxo-C12-HSL in the wild-type PAO1, relA, and complemented mutant strains in MOPS minimal medium. The production of 3-oxo-C12-HSL was only slightly delayed in the mutant strain compared to the wild type (Fig. 3A), a result similar to that obtained by Van Delden et al. (51) in this medium. Similarly, relA had only a small effect on the levels of C4-HSL (Fig. 3B). Overexpression of the P. aeruginosa relA gene did not significantly affect autoinducer production.
FIG. 3.
3-Oxo-C12-HSL and C4-HSL levels in wild-type, relA mutant. and relA-overexpressing strains. 3-Oxo-C12-HSL (A and C) and C4-HSL (B and D) levels were measured in 50 μl of clarified culture supernatants of cultures grown in MOPS minimal medium supplemented with Casamino Acids containing either a high (1 mM; A and B) or low (0.8 μM; C and D) concentration of magnesium. The results are plotted versus the optical density of the culture at 540 nm to ensure comparison of similar culture densities.
During the course of these studies, it was reported by Guina et al. (17) that growth in low-magnesium conditions results in induction of RelA as well as enhanced LasI production. It is possible that the induction of RelA in low magnesium could contribute to LasI expression and perhaps increase 3-oxo-C12-HSL production. Therefore, the effect of relA on autoinducer production in low magnesium was investigated. Growth in MOPS medium containing 0.8 μM magnesium resulted in high levels of 3-oxo-C12-HSL-production, even at a low cell density (Fig. 3C). The levels of 3-oxo-C12-HSL in the relA mutant were decreased compared to that of the wild type, suggesting that the ability to produce ppGpp and pppGpp could contribute to enhanced 3-oxo-C12-HSL in low magnesium (Fig. 3C). However, the production of C4-HSL was not affected as much by relA in these assays (Fig. 3D), which is consistent with the observations of Guina et al. (17), who found that RhlI levels were not enhanced during growth in low magnesium.
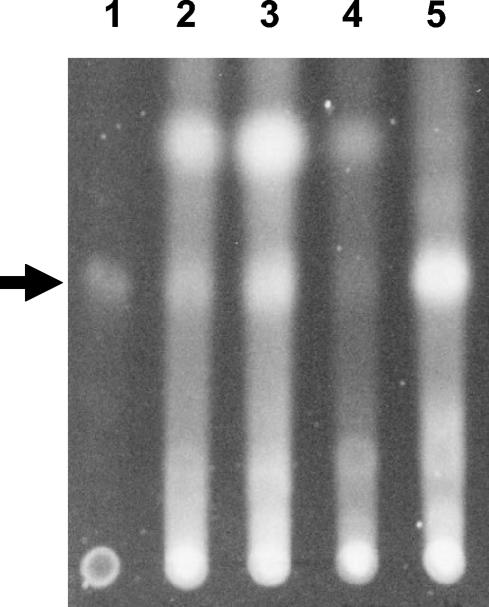
We also tested the effect of a high concentration (400 μM) of serine hydroxamate on the production of the Pseudomonas quinolone system (PQS). The PQS system is a third signal (2-heptyl-3-hydroxyl-4-quinolone) that plays a role in the regulation of multiple virulence factors and the expression of which is intertwined with that of the LasIR and RhlIR quorum-sensing system. Interestingly, when wild-type PAO1 was treated with serine hydroxamate, the levels of PQS were decreased compared to that of untreated cultures (Fig. 4). This effect was not observed in the relA mutant strain (Fig. 4), suggesting that high levels of serine hydroxamate could repress PQS production in a relA-dependent manner.
FIG. 4.
PQS levels in response to serine hydroxamate induction of the stringent response. PQS was extracted with ethyl acetate from cultures as described in Materials and Methods. Lane 1 contains 50 ng of synthetic PQS. The cultures for lanes 2 and 3 were grown without serine hydroxamate and the cultures for lanes 4 and 5 were grown with serine hydroxamate. Lanes: 2 and 4, strain PAO1; 3 and 5, strain PAO1 relA. PQS is indicated by the arrowhead. The results presented are representative of three independent repeats of this experiment.
RelA affects pyocyanin, elastase, and RpoS levels.
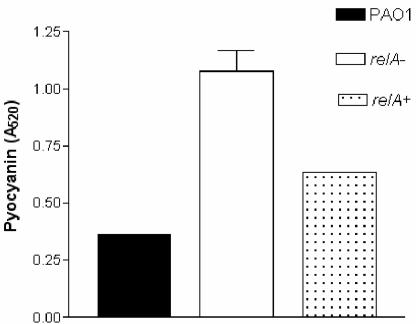
To examine the affect of relA on two virulence factors controlled by the quorum-sensing systems, we assayed pyocyanin and elastase production in strains PAO1 and PAO1 relA. We observed that the relA mutant produced higher levels of pyocyanin than the wild-type strain. Pyocyanin production occurred earlier in the growth phase and was higher at 12 h in the relA mutant strain (Fig. 5). Pyocyanin production is positively regulated by the rhl quorum-sensing system and negatively regulated by the alternative sigma factor RpoS (47). Since the relA strain was unaffected in C4-HSL production, we hypothesized that the increased pyocyanin might be due to decreased RpoS production. Western blotting of total protein extracts from cultures grown in minimal medium showed that the relA mutant produced less RpoS than the wild-type strain and that this decrease could be complemented with pUCPrelA (Fig. 6).
FIG. 5.
Pyocyanin levels are affected by relA. Cultures were grown in medium containing a low concentration of magnesium (0.8 μM). After 12 h of growth, culture supernatants were extracted with chloroform and hydrochloric acid, and pyocyanin levels were expressed as the absorbance at 520 nm.
FIG. 6.
Western blot analysis of RpoS levels in PAO1, PAO1 relA, and PAO1 relA(pUCPrelA). Cultures were sampled at the indicated time points (4, 8, 12, and 24 h). The cells were resuspended to 0.01 A540 units/μl and lysed in SDS-PAGE sample buffer. Cell lysates (10 μl) were separated by SDS-PAGE. Following transfer of the proteins to a Nytran membrane, RpoS was detected with anti-RpoS rabbit antiserum (1:2,000 dilution).
The LasI/R signal 3-oxo-C12-HSL is required for elastase production in P. aeruginosa. In contrast, C4-HSL and PQS significantly enhance elastase production but are not required. We found over a number of growth curves that elastase production was significantly lower (by 44%) in the PAO1 relA mutant (average optical density at 495 nm, 0.322) compared to the prototypical strain PAO1 (average optical density at 495 nm, 0.575) (paired t test, t = 2.743, df = 5, P < 0.05). Lower levels of both 3-oxo-C12-HSL and RpoS produced by this strain are consistent with the decreased elastase production.
Interestingly, the relA mutant showed no differences from the parental strain PAO1 with respect to swarming, swimming, and biofilm formation (data not shown).
relA deletion in a mucoid cystic fibrosis isolate reduces virulence.
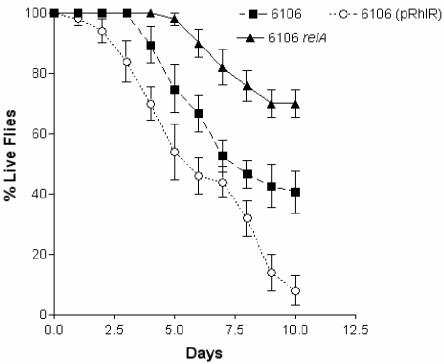
Our results had suggested that RelA may be affecting virulence factor production through the quorum-sensing hierarchy. We wanted to determine if this relA mutation had an effect on fly killing independent of the quorum-sensing systems. We also examined the effect of relA on virulence in a cystic fibrosis strain lacking functional quorum-sensing genes. The cystic fibrosis isolate 6106 is deficient in the production of autoinducers and proteases. Sequence analysis shows that it carries mutations in lasB, lasR, and rhlR (10; K. Stuber and D. G. Storey, unpublished data). A relA derivative of this strain along with the parent and the parent strain carrying a functional rhlR gene in multicopy were tested in the D. melanogaster feeding assay (Fig. 7). Similar to many cystic fibrosis isolates, this strain is less virulent than strain PAO1, necessitating the use of higher numbers of bacteria to achieve fly killing. Nevertheless, the relA mutant of strain 6106 was reduced in fly killing compared to the parent strain. The addition of rhlR to strain 6106 resulted in enhanced fly killing. These results demonstrate that the rhl quorum-sensing system affects virulence in this model but also that the stringent response can impact virulence independently of quorum sensing.
FIG. 7.
D. melanogaster killing by cystic fibrosis isolate 6106, 6106 relA, and 6106(pRhlR). The cultures tested were grown for 12 h in 5 ml of LB broth to generate sufficient quantities of bacteria to kill the flies. Approximately 9.0 × 109 bacteria suspended in sucrose were placed on each filter, and the survival of the flies was monitored over 10 days.
DISCUSSION
In these studies, we have shown that disruption of the relA gene of P. aeruginosa severely limits the production of ppGpp and pppGpp under conditions of amino acid starvation. This finding is similar to that for Vibrio cholerae, in which a relA mutation abolishes ppGpp and pppGpp production (20) and is in contrast to Salmonella enterica serovar Typhimurium and E. coli, in which both relA and spoT must be mutated to get a similar effect (39, 56). The ability to produce the nucleotides ppGpp and pppGpp is likely relevant during adaptation to different host environments, even for a predominantly extracellular pathogen such as P. aeruginosa. Indeed, mutants of P. aeruginosa that lack the relA gene were less virulent in an insect model of infection. We demonstrated for the first time that the relA gene is important for an extracellular pathogen such as P. aeruginosa to cause infection.
We used the D. melanogaster infection model, which has previously been used to identify virulence genes in P. aeruginosa (6, 9, 13). Furthermore, we show that the production of two P. aeruginosa virulence factors, pyocyanin and elastase, was altered in a relA mutant. Pyocyanin levels were increased and elastase levels were decreased. This is an important finding, as it suggests that even a slight alteration in expression of these virulence factors may result in decreased killing in the D. melanogaster infection assay. Pyocyanin has been shown to have both inhibitory and stimulatory effects on B and T cells (49) as well as being important in aiding in the development of persistent infections caused by P. aeruginosa (50). Thus, increased production of pyocyanin in the D. melanogaster feeding model, which is a more chronic type of infection, may itself lessen the virulence of the bacterium.
Our studies suggest that the stringent response might interact with the quorum-sensing systems, albeit in a manner somewhat different from what was expected based on previous studies (17, 51). In low-magnesium conditions, the relA gene contributed to enhanced production of 3-oxo-C12-HSL (Fig. 3). However, the PQS signal appeared to be repressed by ppGpp and pppGpp, as PQS production was lowered by the addition of serine hydroxamate in wild-type but not relA strains (Fig. 4).
Conversely, the effect of relA on the production of C4-HSL was not as pronounced. This may be a result of the effect of the stringent response on multiple pathways that affect C4-HSL. For instance, we have shown that the stringent response affected the production of RpoS. RpoS negatively regulates rhlI expression (53). Furthermore, PQS positively regulates rhlI expression (31), and our studies have also shown that the stringent response can repress PQS production (Fig. 4). Enhanced RpoS and decreased PQS would be expected to govern the production of C4-HSL during the stringent response. Conversely, possible increases in 3-oxo-C12-HSL as a result of the stringent response might trigger enhanced rhlR and subsequently C4-HSL production. Therefore, the net change in C4-HSL as a result of the stringent response appears to be small.
These findings may be relevant to the lung infections associated with cystic fibrosis. The D. melanogaster infection assays suggest that the stringent response may be important in establishing an infection, perhaps because of its effects on cell-cell signaling but also likely through quorum-sensing-independent pathways. It is possible that ppGpp and pppGpp production might be important in adaptation of the pathogen to the airway of cystic fibrosis patients. For instance, Guina et al. (17) found that growth in low magnesium induces expression of the RelA protein, which would likely increase the production of ppGpp. Interestingly, growth in low magnesium elicits changes in lipopolysaccharide structure that mimic the changes that take place in the airway of cystic fibrosis patients (11). This suggests that some environmental signal present in the lung of cystic fibrosis patients is also present in this medium. It is possible that bacteria in the airways of cystic fibrosis patients do experience low-magnesium conditions. Low magnesium availability might occur because the high level of DNA within the airways of cystic fibrosis patients, which contributes to mucus viscosity, could also act as a chelator of positively charged ions such as magnesium.
One difference between our results and those reported by Van Delden et al. (51) was the effect of overexpression of the relA gene on the quorum-sensing systems. In their study, the E. coli relA gene was expressed in P. aeruginosa, which resulted in increased transcription of the las and rhl quorum-sensing genes, as assessed with promoter fusions. Our study used the P. aeruginosa relA gene, which may have altered ppGpp and pppGpp levels differently. We avoided the use of reporter fusions carried on plasmids, as the stringent response could affect the copy number of such plasmids, potentially interfering with the measurements (22-24). We measured ppGpp and pppGpp production by the relA strains created in these studies to ensure that the levels of these nucleotides were being affected (Fig. 1). No such measurements were reported by Van Delden et al. (51), which makes comparisons difficult. Therefore, it is possible that the experimental conditions used by Van Delden et al. elicited changes in the production of ppGpp and pppGpp of a different magnitude, which were able to affect autoinducer production. The concentrations of ppGpp and pppGpp produced as a result of relA overexpression in our studies may have been too great or too small to produce a similar effect. However, magnesium limitation did cause increases in 3-oxo-C12-HSL production which were partly dependent on relA (Fig. 3), suggesting that the levels of ppGpp and pppGpp under these conditions were appropriate for an effect on 3-oxo-C12-HSL.
In contrast to the effect of relA on 3-oxo-C12-HSL in low magnesium, serine hydroxamate induction of the stringent response actually repressed the production of PQS (Fig. 4). Again, this might be a consequence of different ppGpp and pppGpp levels elicited with the use of serine hydroxamate versus low magnesium to induce the stringent response. Guina et al. (17) found that growth in magnesium-limited conditions results in enhanced RelA production and PQS synthesis but did not examine the contribution of the stringent response to PQS production directly. They also found that some isolates from recently infected cystic fibrosis patients produced higher levels of PQS.
We also observed that relA affects the production of RpoS. Suh et al. (47) previously reported that RpoS regulates numerous genes that play a role in the virulence of P. aeruginosa, including exotoxin A. Deletion of relA did not abolish RpoS expression, suggesting that there may not be a strict requirement for ppGpp for expression of RpoS-regulated genes in P. aeruginosa.
The relationships between the stringent response, quorum-sensing systems, and the RpoS sigma factor are complex and remain unclear. Latifi et al. (30) initially reported that rpoS transcription is triggered by C4-HSL and RhlR. This is supported by Wagner et al. (52), who showed that in a quorum-sensing mutant (strain PAO-JP2), the addition of exogenous autoinducers enhanced rpoS transcription approximately threefold under certain growth conditions. However, Whiteley et al. (53) presented data supporting the opposite conclusion, that rpoS transcription is not enhanced by C4-HSL and rhlR but that an rpoS mutant produces higher levels of C4-HSL. Those data suggest that RpoS negatively regulates C4-HSL production. Conversely, Kojic and Venturi (26) and Bertani et al. (1) reported that a P. aeruginosa psrA mutant produces substantially less RpoS yet displays no differences in either 3-oxo-C12-HSL or C4-HSL levels. Our data show that the absence of ppGpp and pppGpp can reduce RpoS levels.
Overall, our results suggest a link between the stringent response and the virulence of the extracellular organism P. aeruginosa. It appears that this link is at least partially dependent on the las, rhl, and/or quinolone signaling systems. The result of this disruption of the quorum-sensing hierarchy is an alteration in the production levels of two virulence factors, pyocyanin and, in particular, elastase. It also appears that relA has an influence that is independent of the quorum-sensing system, as shown by the attenuated fly killing by isolate 6106, which lacks the quorum-sensing system. Further study is needed to find out what stage of infection is affected by the stringent response and whether relA and spoT together might play a larger role in infection.
Acknowledgments
This work was supported by Canadian Institute of Health Research Grant (CIHR) MOP-43901 to D.G.S. D.L.E. was supported by a Canadian Cystic Fibrosis Foundation Studentship. J.L.L. and E.C.P. were supported by a research grant from the National Institutes of Allergy and Infectious Diseases (grant R01-AI46682 to E.C.P.).
We are grateful to D. A. D’Argenio for help in setting up the Drosophila infection assays.
Editor: V. J. DiRita
REFERENCES
- 1.Bertani, I., M. Sevo, M. Kojic, and V. Venturi. 2003. Role of GacA, LasI, RhlI, Ppk, PsrA, Vfr and ClpXP in the regulation of the stationary phase sigma factor rpoS/RpoS in Pseudomonas. Arch. Microbiol. 180:264-271. [DOI] [PubMed] [Google Scholar]
- 2.Brown, L., D. Gentry, T. Elliott, and M. Cashel. 2002. DksA affects ppGpp induction of RpoS at a translational level. J. Bacteriol. 184:4455-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calfee, M. W., J. P. Coleman, and E. C. Pesci. 2001. Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 98:11633-11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cashel, M. 1994. Detection of ppGpp and pppGpp accumulation patterns in Escherichia coli mutants, p. 341-356. In K. W. Adolph (ed.), Methods in molecular genetics, vol. 3, part A. Academic Press, New York, N.Y. [Google Scholar]
- 5.Cashel, M., D.R. gentry, V. J. Hernadez, and D. Vinella. 1996. The stringent response, p. 1458-1496. In F. C. Neidhardt et al., (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 6.Chugani, S. A., M. Whiteley, K. M. Lee, D. D'Argenio, C. Manoil, and E. P. Greenberg. 2001. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 98:2752-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collier, D. N., L. Anderson, S. L. McKnight, T. L. Noah, M. Knowles, R. Boucher, U. Schwab, P. Gilligan, and E. C. Pesci. 2002. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol. Lett. 215:41-46. [DOI] [PubMed] [Google Scholar]
- 8.Crawford, E. W., Jr., and L. J. Shimkets. 2000. The stringent response in Myxococcus xanthus is regulated by socE and the csgA C-signaling protein. Genes Dev. 14:483-492. [PMC free article] [PubMed] [Google Scholar]
- 9.D'Argenio, D. A., L. A. Gallagher, C. A. Berg, and C. Manoil. 2001. Drosophila as a model host for Pseudomonas aeruginosa infection. J. Bacteriol. 183:1466-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson, D. L., R. Endersby, A. Kirkham, K. Stuber, D. D. Vollman, H. R. Rabin, I. Mitchell, and D. G. Storey. 2002. Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect. Immun. 70:1783-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561-1565. [DOI] [PubMed] [Google Scholar]
- 12.Essar, D. W., L. Eberly, A. Hadero, and I. P. Crawford. 1990. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 172:884-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fauvarque, M. O., E. Bergeret, J. Chabert, D. Dacheux, M. Satre, and I. Attree. 2002. Role and activation of type III secretion system genes in Pseudomonas aeruginosa-induced Drosophila killing. Microb. Pathog. 32:287-295. [DOI] [PubMed] [Google Scholar]
- 14.Gallant, J. A. 1979. Stringent control in E. coli. Annu. Rev. Genet. 13:393-415. [DOI] [PubMed] [Google Scholar]
- 15.Gambello, M. J., and B. H. Iglewski. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 173:3000-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentry, D. R., V. J. Hernandez, L. H. Nguyen, D. B. Jensen, and M. Cashel. 1993. Synthesis of the stationary-phase sigma factor sigma S is positively regulated by ppGpp. J. Bacteriol. 175:7982-7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guina, T., S. O. Purvine, E. C. Yi, J. Eng, D. R. Goodlett, R. Aebersold, and S. I. Miller. 2003. Quantitative proteomic analysis indicates increased synthesis of a quinolone by Pseudomonas aeruginosa isolates from cystic fibrosis airways. Proc. Natl. Acad. Sci. USA 100:2771-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammer, B. K., and M. S. Swanson. 1999. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33:721-731. [DOI] [PubMed] [Google Scholar]
- 19.Hammer, B. K., E. S. Tateda, and M. S. Swanson. 2002. A two-component regulator induces the transmission phenotype of stationary-phase Legionella pneumophila. Mol. Microbiol. 44:107-118. [DOI] [PubMed] [Google Scholar]
- 20.Haralalka, S., S. Nandi, and R. K. Bhadra. 2003. Mutation in the relA gene of Vibrio cholerae affects in vitro and in vivo expression of virulence factors. J. Bacteriol. 185:4672-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris, B. Z., D. Kaiser, and M. Singer. 1998. The guanosine nucleotide ppGpp and pppGpp initiates development and A-factor production in Myxococcus xanthus. Genes Dev. 12:1022-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hecker, M., A. Schroeter, and F. Mach. 1983. Replication of pBR322 DNA in stringent and relaxed strains of Escherichia coli. Mol. Gen. Genet. 190:355-357. [DOI] [PubMed] [Google Scholar]
- 23.Herman, A., A. Wegrzyn, and G. Wegrzyn. 1994. Differential replication of plasmids during stringent and relaxed response of Escherichia coli. Plasmid 32:89-94. [DOI] [PubMed] [Google Scholar]
- 24.Herman, A., and G. Wegrzyn. 1995. Effect of increased ppGpp concentration on DNA replication of different replicons in Escherichia coli. J. Basic Microbiol. 35:33-39. [DOI] [PubMed] [Google Scholar]
- 25.Hoang, T. T., A. J. Kutchma, A. Becher, and H. P. Schweizer. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59-72. [DOI] [PubMed] [Google Scholar]
- 26.Kojic, M., and V. Venturi. 2001. Regulation of rpoS gene expression in Pseudomonas: involvement of a TetR family regulator. J. Bacteriol. 183:3712-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhar, I., J. P. van Putten, D. Zgur-Bertok, W. Gaastra, and B. J. Jordi. 2001. Codon-usage based regulation of colicin K synthesis by the stress alarmone ppGpp. Mol. Microbiol. 41:207-216. [DOI] [PubMed] [Google Scholar]
- 28.Kuroda, A., H. Murphy, M. Cashel, and A. Kornberg. 1997. Guanosine tetra- and pentaphosphate promote accumulation of inorganic polyphosphate in Escherichia coli. J. Biol. Chem. 272:21240-21243. [DOI] [PubMed] [Google Scholar]
- 29.Kvint, K., A. Farewell, and T. Nystrom. 2000. RpoS-dependent promoters require guanosine tetraphosphate for induction even in the presence of high levels of sigma(s). J. Biol. Chem. 275:14795-14798. [DOI] [PubMed] [Google Scholar]
- 30.Latifi, A., M. Foglino, K. Tanaka, P. Williams, and A. Lazdunski. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21:1137-1146. [DOI] [PubMed] [Google Scholar]
- 31.McKnight, S. L., B. H. Iglewski, and E. C. Pesci. 2000. The Pseudomonas quinolone signal regulates Rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 182:2702-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 33.Murray, K. D., and H. Bremer. 1996. Control of spoT-dependent ppGpp synthesis and degradation in Escherichia coli. J. Mol. Biol. 259:41-57. [DOI] [PubMed] [Google Scholar]
- 34.Ochsner, U. A., and J. Reiser. 1995. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:6424-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ojha, A. K., T. K. Mukherjee, and D. Chatterji. 2000. High intracellular level of guanosine tetraphosphate in Mycobacterium smegmatis changes the morphology of the bacterium. Infect. Immun. 68:4084-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson, J. P., K. M. Gray, L. Passador, K. D. Tucker, A. Eberhard, B. H. Iglewski, and E. P. Greenberg. 1994. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA 91:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearson, J. P., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pesci, E. C., Milbank, J. B., Pearson, J. P., McKnight, S., Kende, A. S., Greenberg, E. P., and Iglewski, B. H. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pizarro-Cerda, J., and K. Tedin. 2004. The bacterial signal molecule, ppGpp, regulates Salmonella virulence gene expression. Mol. Microbiol. 52:1827. [DOI] [PubMed] [Google Scholar]
- 40.Primm, T. P., S. J. Andersen, V. Mizrahi, D. Avarbock, H. Rubin, and C. E. Barry 3rd. 2000. The stringent response of Mycobacterium tuberculosis is required for long-term survival. J. Bacteriol. 182:4889-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rashid, M. H., K. Rumbaugh, L. Passador, D. G. Davies, A. N. Hamood, B. H. Iglewski, and A. Kornberg. 2000. Polyphosphate kinase is essential for biofilm development, quorum sensing, and virulence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 97:9636-9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schad, P. A., R. A. Bever, T. I. Nicas, F. Leduc, L. F. Hanne, and B. H. Iglewski. 1987. Cloning and characterization of elastase genes from Pseudomonas aeruginosa. J. Bacteriol. 169:2691-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schweizer, H. D. 1993. Small broad-host-range gentamycin resistance gene cassettes for site-specific insertion and deletion mutagenesis. BioTechniques 15:831-834. [PubMed] [Google Scholar]
- 44.Schweizer, H. P. 1991. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene 97:109-121. [DOI] [PubMed] [Google Scholar]
- 45.Srinivasan, S., J. Ostling, T. Charlton, R. de Nys, K. Takayama, and S. Kjelleberg. 1998. Extracellular signal molecule(s) involved in the carbon starvation response of marine Vibrio sp. strain S14. J. Bacteriol. 180:201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 47.Suh, S. J., L. Silo-Suh, D. E. Woods, D. J. Hassett, S. E. West, and D. E. Ohman. 1999. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J. Bacteriol. 181:3890-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor, C. M., M. Beresford, H. A. Epton, D. C. Sigee, G. Shama, P. W. Andrew, and I. S. Roberts. 2002. Listeria monocytogenes relA and hpt mutants are impaired in surface-attached growth and virulence. J. Bacteriol. 184:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulmer, A. J., J. Pryjma, Z. Tarnok, M. Ernst, and H. D. Flad. 1990. Inhibitory and stimulatory effects of Pseudomonas aeruginosa pyocyanine on human T and B lymphocytes and human monocytes. Infect. Immun. 58:808-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Usher, L. R., R. A. Lawson, I. Geary, C. J. Taylor, C. D. Bingle, G. W. Taylor, and M. K. Whyte. 2002. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J. Immunol. 168:1861-1868. [DOI] [PubMed] [Google Scholar]
- 51.van Delden, C., R. Comte, and A. M. Bally. 2001. Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J. Bacteriol. 183:5376-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner, V. E., D. Bushnell, L. Passador, A. L. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whiteley, M., M. R. Parsek, and E. P. Greenberg. 2000. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J. Bacteriol. 182:4356-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winson, M. K., M. Camara, A. Latifi, M. Fogliono, S. R. Chhabra, M. Daykin, M. Bally, V. Chapon, G. P. C. Salmono, B. W. Bycroft, A. Lazowski, G. S. A. B. Stewart, and P. Williams. 1995. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:9427-9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao, H., M. Kalman, K. Ikehura, S. Zemel, G. Glaser, and M. Cashel. 1991. Residual guanosine 3′,5′ bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 266:5980-5990. [PubMed] [Google Scholar]
- 56.Zhang, H-B., C. Wang, and L-H. Zhang. 2004. The quormone degradation system of Agrobacterium tumefaciens is regulated by starvation signal and stress alarmone ppGpp and pppGpp. Mol. Microbiol. 52:1389-1401. [DOI] [PubMed] [Google Scholar]