Supplemental Digital Content is available in the text.
Keywords: heart failure, hemodynamics, physiology, therapeutics
Abstract
Background—
Heart failure with preserved ejection fraction has a complex pathophysiology and remains a therapeutic challenge. Elevated left atrial pressure, particularly during exercise, is a key contributor to morbidity and mortality. Preliminary analyses have demonstrated that a novel interatrial septal shunt device that allows shunting to reduce the left atrial pressure provides clinical and hemodynamic benefit at 6 months. Given the chronicity of heart failure with preserved ejection fraction, evidence of longer-term benefit is required.
Methods and Results—
Patients (n=64) with left ventricular ejection fraction ≥40%, New York Heart Association class II–IV, elevated pulmonary capillary wedge pressure (≥15 mm Hg at rest or ≥25 mm Hg during supine bicycle exercise) participated in the open-label study of the interatrial septal shunt device. One year after interatrial septal shunt device implantation, there were sustained improvements in New York Heart Association class (P<0.001), quality of life (Minnesota Living with Heart Failure score, P<0.001), and 6-minute walk distance (P<0.01). Echocardiography showed a small, stable reduction in left ventricular end-diastolic volume index (P<0.001), with a concomitant small stable increase in the right ventricular end-diastolic volume index (P<0.001). Invasive hemodynamic studies performed in a subset of patients demonstrated a sustained reduction in the workload corrected exercise pulmonary capillary wedge pressure (P<0.01). Survival at 1 year was 95%, and there was no evidence of device-related complications.
Conclusions—
These results provide evidence of safety and sustained clinical benefit in heart failure with preserved ejection fraction patients 1 year after interatrial septal shunt device implantation. Randomized, blinded studies are underway to confirm these observations.
Clinical Trial Registration—
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01913613.
Epidemiological and observational studies have demonstrated that approximately half of the current heart failure (HF) burden is represented by patients who have preserved ejection fraction (HFpEF).1–3 The pathophysiologic basis that underpin the symptoms of HFpEF are complex, including both central hemodynamic and peripheral vascular and muscular mechanisms.4,5 In particular, elevated left atrial (LA) pressure is considered a key driver of symptoms in HF,6 and in HFpEF, this may be particularly evident during physical activity.7–10
The influence of a range of pharmacological approaches on morbidity and mortality in HFpEF has been examined in large randomized trials with the limited effect.11–14 Many of these agents were tested based on previous evidence of efficacy in patients with HF with reduced ejection fraction. These include the use of renin–angiotensin–aldosterone antagonists to notionally reduce myocardial fibrosis and hypertrophy and β-blockers. The effectiveness of vasodilators including nitric oxide donors and cyclic GMP modulators has also been examined with variable effects.15–18
On the basis of the limited success of pharmacological management of HFpEF to date, an interatrial shunt device (IASD; Corvia Medical Inc., Tewksbury, MA) was developed to reduce the LA pressure in HFpEF. The REDUCE LAP-HF study (Reduce Elevated Left Atrial Pressure in Patients With Heart Failure) was designed to evaluate device performance and safety of the transcatheter, transvenous interatrial shunt device in symptomatic patients with HFpEF.19,20 Specifically, the device was engineered to reduce elevated LA pressure, particularly associated with exertion, while avoiding excessive left to right shunting.19,20 At 6 months, this approach was associated with improved functional and exercise capacity, with reduced exercise normalized pulmonary capillary wedge pressure, whereas modest increases in right heart cardiac output and right atrial pressure were observed.21 In this context, we sought to establish the longer-term effects of the interatrial shunt device. This report describes the clinical and echocardiographic outcomes 1 year after IASD implantation, together with detailed rest and exercise hemodynamic data in a subset of patients.
Methods
The REDUCE LAP-HF study is a multicenter prospective, nonrandomized, open-label, single-arm study designed to investigate the safety and performance of a novel transcatheter interatrial shunt device. The study design and primary results have been described in detail elsewhere.21,22 We report here the 1-year outcomes of the cohort of implanted patients (n=64), with particular regard to device performance, safety, and the durability of clinical and hemodynamic effects. All patients provided written informed consent, and the study was conducted with the approval of competent authorities and institutional ethics review committees. Interpretation of the results and preparation of the article was the responsibility of the steering committee and principal investigators. The study was monitored by an independent Clinical Events Committee and Data Safety Monitoring Board. The sponsor played no role in the collection, analysis, or interpretation of the data.
Patient Population
We report here the 1-year outcomes of the cohort of patients who underwent successful IASD implantation, as described previously.21 Briefly, HFpEF was defined as symptomatic HF (New York Heart Association class II-ambulant class IV), a left ventricular ejection fraction >40%, and an elevated pulmonary capillary wedge pressure (PCWP) at rest (>15 mm Hg) or during supine bicycle exercise (>25 mm Hg) measured by right heart catheterization.22 Exclusion criteria included patients with moderate or greater right heart dysfunction and patients with significant valvular disease including >grade 2+mitral regurgitation, ≥grade 2+tricuspid regurgitation, ≥grade 2+aortic regurgitation, or ≥moderate aortic stenosis (aortic valve area ≤1.1 cm2).
One-Year Follow-Up
Twelve months after device implantation, patients underwent clinical and echocardiographic assessment per protocol. Clinical parameters included 6-minute walk distance, New York Heart Association class, and quality of life evaluation using the Minnesota Living with Heart Failure questionnaire. Transthoracic echocardiography was performed at the 12-month visit and as appropriate technically adequate images were analyzed at an independent core laboratory located at the University of Pennsylvania (PA). Parameters measured included left and right heart chamber sizes, tricuspid annular plane systolic excursion, and E/e′.
A subset of patients (n=18) underwent repeat right heart catheterization at 12 months according to individual site capacity and patient willingness at 8 of 21 participating sites. Assessment of cardiac output and central hemodynamics (right atrial pressure, pulmonary artery pressure, and PCWP) was performed at rest and during supine bicycle exercise. The exercise protocol was as previously used, comprising symptom-limited supine bicycle exercise commenced at 20 W with 20-W increments every 3 minutes until the patient achieved maximum effort. To account for the hemodynamic effect of differences in workload, we also calculated the workload corrected PCWP, as previously described.7 Blood samples were collected from the pulmonary artery and vena cavae at baseline and follow-up study to measure oxygen saturation and to evaluate left to right shunting as reflected by the Qp:Qs ratio. Hemodynamic records were analyzed by an independent core laboratory (PVLoops LLC, NY).
Statistical Methods
Normally distributed data are presented as mean±SD and non-normal data as median and 25th–75th percentile range. Between-groups and within-subject comparisons were performed using an unpaired or paired Student t test, respectively. Nonparametric tests were performed using a Mann–Whitney or signed-rank test as appropriate (for unpaired or paired data). Repeated-measures ANOVA with post hoc testing performed using Bonferroni testing for normally distributed data. Repeated-measures analysis of non-normal data was performed using Friedman test. Data provided for specific parameters represent only those cases in which measurements were available at all time points (baseline, 6 months, and 12 months). The null hypothesis was rejected at P<0.05. Statistical analysis was conducted using SPSS version 22.
Results
Clinical Outcomes, Quality of Life, and Functional Capacity
Of the 64 patients who underwent successful implantation all survived to 6 months as previously reported.21 During the period 6 months to 1 year, 3 patients died representing an overall 1-year survival of 95%. One patient died of combined pneumonia and renal failure, 1 patient had a fatal stroke (in an individual with CHA2DS2-VASc score of 6) and the cause of death in the third patient was undetermined (no previous adverse events had been reported). One patient did not return for the 12-month follow-up. There were a total of 17 HF hospitalizations, occurring in 13 patients over the first year. Of these, 10 HF hospitalizations events occurred within the first 6 months, in 10 patients. At 12 months, there were sustained significant improvements in New York Heart Association class and quality of life (Minnesota Living with Heart Failure) score as shown in Figure 1. Similarly, a sustained improvement in 6-minute walk distance was observed at 12 months compared with baseline (363±93 versus 331±90 m; P=0.001; Figure 1; n=55), and the 12-month 6-minute walk distance was similar to that seen 6 months after device implantation.
Figure 1.
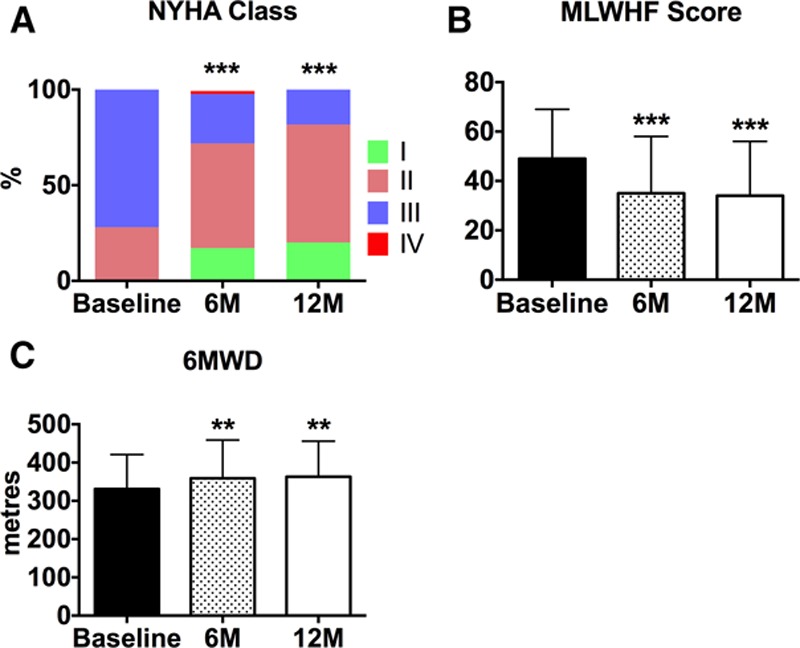
A, Bar graph represents the effects of interatrial septal shunt device implantation on New York Heart Association (NYHA) class (n=60), (B) Minnesota Living with Heart Failure Score (MLWHF; n=60), and (C) 6-min walk distance (6MWD; n=55). **P<0.01, ***P<0.001 vs baseline. B and C, Data represent mean±SD.
Echocardiography
Color flow Doppler imaging confirmed the presence of ongoing left to right shunting at 12 months post device implant, in all patients with adequate image quality and technique (n=48). At 12 months, left ventricular ejection fraction was unchanged, whereas right ventricular ejection fraction remained significantly elevated as observed at the 6-month follow-up (Figure 2A and 2B). In conjunction, there were modest but stable reductions in the left ventricular end-diastolic volume index with a concomitant rise in the right ventricular end-diastolic index (Figure 2C and 2D). By contrast, there were no progressive changes in the left or right atrial volume indexes between 6 and 12 months (Figure 2E and 2F). The E/e′ remained stable from baseline to 6 and 12 months: 13.4±5.5, 13.1±7.6, and 12.2±4.2 (n=36), respectively. Of interest, although tricuspid annular plane systolic excursion was unchanged from baseline to 6 months (2.0±0.4 versus 2.0±0.4 cm), it was significantly increased at 12 months to 2.2±0.4 cm (P<0.05 versus baseline and 6 months; n=36).
Figure 2.
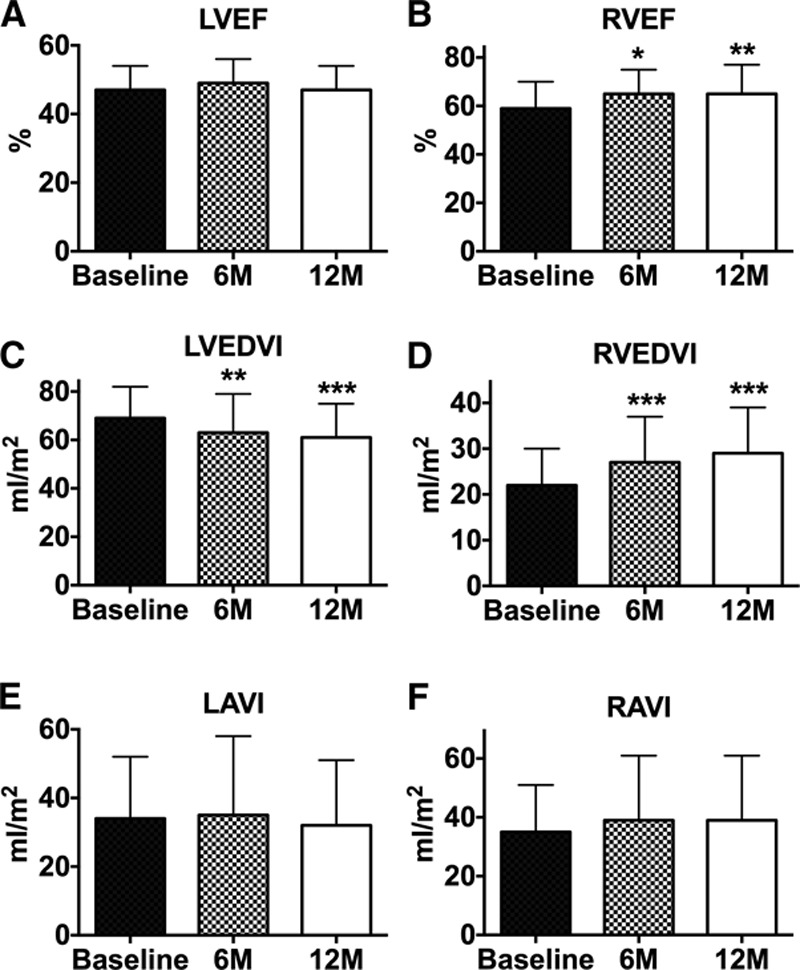
Bar graphs representing (A) left ventricular ejection fraction (LVEF; n=53), (B) right ventricular ejection fraction (RVEF; n=47), (C) left ventricular end-diastolic volume index (LVEDVI; n=53), (D) right ventricular end-diastolic index (RVEDVI; n=48), (E) left atrial volume index (LAVI; n=53), and (F) right atrial volume index (RAVI; n=47). *P<0.05, **P<0.01, ***P<0.001 vs baseline. Data are mean±SD.
Invasive Hemodynamics and Exercise Testing
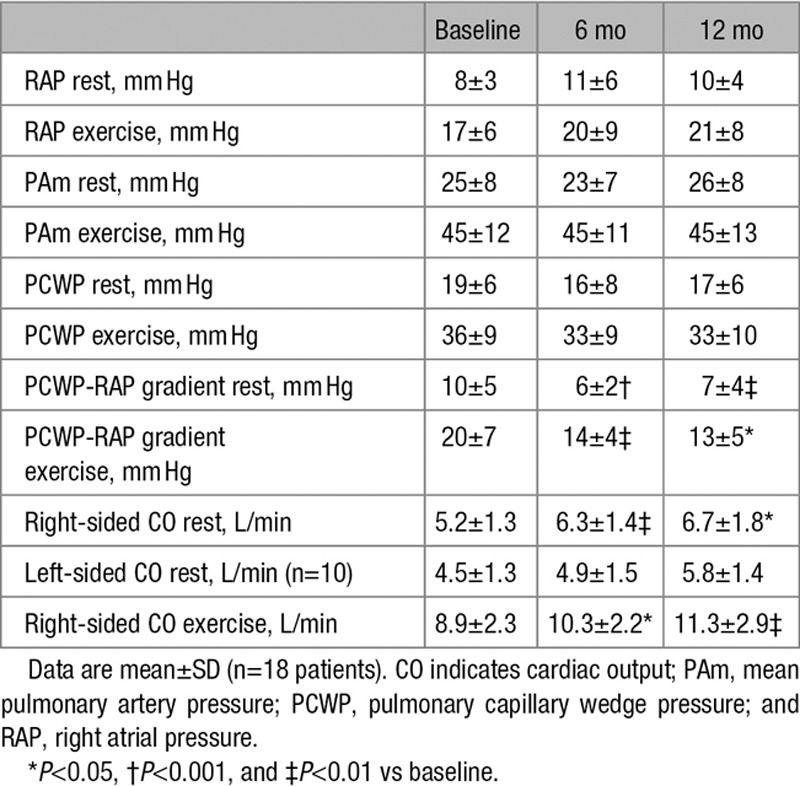
To further evaluate the longer-term influence of the IASD, rest and exercise right heart catheterization was conducted optionally in a subset (n=18) of patients at 12 months, thereby providing serial hemodynamic measures from baseline to 6 and 12 months. Patients undergoing cardiac catheterization at 12 months did not differ statistically from those who did not undergo catheterization with regard to demographic, clinical, or echocardiographic features (Table I in the Data Supplement). Within this cohort, exercise time increased significantly from baseline to 6 months (8.2±3.4 versus 9.7±3.2 minutes; P<0.05), and this increase was sustained at 12 months (10.4±4.2 minutes; P<0.05 versus baseline). Similarly, there was an increase in the supine cycling peak work capacity from baseline to 6 months (48±19 versus 60±16 watts; P<0.01; n=17), and this increase was sustained at 12 months (55±15 watts; P<0.01 versus baseline). The increase in supine exercise cycling peak workload was achieved without an increase in PCWP.
Over the course of follow-up, there were no significant changes in resting or exercise systemic blood pressure (data not shown). As shown in the Table, there were no significant changes in the right atrial pressure, pulmonary artery pressure, or PCWP at rest or during exercise in the cohort of subjects who underwent cardiac catheterization at each time point. Implantation of the shunt device reduced the pressure gradient between the left and right atrium, as assessed by the PCWP to RA pressure gradient (Table), and this reduction was sustained through to 12 months. As shown above, there was a significant increase in the workload capacity, and this occurred in the absence of a change in the peak PCWP. In keeping with previous studies, we calculated the workload indexed PCWP.7 As shown in Figure 3, IASD placement was associated with a sustained significant reduction in workload indexed PCWP >12 months. There was a significant increase in total right-sided cardiac output after IASD implantation, as measured by thermodilution, and this continued through 12 months (Table). Left-sided cardiac output, measured by oximetry, was unchanged (Table). The Qp:Qs ratio in patients undergoing cardiac catheterization at 12 months was 1.25±0.25, which was unchanged from that at 6 months (1.27±0.24). Among the patients undergoing cardiac catheterization, assessment of the Qp:Qs confirmed the presence of continued left to right shunting in 6 patients in whom echocardiographic assessment was not possible. The pulmonary vascular resistance was also unchanged (data not shown).
Figure 3.
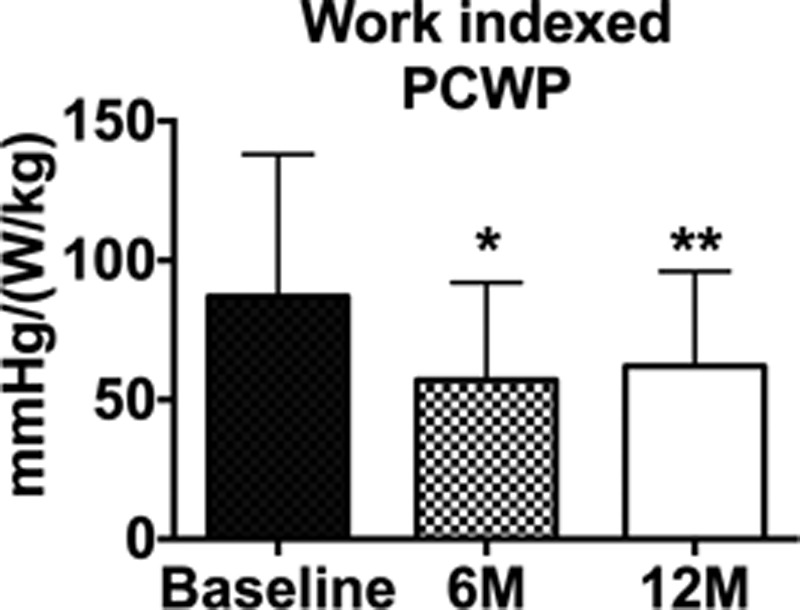
Bar graph showing workload indexed peak exertion wedge pressure before and after interatrial shunt device placement (n=16). *P<0.05, **P<0.01 vs baseline. Data are mean±SD. PCWP indicates pulmonary capillary wedge pressure.
Table.
Serial Hemodynamic Measurements
Discussion
To directly alleviate the contribution of impaired diastolic function and the consequent exertion mediated rise in the LA pressure, the effect of an iatrogenic interatrial shunt has recently been studied by 2 groups including our own.21,23 Computer modeling indicated that an 8-mm shunt opening could effectively reduce LA pressure in simulated HFpEF at rest and during exercise.19 The short-term, nonrandomized studies demonstrated significant positive effects on quality of life and functional capacity that were accompanied with modest reductions in pulmonary capillary wedge pressure either at rest or during exertion.21,23
In the current report, we demonstrated the presence of sustained and meaningful clinical benefit as reflected by continued positive changes in quality of life score, 6-minute walk distance, and New York Heart Association class. In conjunction, echocardiographic and oximetric studies confirmed the presence of ongoing IASD patency with evidence of left to right shunting and a reduction in the pressure differential between left and right atrium, similar in degree to that observed at 6 months. At the 6-month time point, there was evidence of small but significant increases in right ventricular end-diastolic volumes, but these remained stable at 12 months. In parallel, there was a small, stable reduction in the left ventricular end-diastolic volume. Left and right atrial volumes were not changed, and LV mass remained stable (data not shown). Serial echocardiographic parameters could not be assessed by the core laboratory in a minority of the subjects because of inadequate image quality, potentially limiting our ability to detect subtle changes in chamber volumes. Taken together, however, these data suggest that there was no evidence of adverse remodeling after IASD placement, in keeping with the small size of the shunt.
We also performed rest and exercise hemodynamic follow-up in a limited cohort to address the potential concern that chronically increased right-sided blood flow could adversely affect pulmonary pressures and to investigate whether IASD placement continued to favorably affect LA pressure dynamics. The PCWP to RA pressure gradient reduction was sustained through to 12 months. The Qp:Qs ratio at 12 months was unchanged from that at 6 months. There was a sustained increase in right-sided cardiac output, with no change in resting left-sided cardiac output as measured by oximetry. The apparent absence of a reduction in left-sided cardiac output could potentially explained by volume retention although body weight was unchanged. The present data do not indicate any evidence of increased pulmonary pressure or pulmonary vascular resistance. Although there was no evidence of an effect of pulmonary capillary wedge pressure at rest or exercise, there was evidence of a sustained beneficial effect on bicycle exercise duration, workload, and on the workload corrected wedge pressure. This latter parameter has been shown to be an important prognostic factor.24
Although the degree of functional impairment and impact on survival is similar across both HF with reduced ejection fraction and HFpEF, the pathophysiology of HFpEF differs in many ways from HF with reduced ejection fraction. Patients are typically older and comorbidities including atrial fibrillation, hypertension, obesity, frailty, chronic lung disease, and chronic kidney disease are all more frequent. The underpinning myocardial biology is also probably more complex than in HF with reduced ejection fraction and likely more problematic in regard to the potential for reverse remodeling.25 Together, these factors may provide an explanation for the lack of a convincing long-term effects of pharmacological interventions to date in HFpEF patients.26 Within this context, a safe and effective device-based therapy provides a possible advantage. The maintained positive effect on clinical status and functional capacity coupled with evidence of sustained shunt patency and improved central hemodynamic also confirms the key role for elevated filling pressures as a driver of symptoms in HFpEF. Furthermore, by the nature of the mechanism of action of the shunt device, it would be unlikely that the results observed relate to an effect on the peripheral circulation.
The current study has some important limitations. First, the trial was a nonrandomized open-label study, and the key clinical outcome variables, while important for this patient population, were of a subjective or effort-dependent nature. As such, we cannot exclude the possibility of a placebo effect; however, it is of note that the early effects observed within the first 6 months were sustained to the same extent at the 12-month observation period. Second, although stroke occurred in 1 individual at high preexisting risk for a cerebrovascular event, it is not possible to definitively exclude a potential device-related event although it was not adjudicated as device-related by the Clinical Events Committee. Third, in some cases, information at each observation point was not available, potentially leading to bias; however, the number of complete within-subject data sets across time was minimally reduced. Finally, only a subgroup of patients underwent hemodynamic assessment at 12 months, preventing reporting of longitudinal changes in the entire cohort.
Taken together, this study provides the longest experience with an interatrial shunt device specifically developed for the management HFpEF. The data provide additional longitudinal support for the safety and efficacy of this approach. Randomized trials, with blinded patients and physician assessors, are currently underway and are required to validate the utility of this novel therapy.27
Acknowledgments
We thank the REDUCE LAP-HF (Reduce Elevated Left Atrial Pressure in Patients With Heart Failure) coinvestigators and study coordinators for their technical expertise and diligence.
Sources of Funding
The study was funded by Corvia Medical.
Disclosures
None.
Supplementary Material
Footnotes
The Data Supplement is available at http://circheartfailure.ahajournals.org/lookup/suppl/doi:10.1161/CIRCHEARTFAILURE.116.003662/-/DC1.
CLINICAL PERSPECTIVE
Approximately half of all patients with heart failure are found to have a preserved left ventricular ejection fraction. The pathophysiology of heart failure with preserved ejection fraction is complex, and treatment remains a major therapeutic challenge. Elevated left atrial pressure, particularly during exercise, is a fundamental contributor to morbidity and mortality. Based on this pathophysiologic mechanism, an interatrial shunt device was developed to decompress the left atrium as a therapy for heart failure with preserved ejection fraction. Preliminary studies illustrated the potential clinical and hemodynamic benefit of this approach. The current open-label study in symptomatic patients with left ventricular ejection fraction >40% demonstrates the presence of sustained improvements in New York Heart Association class, quality of life, and 6-minute walk distance, 1 year after device implant. Invasive hemodynamic studies performed in a subset of patients demonstrated a sustained reduction pulmonary capillary wedge pressure at a given workload. Randomized, blinded studies are underway to confirm these observations.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 4.Reddy YN, Borlaug BA. Heart Failure With Preserved Ejection Fraction. Curr Probl Cardiol. 2016;41:145–188. doi: 10.1016/j.cpcardiol.2015.12.002. doi: 10.1016/j.cpcardiol.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomonica A, Burger AJ, Aronson D. Hemodynamic determinants of dyspnea improvement in acute decompensated heart failure. Circ Heart Fail. 2013;6:53–60. doi: 10.1161/CIRCHEARTFAILURE.112.970335. doi: 10.1161/CIRCHEARTFAILURE.112.970335. [DOI] [PubMed] [Google Scholar]
- 7.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 8.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–969. doi: 10.1136/hrt.2010.212787. doi: 10.1136/hrt.2010.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Empel VP, Mariani J, Borlaug BA, Kaye DM. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2014;3:e001293. doi: 10.1161/JAHA.114.001293. doi: 10.1161/JAHA.114.001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 12.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J PEP-CHF Investigators. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 13.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 14.van Veldhuisen DJ, Cohen-Solal A, Böhm M, Anker SD, Babalis D, Roughton M, Coats AJ, Poole-Wilson PA, Flather MD SENIORS Investigators. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: Data From SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure). J Am Coll Cardiol. 2009;53:2150–2158. doi: 10.1016/j.jacc.2009.02.046. doi: 10.1016/j.jacc.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 15.Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E NHLBI Heart Failure Clinical Research Network. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2015;373:2314–2324. doi: 10.1056/NEJMoa1510774. doi: 10.1056/NEJMoa1510774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2015;66:1672–1682. doi: 10.1016/j.jacc.2015.07.067. doi: 10.1016/j.jacc.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 18.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. discussion 380. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaye D, Shah SJ, Borlaug BA, Gustafsson F, Komtebedde J, Kubo S, Magnin C, Maurer MS, Feldman T, Burkhoff D. Effects of an interatrial shunt on rest and exercise hemodynamics: results of a computer simulation in heart failure. J Card Fail. 2014;20:212–221. doi: 10.1016/j.cardfail.2014.01.005. doi: 10.1016/j.cardfail.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Søndergaard L, Reddy V, Kaye D, Malek F, Walton A, Mates M, Franzen O, Neuzil P, Ihlemann N, Gustafsson F. Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur J Heart Fail. 2014;16:796–801. doi: 10.1002/ejhf.111. doi: 10.1002/ejhf.111. [DOI] [PubMed] [Google Scholar]
- 21.Hasenfuß G, Hayward C, Burkhoff D, Silvestry FE, McKenzie S, Gustafsson F, Malek F, Van der Heyden J, Lang I, Petrie MC, Cleland JG, Leon M, Kaye DM REDUCE LAP-HF study investigators. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet. 2016;387:1298–1304. doi: 10.1016/S0140-6736(16)00704-2. doi: 10.1016/S0140-6736(16)00704-2. [DOI] [PubMed] [Google Scholar]
- 22.Hasenfuss G, Gustafsson F, Kaye D, Shah SJ, Burkhoff D, Reymond MC, Komtebedde J, Hünlich M Reduce LAP-HF Trial Investigators. Rationale and Design of the Reduce Elevated Left Atrial Pressure in Patients With Heart Failure (Reduce LAP-HF) Trial. J Card Fail. 2015;21:594–600. doi: 10.1016/j.cardfail.2015.05.008. doi: 10.1016/j.cardfail.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Del Trigo M, Bergeron S, Bernier M, Amat-Santos IJ, Puri R, Campelo-Parada F, Altisent OA, Regueiro A, Eigler N, Rozenfeld E, Pibarot P, Abraham WT, Rodés-Cabau J. Unidirectional left-to-right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: a safety and proof-of-principle cohort study. Lancet. 2016;387:1290–1297. doi: 10.1016/S0140-6736(16)00585-7. doi: 10.1016/S0140-6736(16)00585-7. [DOI] [PubMed] [Google Scholar]
- 24.Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B, Neumann FJ. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3103–3112. doi: 10.1093/eurheartj/ehu315. doi: 10.1093/eurheartj/ehu315. [DOI] [PubMed] [Google Scholar]
- 25.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 26.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 27.Feldman T, Komtebedde J, Burkhoff D, Massaro J, Maurer MS, Leon MB, Kaye D, Silvestry FE, Cleland JG, Kitzman D, Kubo SH, Van Veldhuisen DJ, Kleber F, Trochu JN, Auricchio A, Gustafsson F, Hasenfubeta G, Ponikowski P, Filippatos G, Mauri L, Shah SJ. Transcatheter interatrial shunt device for the treatment of heart failure: rationale and design of the randomized trial to REDUCE Elevated Left Atrial Pressure in Heart Failure (REDUCE LAP-HF I). Circ Heart Fail. 2016;9:e003025. doi: 10.1161/CIRCHEARTFAILURE.116.003025. [DOI] [PubMed] [Google Scholar]


