Abstract
Background
The management of uterine-serous-carcinoma (USC) no longer amenable to treatment with surgery, radiation and/or chemotherapy remains dismal. Alternative therapeutic options are desperately needed.
Case
We describe the case of a heavily pretreated 74-year-old patient with a recurrent USC overexpressing HER2/neu at 3 + level by IHC treated with the anti-HER2/neu antibody-drug-conjugate (ADC) trastuzumab-emtansine (TDM-1-Kadcyla-Genentech/Roche). She experienced a remarkable clinical response to TDM-1 with a complete resolution of a large metastatic, radiation/chemotherapy resistant tumor deposit in her abdominal wall muscle confirmed by multiple CAT scans and a prolonged systemic control of her disease.
Conclusion
TDM-1 may represent a novel treatment option for recurrent/metastatic HER2/neu-positive USC patients refractory to salvage-treatment.
Keywords: Uterine serous carcinoma, Trastuzumab-emtansine, HER2/neu
Highlights
-
•
USC patients have limited therapeutic options when the disease becomes resistant to chemotherapy.
-
•
About 35% of USC overexpress the HER2/neu receptor at 3 + level by IHC and/or are c-erbB2 gene amplified.
-
•
Trastuzumab emtansine (T-DM1, Kadcyla) is a novel antibody-drug conjugate.
-
•
T-DM1 may represent a novel treatment modality in USC patients with recurrent, HER2/neu + disease.
1. Introduction
USC is a rare histological type of endometrial cancer characterized by a poor prognosis. While USC constitutes only 10% of cases, it accounts for as many as 40% of deaths (Fader et al., 2010). The 5-year disease-specific survival rate for USC is only 55% (Hamilton et al., 2006), which compares unfavorably to the rate of 89% for grade 1 or 2 endometrioid cancers (Creasman et al., 2006). As many as 46% of patients have extrauterine spread at time of diagnosis, compared to only 26% of patients with type I disease. Five-year overall survival for stage I, II, III, and IV disease is 50–80%, 50%, 20%, and 5–10%, respectively (Boruta et al., 2009). HER2/neu overexpression is detected in about 35% of USC (Buza et al., 2013) and a shorter survival has been independently associated with HER2 expression in USC patients (Santin et al., 2005). Novel, target-specific and effective modalities against USC refractory to standard treatments remain desperately needed.
2. Case report
The patient is a 74-year old woman diagnosed with a mixed uterine serous (USC, 90%) and Clear Cell (CC, 10%) carcinoma in July 2012 approximately 8 months after she was noted to have an elevated CA125 (i.e., November 2011). Her initial pelvic ultrasound at the time of the elevated CA125 was reported as negative. The CA125 elevation persisted and in July 2012 she had a repeat ultrasound, which showed blood in the endometrial wall. An endometrial biopsy performed at that time was positive for USC. She underwent a laparoscopic total hysterectomy-bilateral salpingo-oophorectomy and comprehensive staging including an omentectomy, with a final pathology report consistent with Stage IB mixed USC/CC (Fig. 1). She received one dose of adjuvant carboplatin and paclitaxel. However, secondary to the rapid development of neuropathy, the adjuvant chemotherapy regimen was switched from paclitaxel to docetaxel in combination with carboplatin (ie, cycles 2 to 6). A follow-up Pap smear after completion of chemotherapy was abnormal, and a subsequent biopsy confirmed the presence of a local recurrence at the vaginal cuff. CT scan of the chest, abdomen and pelvis in January 2013 were negative for distant metastases. She was therefore referred for pelvic external beam radiotherapy, which was completed on 4/1/13, followed by brachytherapy to the vaginal cuff, which was completed on 5/22/13.
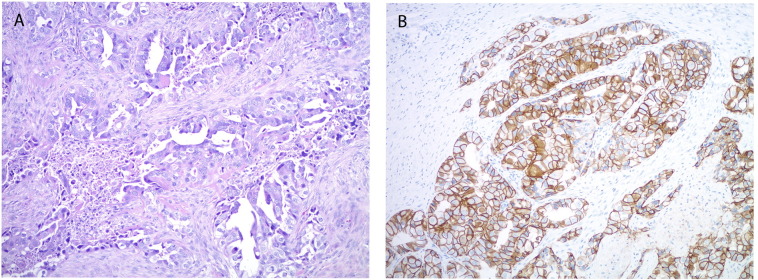
Fig. 1.
Representative microscopic images of recurrent serous endometrial carcinoma in the rectus muscle. A. The tumor shows predominantly glandular architecture with high nuclear grade and pale eosinophilic cytoplasm, consistent with the serous carcinoma component of the patient's endometrial primary. B. Her2 immunohistochemistry demonstrates strong membranous staining involving the complete membrane or showing a lateral/baso-lateral staining pattern. (A. original magnification 100 ×, H&E stain, B. Original magnification 100 ×, Her2 immunostain).
She remained stable until May 2014, when she was noted to have a mass growing in the rectus sheath. She underwent resection on 6/12/14, with pathology consistent with recurrent USC. A repeat CT scan of the chest, abdomen and pelvis on 6/13/14 revealed multiple new sub-centimeter lung nodules, consistent with widespread metastatic disease. She received 6 weeks of consolidative radiation to the abdominal wall, with follow-up CT scan on 9/29/14 showing a reduction in the rectus muscle lesion but an increase in size of the pulmonary nodules. A PET/CT scan performed on 10/09/14 revealed two new areas of recurrent disease in the abdominal wall in addition to the lung nodules, and biopsy of a lung nodule at that time was confirmed positive for metastatic USC.
She was therefore started on systemic chemotherapy consisting of gemcitabine, carboplatin and bevacizumab on 10/16/14. Treatment was complicated by progressive leg weakness, abdominal complaints and anemia, in part from chemotherapy and in part from radiation proctitis. She completed her 6th cycle of chemotherapy on 3/5/15, with a CT scan dated 3/16/15 showing reduction in both the abdominal wall masses and in the lung nodules. She continued on bevacizumab as maintenance therapy, and was then referred for stereotactic radiotherapy to the lung nodules on 7/13/15.
She was stable until October 2015, when a repeat CT scan demonstrated progression of her recurrent disease in the rectus muscle. At this time point she was referred to our institution for a second opinion. Next generation sequencing (NGS) testing (Foundation Medicine, (FM) Inc. Cambridge, MA) and Microsatellite instability (MSI) testing by PCR were performed and demonstrated a MSI stable tumor harboring a c-erbB2 gene amplification as well as mutations in multiple genes commonly reported mutated in USC including but not limited to TP53 (R249T), PIK3CA (H1047R), CHD4 (F1072 L) and PPP2R1A (S256F) (Zhao et al., 2013). On 10/20/2015, after confirmation of HER2/neu expression at 3 + level by IHC (ie, test performed in the Yale Pathology Department at Yale University) (Fig. 1) she was enrolled in a clinical trial with Afatinib. Unfortunately, a CT scan dated 11/30/15 showed progression of her target lesion in the abdominal wall (i.e., enlargement of her recurrent/metastatic rectus muscle disease).
With the goal to inhibit tumor progression and on the basis of the encouraging preliminary results of study NCT01367002 entitled: Randomized Phase II evaluation of carboplatin/paclitaxel with and without trastuzumab in HER2/neu patients with advanced/recurrent USC, [abstract IGCS-0242 describing interim analysis results after 26 events has been presented at the IGCS bi-annual meeting, Lisbon, Portugal, in October 2016], demonstrating a median PFS of 7.3 months in the chemotherapy arm (CP) vs 12.1 months in the trastuzumab arm (CP-T) (HR 0.73; 95% CI 0.33–1.58; p = 0.21), after extensive counseling and informed consent on December 19, 2015 the patient was started on trastuzumab emtansine (TDM-1, Kadcyla, Genentech/Roche) single agent therapy (3,6 mg/kg) every 3 weeks. CA125 level at that time was elevated at 70.9 U/ML.
Patient tolerated TDM-1 treatment well with some delay in the administration of the drug related to G3 thrombocytopenia. CT imaging obtained 1 month after initiation of TDM-1 treatment, (Fig. 2 middle panel) and 3 months thereafter (Fig. 2, right panel) demonstrated a remarkable response to TDM-1 (ie, a partial response after 1 month and a complete resolution after 3 months by RECIST v1.1) of the large metastatic target lesion growing in the rectus muscle. A CT scan obtained at 7 months after treatment initiation demonstrated a sustained clinical response with no appearance of new lesions. Patient's remarkable clinical response persists at the time of the writing of this report after a total of 9 TDM-1 administrations with no Grade 4 side effects reported to date and a normalized CA125.
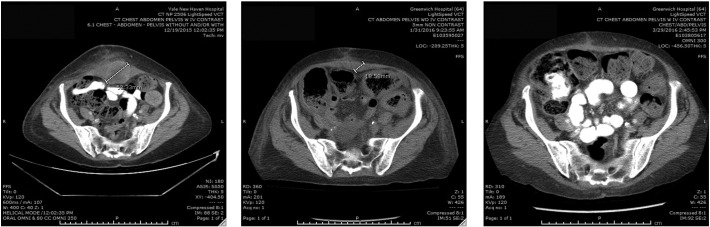
Fig. 2.
Representative CT scans demonstrating activity of TDM-1. Left panel: Pretreatment image with baseline measurement of the target recurrent/metastatic tumor deposit (i.e., mass growing in the rectus muscle). Middle panel: regression of the metastatic tumor deposits described above one month after TDM-1 treatment initiation. Right panel: complete regression of the metastatic tumor deposits described above three months after treatment initiation with TDM-1.
3. Discussion
To our knowledge, this is the first report demonstrating clinical activity of TDM-1 in a HER2/neu overexpressing USC patient with recurrent/chemotherapy resistant disease.
Trastuzumab emtansine (T-DM1, Genentech/Roche) is a novel antibody-drug conjugate that combines trastuzumab with targeted delivery of the antimicrotubule agent DM1. DM1 belongs to the maytansine class of chemotherapeutic agents and on average 3–4 molecules of DM1 are conjugated to each trastuzumab molecule. T-DM1 is internalized by HER2 receptor-mediated endocytosis and as such its action is specific to HER2 expressing cells. After internalization, T-DM1 is then degraded by lysosomes resulting in the release of free intracellular DM-1. DM-1 is a potent microtubule assembly inhibitor and its activity leads to cell death as a result of G2/M phase cell cycle arrest and apoptosis (English et al., 2014). T-DM1 also has the advantage of retaining the mechanism of action of trastuzumab in regards to reducing signaling in the HER2 pathway and initiation of ADCC (English et al., 2014). T-DM1 is the first antibody-drug conjugate receiving United States Food and Drug Administration approval for HER2 positive metastatic breast cancer following the results of the randomized phase III trial of T-DM1 versus lapatinib plus capecitabine in patients previously treated with a taxane and trastuzumab (EMILIA) (Verma et al., 2012).
Recent clinical results using trastuzumab in HER2/neu overexpressing advanced/recurrent USC patients (i.e., an interim analysis after 26 events in USC patients enrolled in study NCT01367002) (NIH Clinical Trials, n.d.) demonstrated an increase in PFS of 4.8 months adding trastuzumab to CP when compared to CP alone (Santin et al., 2016). These clinical data with trastuzumab combined to our recently reported preclinical studies with TDM-1 in primary USC cell lines and USC xenografts strongly suggest that similarly to breast cancer, TDM-1 may represent a potentially effective treatment option for USC patients overexpressing HER2/neu and unresponsive to salvage chemotherapy treatment. In this report we present the first clinical evidence of noteworthy clinical activity of TDM-1 against HER2/neu-positive recurrent/metastatic USC resistant to radiation and chemotherapy.
TDM-1 may represent a novel treatment option for recurrent/metastatic HER2/neu positive USC patients refractory to salvage treatment.
Funding information
This work was supported in part by grants from NIH U01 CA176067-01A1, the Deborah Bunn Alley Foundation, the Honorable Tina Brozman Foundation, the Discovery to Cure Foundation and the Fondazione Guido Berlucchi to Alessandro D. Santin. This investigation was also supported by NIH Research Grant CA-16359 from NCI.
Conflict of interest
None.
References
- Boruta D.M., Gehrig P.A., Fader A.N. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol. Oncol. 2009;115:142–153. doi: 10.1016/j.ygyno.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Buza N., English D.P., Santin A.D., Hui P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Mod. Pathol. 2013 Dec;26(12):1605–1612. doi: 10.1038/modpathol.2013.113. [DOI] [PubMed] [Google Scholar]
- Creasman W.T., Odicino F., Maisonneuve P. Carcinoma of the corpus uteri–FIGO 26th annual report on the results of treatment in gynecological cancer. Int. J. Gynaecol. Obstet. 2006;95:S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- English D.P., Bellone S., Schwab C.L. T-DM1, a novel antibody-drug conjugate, is highly effective against primary HER2 overexpressing uterine serous carcinoma in vitro and in vivo. Cancer Med. 2014 Oct;3(5):1256–1265. doi: 10.1002/cam4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader A.N., Boruta D., Olawaiye Uterine papillary serous carcinoma: epidemiology, pathogenesis and management. Curr. Opin. Obstet. Gynecol. 2010;22(1):21–29. doi: 10.1097/GCO.0b013e328334d8a3. [DOI] [PubMed] [Google Scholar]
- Hamilton C.A., Cheung M.K., Osann K. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br. J. Cancer. 2006;94(5):642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Clinical Trials Evaluation of carboplatin/paclitaxel with or without trastuzumab (Herceptin) in uterine serous cancer. http://clinicaltrials.gov/ct2/show/NCT01367002
- Santin A.D., Bellone S., Van Stedum S. Amplification of c-erbB2 oncogene: a major prognostic indicator in uterine serous papillary carcinoma. Cancer. 2005;104:1391–1397. doi: 10.1002/cncr.21308. [DOI] [PubMed] [Google Scholar]
- Santin A.D., Nickles-Fader A., Siegel E. Abstract IGCS-0243, 16th Biennial Meeting of the International Gynecologic Cancer Society (IGCS), Lisbon, Portugal, October 29–31. 2016. Randomized phase II trial of carboplatin-paclitaxel (CP) compared to carboplatin-paclitaxel-trastuzumab (CP-T) in advanced (stage III-IV) or recurrent uterine serous carcinoma: results of interim analysis ( NCT01367002) [Google Scholar]
- Verma S., Miles D., Gianni L. For the EMILIA study group. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Choi M., Overton J.D. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc. Natl. Acad. Sci. U. S. A. 2013;110:2916–2921. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]