Supplemental Digital Content is available in the text.
Keywords: brain ischemia, hypotension, nimodipine, subarachnoid hemorrhage, sustained release formulation
Abstract
Background and Purpose—
We conducted a randomized, open-label, phase 1/2a, dose-escalation study of intraventricular sustained-release nimodipine (EG-1962) to determine safety, tolerability, pharmacokinetics, and clinical effects in aneurysmal subarachnoid hemorrhage.
Methods—
Subjects with aneurysmal subarachnoid hemorrhage repaired by clipping or coiling were randomized to EG-1962 or enteral nimodipine. Subjects were World Federation of Neurological Surgeons grade 2 to 4 and had an external ventricular drain. Cohorts of 12 subjects received 100 to 1200 mg EG-1962 (9 per cohort) or enteral nimodipine (3 per cohort). The primary objective was to determine the maximum tolerated dose.
Results—
Fifty-four subjects in North America were randomized to EG-1962, and 18 subjects were randomized to enteral nimodipine. The maximum tolerated dose was 800 mg. One serious adverse event related to EG-1962 (400 mg) and 2 EG-1962 dose-limiting toxicities were without clinical sequelae. There was no EG-1962-related hypotension compared with 17% (3/18) with enteral nimodipine. Favorable outcome at 90 days on the extended Glasgow outcome scale occurred in 27/45 (60%, 95% confidence interval 46%–74%) EG-1962 subjects (5/9 with 100, 6/9 with 200, 7/9 with 400, 4/9 with 600, and 5/9 with 800 mg) and 5/18 (28%, 95% confidence interval 7%–48%, relative risk reduction of unfavorable outcome; 1.45, 95% confidence interval 1.04–2.03; P=0.027) enteral nimodipine subjects. EG-1962 reduced delayed cerebral ischemia (14/45 [31%] EG-1962 versus 11/18 [61%] enteral nimodipine) and rescue therapy (11/45 [24%] versus 10/18 [56%]).
Conclusions—
EG-1962 was safe and tolerable to 800 mg, and in this, aneurysmal subarachnoid hemorrhage population was associated with reduced delayed cerebral ischemia and rescue therapy. Overall, the rate of favorable clinical outcome was greater in the EG-1962-treated group.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01893190.
Nimodipine is used throughout the world to improve outcome after aneurysmal subarachnoid hemorrhage (aSAH).1 Its use, however, is limited by systemic hypotension that occurs in ≤56% of patients.2 Plasma concentrations can exceed those associated with hypotension, yet cerebrospinal fluid (CSF) concentrations remain below the optimal therapeutic threshold.3,4 Hypotension is deleterious to patients with aSAH because it lowers cerebral blood flow and cerebral perfusion pressure and worsens delayed cerebral ischemia (DCI).5
We hypothesized that EG-1962 (nimodipine in a biodegradable polymer suspended in hyaluronic acid administered as one intraventricular injection that releases nimodipine into the subarachnoid space for at least 21 days) would increase efficacy and reduce systemic side effects compared with enteral nimodipine.6–8 We performed a randomized, controlled, dose-escalation study to determine the safety, maximum tolerable dose (MTD), pharmacokinetics, and clinical effects of EG-1962 in humans (NEWTON [Nimodipine Microparticles to Enhance Recovery While Reducing Toxicity After Subarachnoid Hemorrhage).7
Methods
Study Design and Subjects
Subjects were randomized at 20 neurosurgical centers in the United States and Canada (online-only Data Supplement). The protocol was written by the authors, and the study was approved by institutional review boards. The protocol and written informed consent procedure are published (http://www.clinicaltrials.gov. Unique identifier: NCT01893190).7 Key inclusion criteria were World Federation of Neurological Surgeons (WFNS) grade 2 to 4 subjects with an external ventricular drain (EVD) inserted as standard of care and a ruptured aneurysm repaired by clipping or coiling. After the first cohort was entered, the inclusion criteria were amended to increase the time from aSAH to study drug administration from 48 to 60 hours to facilitate recruitment. The rationale was that in the first cohort, plasma pharmacokinetics showed a rapid increase in plasma nimodipine concentrations within 24 hours of administration of EG-1962. Nimodipine is approved to reduce DCI after SAH, and this occurs 3 to 14 days after SAH. The approval says to start nimodipine within 4 days of SAH. Therefore, we thought it would be safe to increase the inclusion time to 60 hours. There was no reason to think this would affect the assessment of the MTD. The second cohort was administered EG-1962, 200 mg.
The study was to include a dose-escalation period followed by a treatment period (Figure I in the online-only Data Supplement). The primary objective of the dose-escalation period was to determine the MTD of EG-1962 and of the treatment period to further determine the safety and tolerability of the selected dose of EG-1962 compared with enteral nimodipine. The treatment period was not conducted based on the following. There were only 2 dose-limiting toxicities (DLT) spread among 2 different dose groups, and the number of serious adverse events (SAE) among the dose groups did not seem to have any dependence on dose. Favorable responses did not exhibit a dose–response, in that the rate was already high at the 100 mg dose. The dose–response relationship was assessed by visual inspection of the outcomes by cohort and by e-max and logistic regression models adjusting for WFNS grade and age (dichotomized). Finally, there was a strong dose–response seen in some key pharmacokinetic parameters. The secondary objective was to measure plasma and CSF concentrations of nimodipine; these will be reported separately. Exploratory end points were angiographic vasospasm, DCI, cerebral infarction caused by DCI, rescue therapy, and clinical outcomes at days 30 and 90.7,9 Definitions of these events are published and followed recommendations of a multidisciplinary consensus statement.9 Health economic outcomes will be reported separately. A computed tomography scan was obtained at day 30. The extended Glasgow outcome score (GOSE), Montreal cognitive assessment, modified Rankin scale, Barthel index, and telephone interview of cognitive status were conducted by study coordinators, who were trained in administering the outcome measures, at days 30 and 90 and National Institutes of Health stroke scale at days 7, 14, and 90.10–15
In part 1, cohorts of subjects were randomized (by biased coin method) to receive either EG-1962 or enteral nimodipine with a minimization procedure based on WFNS grade.16 WFNS grade was chosen for this because it is the most powerful prognostic factor for outcome after aSAH.17,18 Randomization was computer generated by an independent company using interactive response technology. Allocation concealment was maintained because study centers did not know what other subjects entered into a given cohort had received. Up to 6 dose-level cohorts of EG-1962 were to be assessed during the dose-escalation part. Each dose-level cohort enrolled 12 subjects in a 3:1 (EG-1962:enteral nimodipine) ratio. The starting dose of EG-1962 was 100 mg, and the maximum dose was 1200 mg. These doses were based on preclinical studies and were allowed under an Investigational New Drug Application to the United States Food and Drug Administration and a Clinical Trial Application to Health Canada.8 EG-1962 (100 mg nimodipine/mL [Evonik Industries, Birmingham, AL] suspended in hyaluronic acid [Fidia Farmaceutici SPA, Abano Terme, Italy]) was administered as one intraventricular injection. After reconstitution in the pharmacy, it was transported to the subject’s bedside and remixed. Up to 5 mL CSF was aspirated from the EVD under sterile conditions. The syringe containing EG-1962 was then administered and flushed through the EVD with sterile, preservative-free 0.9% NaCl. It was recommended that the EVD remain closed and that CSF be drained only if clinically indicated. Once randomized, subjects assigned to EG-1962 no longer received enteral nimodipine. An independent data safety monitoring committee reviewed the safety, occurrence of predefined DLT, stopping rules, plasma nimodipine pharmacokinetics, and tolerability data up to day 14 for each cohort. A decision was then made to increase the dose by some amount to determine that the MTD was reached or to enter another cohort with a lower dose. With the exception of the doses for cohort 1 (100 mg) and cohort 6 (1200 mg), the EG-1962 dose to be administered in cohorts 2, 3, 4, and 5 could be modified based on the data safety monitoring committee recommendation. Safety and DLT information was reported by the investigators up until day 90. The MTD was reached if ≥3 subjects receiving EG-1962 had a DLT or if the 1200 mg dose was reached. Management of subjects, DLT, and adverse events was outlined in the protocol and followed guidelines for management of aSAH.7,19,20
Statistical Analysis
Cohort size estimates were based on the analysis of published aSAH data and supported a dose-escalation scheme with 9 EG-1962 and 3 enteral nimodipine subjects per cohort.21 The safety data set (all subjects randomized who received EG-1962 or enteral nimodipine and were assessed for safety at least once) included 72 subjects. Exploratory outcomes were based on a modified intent to treat (all subjects who were included in the safety analysis set and who had GOSE assessments at day 30 or 90). The modified intent to treat set excluded 1200 mg EG-1962 subjects because this dose was above the MTD. Only 3 subjects received the full dose, and some also received enteral nimodipine, which renders outcomes uninterpretable.
Demographics and safety data are reported as descriptive statistics (means, standard deviation). The first exploratory end point was the GOSE, analyzed as a dichotomous outcome (favorable outcome 6–8 and unfavorable outcome 1–5). This cut point was based on analysis of existing data.7 The GOSE was compared between subjects in all cohorts combined randomized to enteral nimodipine and those randomized to each of the EG-1962 dose groups and to the combined EG-1962 group. The prespecified analysis used Fisher exact test and the Cochran Mantel Haenszel test adjusted by WFNS grade (dichotomized as 2 versus 3+4) and age (dichotomized as <60 or ≥60) because these are important admission prognostic factors for outcome and to adjust for differences between cohorts in WFNS grade and age.17,21 Exact 95% (2-sided) confidence intervals (CI) for proportions and approximate 95% CI were used for relative risk ratios. Missing day-90 outcome was scored as the day-30 outcome.17 We assessed interactions of study group with WFNS grade and age, though the power is limited by the small sample size. Analysis was in SAS (version 9.2) and STATA (version 14.0).
Results
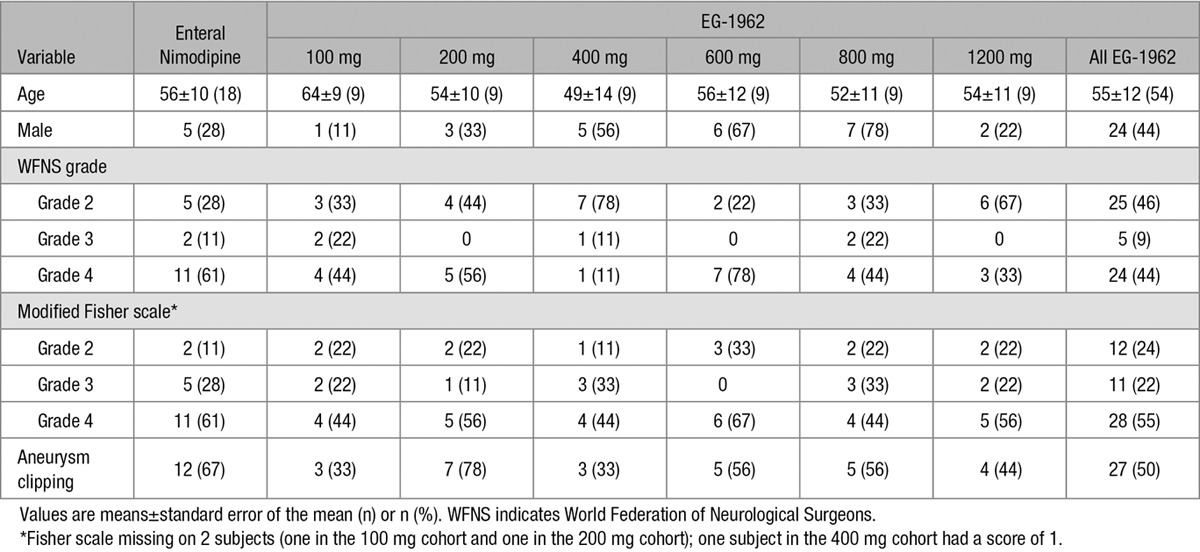
Nine hundred and ninety-eight patients were prescreened (Figure I in the online-only Data Supplement). Seventy-two subjects completed the North American study between October 28, 2013, and July 31, 2015; 54 subjects were randomized to EG-1962 and 18 to enteral nimodipine (Figure 1). Demographics and baseline characteristics were consistent with patients with aSAH (Table 1).
Figure 1.
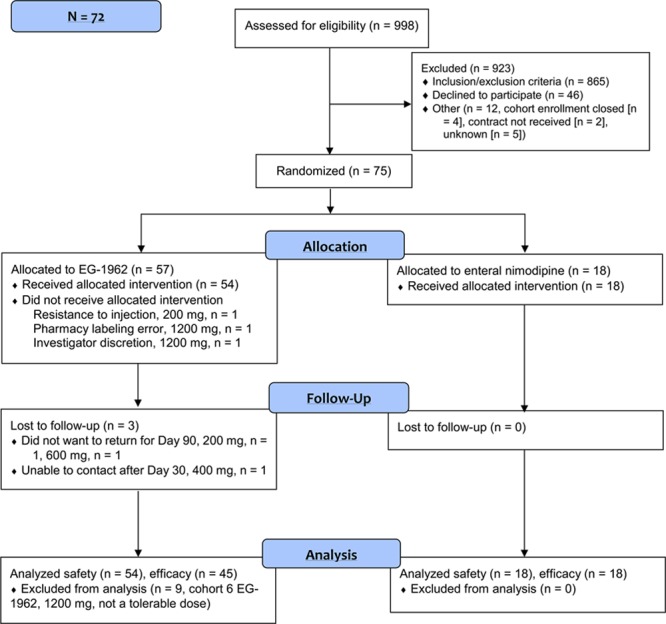
Study profile.
Table 1.
Subject Demographics
No safety concerns limited dose escalation to 1200 mg. The maximum tolerated dose was 800 mg; 1200 mg was not tolerable because of the injection volume. Two DLT were reported, one each in cohort 3 (400 mg) and cohort 5 (800 mg). Both were elevated intracranial pressure (ICP). Elevated ICP was considered a DLT if ICP was >30 mm Hg compared with the ICP recorded just prior to the administration of EG-1962 within 24 hours after EG-1962 administration and lasting over 4 hours despite appropriate therapy. In one case, the increased ICP was not associated with neurological deterioration, and the subject had a day-90 GOSE of upper moderate disability (favorable outcome). In the second case, there was transient neurological deterioration starting 13.5 hours after administration of EG-1962. The subject had a GOSE of lower good recovery (favorable outcome) at day 90.
There were 3 deaths (6%) in the EG-1962-treated subjects (2 in the 200 mg cohort and 1 in the 800 mg cohort) and 1 death (6%) in the enteral nimodipine group (in the 1200 mg cohort). None of these deaths were considered by the investigator to be related to study drug, and all were a consequence of the initial brain injury from the aSAH, rebleeding, and withdrawal of care.
Through and including cohort 6 (1200 mg), 30 of 54 (56%) subjects treated with EG-1962 and 13 of 18 (72%) subjects treated with enteral nimodipine were reported to have treatment-emergent SAE (Table 2). Central nervous system events, including cerebral vasoconstriction, DCI, cerebral infarction, and intracranial hemorrhage, were all more frequent in the enteral nimodipine group (Table 2).
Table 2.
Adverse Events
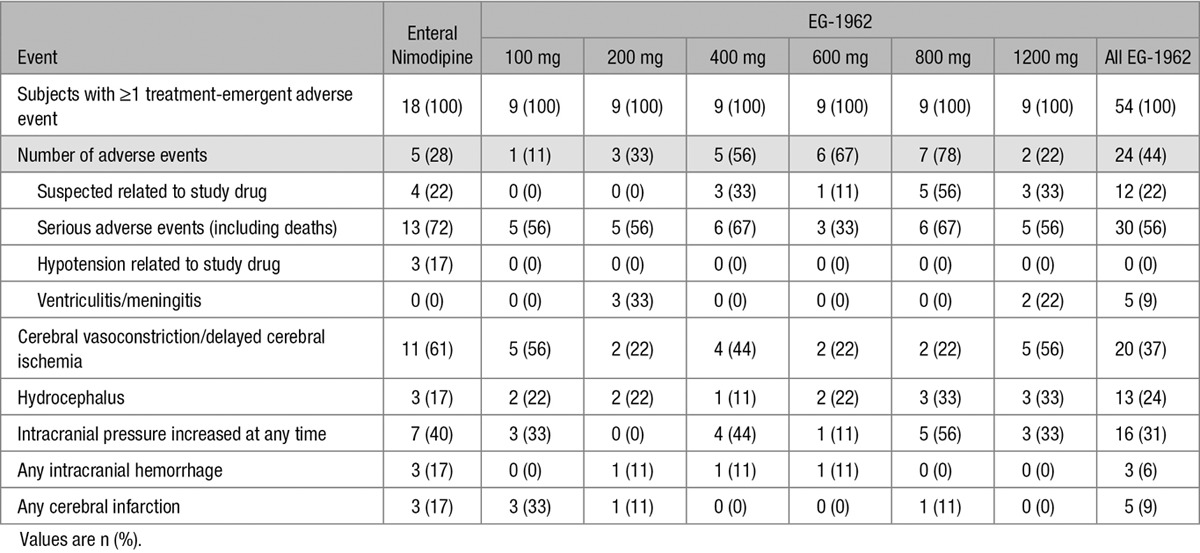
The overall incidence of study medication–related adverse events was similar between the EG-1962 (12 [22%] with 13 events) and enteral nimodipine groups (4 [22%] with 4 events). Three of the 4 adverse events related to enteral nimodipine were hypotension. Favorable outcome occurred in 1 of 4 of these subjects. In the EG-1962 treatment group, 9 of 12 study medication–related adverse events were increased ICP within 24 hours of administration of EG-1962. Two were EVD malfunctions, and 1 was a possible transient allergic reaction with no clinical sequelae. Favorable outcome occurred in 9 of 12 (75%) of these subjects. In the EG-1962-treated subjects, 1 SAE was considered by the investigator to be related to EG-1962 (possible transient allergic reaction with no clinical sequelae).
The occurrence of increased ICP was not dose related. The overall incidence of increased ICP was similar between the EG-1962 (16/54, 30%) and enteral nimodipine (7/18, 39%) groups as was the rate reported as SAE (7% and 6% for the EG-1962 and enteral nimodipine groups, respectively). In the EG-1962 group, 12/13 (92%) subjects with increased ICP had a favorable outcome on the GOSE compared with 2/7 (29%) subjects in the enteral nimodipine group. Thirteen of 54 (24%) subjects had increased ICP within 24 hours of administration of EG-1962; 10 (77%) subjects had a favorable outcome. In the enteral nimodipine group, 3/18 (17%) subjects experienced increased ICP within 24 hours of randomization; 1 (33%) had a favorable outcome. In the EG-1962-treated group, 6/54 (11%) subjects experienced increased ICP after 24 hours; 3 (50%) had a favorable outcome. In the enteral nimodipine group, 4/18 (22%) subjects experienced late increased ICP; 1 (25%) had a favorable outcome.
There were 5 cases of CSF culture–positive ventriculitis/meningitis in subjects treated with EG-1962 (5/54, 9%) and none in those treated with enteral nimodipine. There were 3 in cohort 2 (200 mg EG-1962) and 2 in cohort 6 (1200 mg). None were considered related to EG-1962. The day-90 outcomes were favorable in 2/3 (67%) subjects who received 200 mg and in 2/2 (100%) subjects who received 1200 mg.
There was a higher incidence of hypotension reported in the enteral nimodipine group (6/18, 33%) than in the EG-1962 group (3/54, 6%). In the enteral nimodipine group, 3 of 6 events of hypotension were reported as related to treatment, whereas in the EG-1962 group, no hypotension was considered related. There were no other clinically important imbalances in adverse events in other organ systems.
The percent of subjects from cohorts 1 to 5 who achieved a favorable clinical outcome was greater in the EG-1962 group (60%, 27/45; 95% CI 46%–74%) compared with that in the enteral nimodipine group (28%, 5/18; 95% CI 7%–48%, relative risk reduction of unfavorable outcome; 1.45, 95% CI 1.04–2.03, P=0.027, not adjusted for multiplicity, Fisher exact test). In cohort 6, 67% of 3 subjects who received the full 1200 mg dose had favorable outcome. Furthermore, 29% (13/45) of subjects treated with EG-1962 had GOSE scores of upper good recovery, whereas 6% (1/18) of the subjects treated with standard-of-care enteral nimodipine achieved that result. All doses yielded higher rates of favorable outcomes compared with that of enteral nimodipine with no dose relationship apparent (Figure 2).
Figure 2.
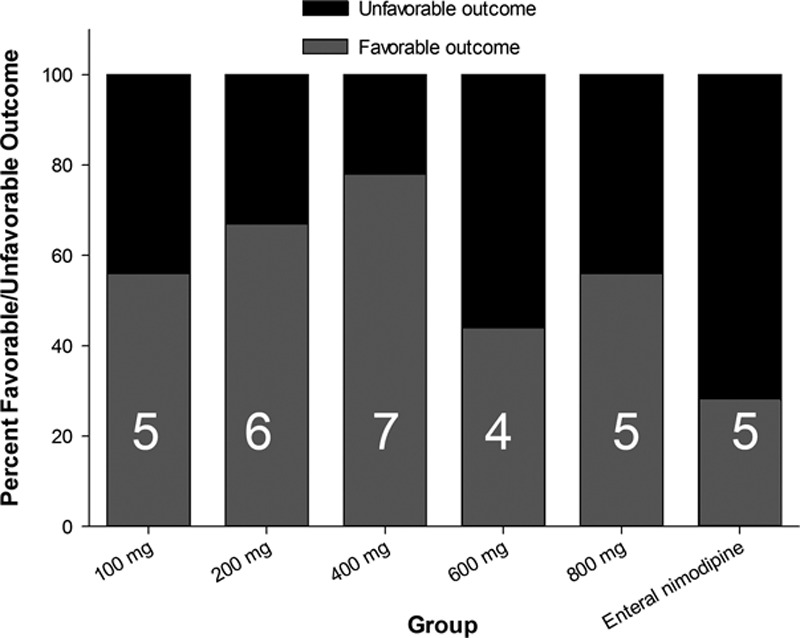
Outcome on the extended Glasgow outcome score (GOSE) at day 90. Numbers are number of subjects with favorable outcomes (GOSE of 6–8) for each cohort (n=9) and enteral nimodipine (n=18). For each dose cohort of EG-1962 compared with enteral nimodipine, controlling for World Federation of Neurological Surgeons (WFNS) grade and age, the adjusted odds ratio was 1.17 (95% confidence interval [CI] 0.87–1.59; P=0.29).
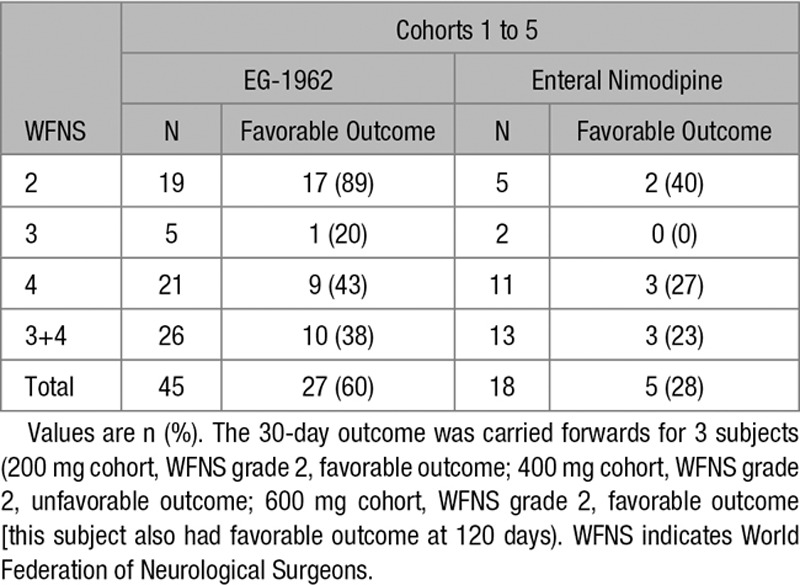
Favorable outcome for subjects treated with EG-1962 compared with those treated with enteral nimodipine was more likely both for subjects with WFNS 2 (89% versus 40%) and for subjects with WFNS 4 (43% versus 27%; Table 3). Exploratory analysis of the NEWTON data confirmed existing literature that the most important prognostic factors for outcome were WFNS grade and age. There were few WFNS grade 3 subjects, and the most optimal grouping for WFNS grade was 2 versus 3+4. Age was analyzed as a dichotomous variable, with the cut point selected based on prior prognostic modeling and at a point producing similar numbers of subjects in each category (<60 or ≥60 years old).17 The pooled relative risk reduction for favorable outcome for all EG-1962 subjects versus enteral nimodipine subjects, adjusting for WFNS grade and age, was 1.84 (95% CI 0.88–3.85; Cochran Mantel Haenszel test). For each dose cohort of EG-1962 compared with enteral nimodipine, controlling for WFNS grade and age, the relative risk reduction, adjusted for WFNS grade and age, was 1.46 (95% CI 0.97–2.20; Cochran Mantel Haenszel test). There were no interactions between treatment group and WFNS grade (2 versus 3+4) or age.
Table 3.
Extended Glasgow Outcome Scale by WFNS Grade
Treatment with EG-1962 at 200 mg or more was associated with less angiographic vasospasm/DCI, infarction because of DCI, and use of rescue therapy compared with enteral nimodipine (Figure 3). Subjects who did develop angiographic vasospasm or DCI were more likely to have favorable outcome if they were treated with EG-1962 (53%) compared with enteral nimodipine (18%).
Figure 3.
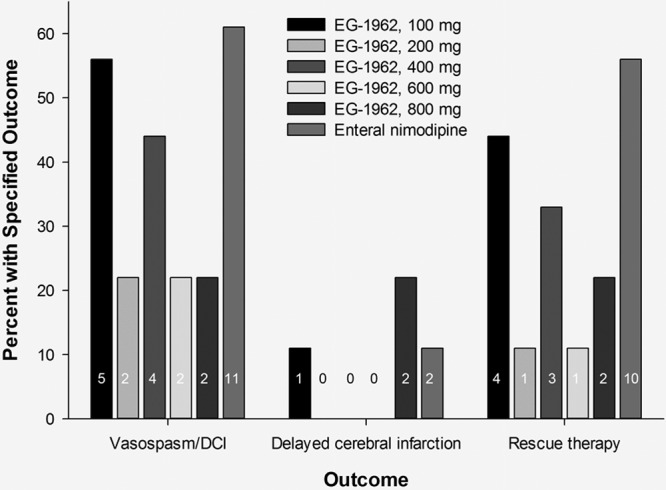
Effect of EG-1962 and enteral nimodipine on angiographic vasospasm, delayed cerebral ischemia, delayed infarction, and rescue therapy. Numbers are number of subjects with the specified outcome for each cohort (n=9) and enteral nimodipine (n=18).
Discussion
Nimodipine was developed to reduce angiographic vasospasm after aSAH.22 At tolerable doses, there was minimal effect on angiographic vasospasm.3,4,23 Current theory is that multiple processes contribute to DCI and delayed infarction, including angiographic vasospasm, cortical spreading ischemia, microthromboembolism, loss of autoregulation, and capillary transit time heterogeneity.24–27 Nimodipine may have improved outcome because it inhibits these deleterious processes, but at tolerable doses has only a small effect on the most widely measured process (angiographic vasospasm). Evidence consistent with this is that at some doses, nimodipine and other dihydropyridines reduce angiographic vasospasm, cortical spreading ischemia, and microthromboembolism.6,28–30 Nimodipine may have improved outcome because it inhibits these deleterious processes, but at tolerable doses has only a small effect on the most widely measured process (angiographic vasospasm). NEWTON tested the hypothesis that intracranial delivery of EG-1962, which achieved high and sustained CSF concentrations in preclinical studies, would increase efficacy without compromising safety.8
The NEWTON study objectives were met. The MTD was determined to be 800 mg because the volume of the 1200 mg injection (12 mL) was not tolerable as a single injection. The only DLT were episodes of transient increased ICP after injection of EG-1962. One SAE, a possible allergic reaction that had no clinical sequelae, was reported as possibly related to EG-1962. EG-1962 was safe with the same or lower risk of central nervous system events such as hemorrhage compared with enteral nimodipine. As hypothesized, no EG-1962-related hypotension occurred, whereas 17% (3/18) of subjects in the enteral nimodipine group experienced dose-limiting hypotension. Meningitis and ventriculitis were the only adverse events more common in the EG-1962 group (5/54, 9%). We attribute this in part to the protocol requesting repeated sampling of CSF for nimodipine pharmacokinetics. This infection rate is comparable to analysis of 33 studies with 9667 cases from the literature that found a pooled infection incidence of 8%.31 This study allowed for institutional practice to be followed for EVD insertion. There was no study-mandated protocol for EVD insertion. Future studies of EG-1962 will mitigate the risk of infection by requiring adherence to protocols for EVD insertion and intraventricular injection and by not obtaining CSF samples unless clinically indicated.31,32
Treatment with EG-1962 was associated with improved outcome on the GOSE, an effect that was robust over different WFNS grades (Table 3). Furthermore, EG-1962 subjects had reduction in angiographic vasospasm, DCI, and need for rescue therapy. Favorable response rates were greater than control at all doses, with no obvious dose relationship. The lack of dose–response is consistent with randomized trials of enteral nimodipine that showed similar outcomes with doses of 30 to 90 mg nimodipine every 4 hours for 21 days.3,4,23 Furthermore, studies of drugs targeting central nervous system disorders frequently do not demonstrate strong dose–responses and have wide ranges of recommended doses.
Limitations of this study include that the number of subjects treated was small, it was unblinded, and we used a modified intent–to-treat analysis for the clinical outcome. There were differences between cohorts in numbers of subjects at baseline with different WFNS grades, which was anticipated and adjusted for in the statistical analysis. Finally, subjects randomized represent a select population of SAH patients. Whether similar results could be obtained by administering EG-1962 by lumbar injection or whether an EVD could be indicated for the primary purpose of administering EG-1962 cannot be determined at this time.
Summary
Intraventricular EG-1962 was safe and tolerable to 800 mg and, in this aSAH population, was associated with reduced DCI and use of rescue therapy. Overall, the rate of favorable outcome was greater in the EG-1962-treated group. A pivotal phase 3, double-blind, double-dummy, randomized clinical study of EG-1962 compared with enteral nimodipine has been started.
Acknowledgments
We thank Integrated Medical Development, LLC, for statistical and study support.
Sources of Funding
The study was funded by Edge Therapeutics, Inc.
Disclosures
Dr Macdonald receives grant support from the Physicians Services Incorporated Foundation, Brain Aneurysm Foundation, Canadian Institutes for Health Research, and the Heart and Stroke Foundation of Canada and is an employee and Chief Scientific Officer of Edge Therapeutics, Inc. Drs Hänggi, Etminan, Aldrich, Mayer, Diringer, Hoh, and Mocco receive consulting fees from Edge Therapeutics, Inc, for serving on the steering committee for this trial and for advising Edge Therapeutics, Inc. Drs Mayer and Aldrich are consultants and receive consulting fees from Actelion Pharmaceuticals, Ltd. Dr Faleck is an employee of Edge Therapeutics, Inc. Dr Steiger reports no conflicts.
Supplementary Material
Footnotes
Presented in part at the International Stroke Conference, Los Angeles, CA, February 17–19, 2016, and at the American Association of Neurological Surgeons meeting, Chicago, IL, April 30 to May 4, 2016.
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.116.014250/-/DC1.
References
- 1.Dorhout Mees SM, Rinkel GJ, Feigin VL, Algra A, van den Bergh WM, Vermeulen M, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2007:CD000277. doi: 10.1002/14651858.CD000277.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandow N, Diesing D, Sarrafzadeh A, Vajkoczy P, Wolf S. Nimodipine dose reductions in the treatment of patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2016;25:29–39. doi: 10.1007/s12028-015-0230-x. doi: 10.1007/s12028-015-0230-x. [DOI] [PubMed] [Google Scholar]
- 3.Allen GS, Ahn HS, Preziosi TJ, Battye R, Boone SC, Boone SC, et al. Cerebral arterial spasm–a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med. 1983;308:619–624. doi: 10.1056/NEJM198303173081103. doi: 10.1056/NEJM198303173081103. [DOI] [PubMed] [Google Scholar]
- 4.Petruk KC, West M, Mohr G, Weir BK, Benoit BG, Gentili F, et al. Nimodipine treatment in poor-grade aneurysm patients. Results of a multicenter double-blind placebo-controlled trial. J Neurosurg. 1988;68:505–517. doi: 10.3171/jns.1988.68.4.0505. doi: 10.3171/jns.1988.68.4.0505. [DOI] [PubMed] [Google Scholar]
- 5.Dankbaar JW, Slooter AJ, Rinkel GJ, Schaaf IC. Effect of different components of triple-H therapy on cerebral perfusion in patients with aneurysmal subarachnoid haemorrhage: a systematic review. Crit Care. 2010;14:R23. doi: 10.1186/cc8886. doi: 10.1186/cc8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasuya H, Onda H, Takeshita M, Okada Y, Hori T. Efficacy and safety of nicardipine prolonged-release implants for preventing vasospasm in humans. Stroke. 2002;33:1011–1015. doi: 10.1161/01.str.0000014563.75483.22. [DOI] [PubMed] [Google Scholar]
- 7.Hänggi D, Etminan N, Macdonald RL, Steiger HJ, Mayer SA, Aldrich F, et al. NEWTON: Nimodipine Microparticles to Enhance Recovery While Reducing Toxicity After Subarachnoid Hemorrhage. Neurocrit Care. 2015;23:274–284. doi: 10.1007/s12028-015-0112-2. doi: 10.1007/s12028-015-0112-2. [DOI] [PubMed] [Google Scholar]
- 8.Hänggi D, Etminan N, Steiger HJ, Johnson M, Peet MM, Tice T, et al. A site-specific, sustained-release drug delivery system for aneurysmal subarachnoid hemorrhage. Neurotherapeutics. 2016;13:439–449. doi: 10.1007/s13311-016-0424-8. doi: 10.1007/s13311-016-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–2395. doi: 10.1161/STROKEAHA.110.589275. doi: 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- 10.Brandt J, Spencer M, Folstein M. The telephone interval for cognitive status. Neuropsychiatr Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 11.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 12.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 13.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 14.Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 15.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 16.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 17.Jaja BN, Cusimano MD, Etminan N, Hanggi D, Hasan D, Ilodigwe D, et al. Clinical prediction models for aneurysmal subarachnoid hemorrhage: a systematic review. Neurocrit Care. 2013;18:143–153. doi: 10.1007/s12028-012-9792-z. doi: 10.1007/s12028-012-9792-z. [DOI] [PubMed] [Google Scholar]
- 18.Jaja BN, Lingsma H, Schweizer TA, Thorpe KE, Steyerberg EW, Macdonald RL SAHIT collaboration. Prognostic value of premorbid hypertension and neurological status in aneurysmal subarachnoid hemorrhage: pooled analyses of individual patient data in the SAHIT repository. J Neurosurg. 2015;122:644–652. doi: 10.3171/2014.10.JNS132694. doi: 10.3171/2014.10.JNS132694. [DOI] [PubMed] [Google Scholar]
- 19.Diringer MN, Bleck TP, Hemphill CJ, III, Menon D, Shutter L, Vespa P, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the neurocritical care society’s multidisciplinary consensus conference. Neurocrit. Care. 2011;15:211–240. doi: 10.1007/s12028-011-9605-9. [DOI] [PubMed] [Google Scholar]
- 20.Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–1737. doi: 10.1161/STR.0b013e3182587839. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 21.Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315–2321. doi: 10.1161/STROKEAHA.107.484360. doi: 10.1161/STROKEAHA.107.484360. [DOI] [PubMed] [Google Scholar]
- 22.Scriabine A, Schuurman T, Traber J. Pharmacological basis for the use of nimodipine in central nervous system disorders. FASEB J. 1989;3:1799–1806. [PubMed] [Google Scholar]
- 23.Pickard JD, Murray GD, Illingworth R, Shaw MD, Teasdale GM, Foy PM, et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ. 1989;298:636–642. doi: 10.1136/bmj.298.6674.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10:44–58. doi: 10.1038/nrneurol.2013.246. doi: 10.1038/nrneurol.2013.246. [DOI] [PubMed] [Google Scholar]
- 25.Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, et al. COSBID study group. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132(Pt 7):1866–1881. doi: 10.1093/brain/awp102. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budohoski KP, Czosnyka M, Smielewski P, Kasprowicz M, Helmy A, Bulters D, et al. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke. 2012;43:3230–3237. doi: 10.1161/STROKEAHA.112.669788. doi: 10.1161/STROKEAHA.112.669788. [DOI] [PubMed] [Google Scholar]
- 27.Østergaard L, Aamand R, Karabegovic S, Tietze A, Blicher JU, Mikkelsen IK, et al. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2013;33:1825–1837. doi: 10.1038/jcbfm.2013.173. doi: 10.1038/jcbfm.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreier JP, Windmüller O, Petzold G, Lindauer U, Einhäupl KM, Dirnagl U. Ischemia triggered by red blood cell products in the subarachnoid space is inhibited by nimodipine administration or moderate volume expansion/hemodilution in rats. Neurosurgery. 2002;51:1457–1465. discussion 1465. [PubMed] [Google Scholar]
- 29.Barth M, Capelle HH, Weidauer S, Weiss C, Münch E, Thomé C, et al. Effect of nicardipine prolonged-release implants on cerebral vasospasm and clinical outcome after severe aneurysmal subarachnoid hemorrhage: a prospective, randomized, double-blind phase IIa study. Stroke. 2007;38:330–336. doi: 10.1161/01.STR.0000254601.74596.0f. doi: 10.1161/01.STR.0000254601.74596.0f. [DOI] [PubMed] [Google Scholar]
- 30.Vergouwen MD, Vermeulen M, de Haan RJ, Levi M, Roos YB. Dihydropyridine calcium antagonists increase fibrinolytic activity: a systematic review. J Cereb Blood Flow Metab. 2007;27:1293–1308. doi: 10.1038/sj.jcbfm.9600431. doi: 10.1038/sj.jcbfm.9600431. [DOI] [PubMed] [Google Scholar]
- 31.Dey M, Stadnik A, Riad F, Zhang L, McBee N, Kase C, et al. CLEAR III Trial Investigators. Bleeding and infection with external ventricular drainage: a systematic review in comparison with adjudicated adverse events in the ongoing Clot Lysis Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase III (CLEAR-III IHV) trial. Neurosurgery. 2015;76:291–300. doi: 10.1227/NEU.0000000000000624. discussion 301. doi: 10.1227/NEU.0000000000000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naff N, Williams MA, Keyl PM, Tuhrim S, Bullock MR, Mayer SA, et al. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial. Stroke. 2011;42:3009–3016. doi: 10.1161/STROKEAHA.110.610949. doi: 10.1161/STROKEAHA.110.610949. [DOI] [PMC free article] [PubMed] [Google Scholar]


