Abstract
Context
Comparative reviews of whole-body magnetic resonance imaging (WB-MRI) and positron emission tomography/computed tomography (CT; with different radiotracers) have shown that metastasis detection in advanced cancers is more accurate than with currently used CT and bone scans. However, the ability of WB-MRI and positron emission tomography/CT to assess therapeutic benefits has not been comprehensively evaluated. There is also considerable variability in the availability and quality of WB-MRI, which is an impediment to clinical development. Expert recommendations for standardising WB-MRI scans are needed, in order to assess its performance in advanced prostate cancer (APC) clinical trials.
Objective
To design recommendations that promote standardisation and diminish variations in the acquisition, interpretation, and reporting of WB-MRI scans for use in APC.
Evidence acquisition
An international expert panel of oncologic imagers and oncologists with clinical and research interests in APC management assessed biomarker requirements for clinical care and clinical trials. Key requirements for a workable WB-MRI protocol, achievable quality standards, and interpretation criteria were identified and synthesised in a white paper.
Evidence synthesis
The METastasis Reporting and Data System for Prostate Cancer guidelines were formulated for use in all oncologic manifestations of APC.
Conclusions
Uniformity in imaging data acquisition, quality, and interpretation of WB-MRI are essential for assessing the test performance of WB-MRI. The METastasis Reporting and Data System for Prostate Cancer standard requires validation in clinical trials of treatment approaches in APC.
Patient summary
METastasis Reporting and Data System for Prostate Cancer represents the consensus recommendations on the performance, quality standards, and reporting of whole-body magnetic resonance imaging, for use in all oncologic manifestations of advanced prostate cancer. These new criteria require validation in clinical trials of established and new treatment approaches in advanced prostate cancer.
Keywords: Advanced prostate cancer, Guidelines, Metastatic castrate-resistant prostate cancer, Whole-body MRI, Diffusion MRI, Response assessment, Clinical trials
Take Home Message
Bone/computed tomography scans are limited for detection and response assessment of bone disease in advanced prostate cancer. METastasis Reporting and Data System for Prostate Cancer is the new performance and quality standard for whole-body magnetic resonance imaging, for use in all oncologic manifestations of advanced prostate cancer.
1. Introduction
Several therapeutic approaches have received recent regulatory approval for use in men with advanced prostate cancer (APC) [1]. In addition to continued androgen deprivation and docetaxel treatments, there are now additional agents with varying mechanisms of action showing survival benefit in this patient population. These include agents that target the androgen axis (eg, enzalutamide and abiraterone), stimulate the immune system (eg, sipuleucel-T), have a chemotherapeutic effect (eg, docetaxel and cabazitaxel), and the alpha-particle emitter that directly targets bone metastases (eg, radium-223) [2], [3]. Response to these agents is not universal with significant proportions of patients having primary resistance, and virtually all patients developing secondary resistance at variable times after treatment commencement. The optimal use of these therapies remains debatable [4], [5] with the presence, volume, and location of metastases being important determinants.
The imaging depicted metastatic state is key to patient management for biomarker (BM) development and for therapeutic clinical trials [6], [7], [8]. Imaging BMs provide information on disease volume and distribution, likely prognosis, changes in biologic behaviour, therapy-induced changes (both benefits and nonbenefits), durations of response, emergence of treatment resistance, and the host's reaction to the therapies administered. Numerous investigators have subcategorised the metastatic state in APC, by tissue involvement (bone/lymph nodes/visceral), by skeletal location (axial/peripheral), by number of lesions (oligometastatic or polymetastatic), and by the overall burden/volume of disease, because these have been shown to have prognostic and therapeutic value as summarised in Table 1.
Table 1.
Imaging impacts on advanced prostate cancer management
| Impact statements | References |
|---|---|
| Imaging defines clinical groups for drug and biomarker development and clinical states for therapy recommendations | Scher et al. [8]; Cookson et al. [25] |
| The anatomic location of metastases in CRPC is highly prognostic, adding to prognostic models predicting overall survival to docetaxel treatment | Halabi et al. [6], [26] |
| The presence of visceral disease and/or large volume nodal metastases precludes use of radium-223 | Parker et al. [27] |
| Therapeutic benefits using androgen axis directed treatments in asymptomatic/mildly symptomatic, chemotherapy naïve, metastatic prostate cancer patients are often greater for those with better performance status and lower disease volume on bone and CT scans | de Bono et al. [28]; Beer et al. [29]; James et al. [30]; Ryan et al. [31]; Evans et al. [7] |
| High volume disease patients on imaging have worse survival than lower volume disease patients (no matter which imaging test is used to make the determination) | Dennis et al. [32]; Sweeney et al. [33]; Ceci et al. [34]; Tait et al. [35]; Sabbatini et al. [36]; Perez-Lopez et al. [37]; Evans et al. [7] |
| The presence of high volume and visceral disease on imaging is an indication for intensified combination therapy, including chemotherapy in fit patients | Sweeney et al. [33]; Aparicio et al. [38]; James et al. [30] |
| Shorter imaging durations of response to abiraterone and docetaxel treatments using bone scans and the more objective size based RECIST criteria for soft tissue disease, are associated with worse overall survival | Morris et al. [39]; Sonpavde et al. [40] |
CRPC = castration resistant prostate cancer; CT = computed tomography; RECIST = Response Evaluation Criteria in Solid Tumours.
Recent reviews have identified the potential of whole-body magnetic resonance imaging (WB-MRI) and positron-emission tomography/computed tomography (PET/CT), to address the unmet need for robust imaging that allows tumour detection and therapy evaluations in APC [9], [10]. In particular, it has been noted that WB-MRI can detect bone metastases with higher sensitivity than bone scans and with at least comparable performance to choline PET/CT [11]. Importantly, WB-MRI provides a clearer categorisation of bone metastases response, unlike bone scans and sodium fluoride PET/CT scans that only identify disease progression. More accurate assessments of therapy response (including the detection of primary and secondary resistance and heterogeneity of response) could aid in the rationale development of targeted therapies [8]. Two recent reviews have indicated that WB-MRI is suitable for wider deployment in disease detection settings, given its established test performance, potential for wide availability, and multi-organ evaluation capabilities [9], [10]. The European Organisation for Research and Treatment of Cancer position paper concluded that MRI offers a good “one size fits all” solution for assessing therapy effectiveness in APC [10]. The need for standardisation of WB-MRI technology has also been expressed by multiple groups [5], [9], [10], [12], [13].
2. Evidence acquisition
2.1. WB-MRI for clinical practise and clinical trials in APC
An expert panel of radiologists, nuclear medicine physicians, and medical physicists, with the largest experience of imaging in APC, conducted a review of the performance, merits, and limitations of currently available imaging methods [9]. As part of the imaging review, guidelines were formulated on the performance standards for WB-MRI in the assessment of multi-organ involvement by APC.
The METastasis Reporting and Data System for Prostate Cancer (MET-RADS-P) imaging recommendations are designed to promote standardisation and diminish variations in the acquisition, interpretation, and reporting of WB-MRI in APC.
MET-RADS-P allows patients to be subclassified into clinical subgroups depending on the pattern of metastatic spread (bone, nodal, visceral, and local) for clinical trials entry, as recommended by the Prostate Cancer Clinical Trials Working Group (PCWG) [8]. Importantly, MET-RADS-P promotes the collection of robust BM data informing on clinically relevant therapy objectives, including: (1) information on disease control with separate documentation of progression in existing lesions and the development of new disease, and (2) the ability to document delays in disease progression including time to development of first metastasis and time to progression for nonmetastatic castrate resistant prostate cancer (M0-CRPC) [8]. MET-RADS-P also allows the capture of discordant (mixed) imaging responses and thereby provides opportunities to assess changing tumour biology, via molecular characterisation of biopsy tissue samples that may be obtained from anatomic sites on the basis of spatial characteristics and imaging response heterogeneity [14], [15].
The specific aims of the MET-RADS-P recommendations are to:
-
•
Establish minimum acceptable technical parameters for WB-MRI data acquisition
-
•
Develop standardised data collection methods that enables detailed descriptions of the disease phenotype based on imaging patterns of metastatic spread (PCWG compliance)
-
•
Develop comprehensive response criteria that assess bone, soft tissue, and local disease (PCWG compliance)
-
•
Provide methods for recording the presence, location, and extent of mixed imaging responses (to enable tumour biopsy sampling to gain insights into mechanisms of resistance; PCWG compliance)
-
•
Summarise the likelihood of response in bone, soft tissues, and local disease that may be used to direct patient management
-
•
Enable data collection for outcome monitoring in the context of clinical trials (PCWG compliance)
-
•
Allow the education of radiologists on WB-MRI reporting in order to reduce variability in imaging interpretations
-
•
Enhance communication with referring clinicians
-
•
Promote quality assurance and research in APC (PCWG compliance)
There are differences between MET-RADS-P proposals for clinical trials compared with the recent recommendations of PCWG (which recommends bone scan and CT assessments) [8]. These take into account the lower spatial resolution (but higher contrast resolution) of WB-MRI images (compared with CT scans) and the greater complexity of evaluating and documenting WB-MRI findings. Differences include the minimum size of disease that is considered measureable for response categorisations (≥ 1.5 cm for WB-MRI compared with ≥ 1 cm for CT scanning, unless high spatial resolution MRI methods are used), the measurement of five non-nodal, soft tissue lesions (in total) instead of five lesions per involved organ system, and the introduction of MRI-based response criteria that are applicable for bone metastases [16], [17]. Detailed comparisons between MET-RADS-P and PCWG recommendations are also highlighted in the Supplementary data.
3. Evidence synthesis
3.1. General considerations
For the purposes of MET-RADS-P assessments, specific imaging of the prostate or prostatectomy bed is not an essential requirement. When there are questions regarding persistent or recurrent local disease, a dedicated MRI can be performed to gauge local disease extent and assess complications.
Routine examinations of the brain are not required for adenocarcinoma histology. The brain should be assessed in patients with small cell/neuroendocrine tumours. There should be a low threshold for skull base imaging when there is likely to be neurological compromise.
Details of machine set-up, sequence specifications, quality assurance procedures, quality control, and radiographic aspects are detailed in the Supplementary data and Table 2.
Table 2.
Sequence components for whole-body magnetic resonance imaging examinations
| Sequence description | Core protocol | Extensions for comprehensive assessments | |
|---|---|---|---|
| 1 | Whole spine–sagittal, T1 W, TSE, 4–5 mm slice thickness | Yes | – |
| 2 | Whole spine–sagittal, STIR (preferred) or fat suppressed T2 W, 4–5 mm slice thickness | Yes | – |
| 3 | Whole body (vertex to mid thighs)–T1 W, GRE Dixon technique. Fat image reconstructions are mandatory • A 3D FSE T1 W sequence offering multiplanar capability may be performed as an alternative to replace sequences 1 and 3 |
Axial (5 mm)a or coronal (2 mm) | Axial and coronal |
| 4 | Whole body (skull base to mid-thighs)–axial, diffusion weighted, STIR fat suppression, 5–7 mm contiguous slicing, multiple stations • ADC calculations with mono-exponential data fitting • Coronal b800–1000 multiplanar reconstructionsb • 3D-MIP reconstructions of highest b-value imagesc |
2 b-values (b50–100 s/mm2 and b800-–1000 s/mm2) | 3 b-values (additional b500–600 s/mm2) |
| 5 | Whole body (vertex to mid thighs)–axial, T2 W, TSE without fat-suppression, 5 mm contiguous slicing, multiple stations, preferably matching the diffusion weighted images | Option | Yes |
| 6 | Regional assessments including dedicated prostate, small field of view spine, brain studies, and contrast enhancement | No | Yes |
ADC = apparent diffusion coefficient; FSE = fast spin echo; GRE = gradient echo; MIP = maximum intensity projection; STIR = short tau inversion recovery; TSE = turbo spin echo; W = weighted; 3D = three dimensional.
5–7 mm, axial imaging may be chosen to match section thickness of diffusion weighted imaging to facilitate image review.
b800–1000 images from all diffusion imaging stations are grouped and reconstructed as contiguous, two-dimensional coronal, 5-mm slices.
Whole body three-dimensional maximum intensity projection images, displayed as rotating images, using an inverted grayscale.
WB-MRI acquisitions can be tailored to the evaluation of bones and lymph nodes (core WB-MRI protocol; Table 2, Fig. 1) or more comprehensive assessments of the whole body can be undertaken (Fig. 2).
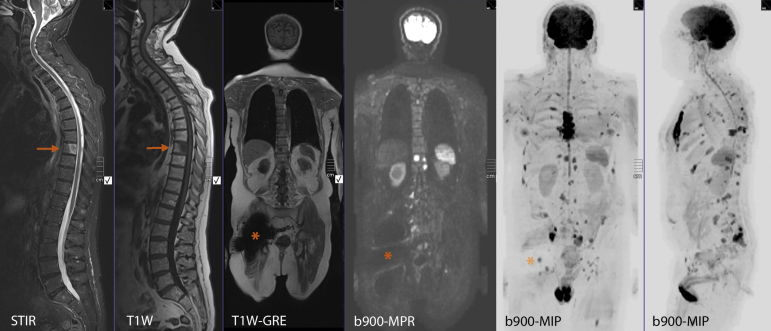
Fig. 1.
Typical core whole-body magnetic resonance imaging (MRI) protocol depicting extensive metastatic bone disease. Clinical details: 76-yr-old man previously treated with low dose rate brachytherapy for prostate cancer, now with biochemical recurrence (prostate-specific antigen 8.9 ng/ml). Good performance status: PS-1. Clinical question: suitability for salvage therapy. Typical core whole-body MRI examination undertaken using a 1.5T scanner (30 min). Panels 1 and 2: sagittal short tau inversion recovery and T1 weighted (W) turbo spin-echo images of the spine showing a metastasis in the T8 vertebral body (arrow). The lower signal in the centre of the lesion on the short tau inversion recovery image is consistent with mineralisation. No other spinal lesions are visible. Panel 3: coronal T1W gradient-recalled echo sequence shows the presence of a metal artefact from a right hip replacement (asterisk). Panel 4: coronal b900 diffusion weighted image (multiplanar reconstruction [MPR] from a stacked series of axially acquired b900 images) showing multiple hyperintense foci consistent with bone metastases. Note that the artefact from the right hip replacement (asterisk) is larger than on the T1W-gradient-recalled echo but only obscures the image locally. Panels 5 and 6: the diffusion weighted b900 image stack was reconstructed as a three-dimensional maximum intensity projection (MIP) image and displayed using an inverted blue scale. Coronal and sagittal projection MIP images show multiple bone metastases (as dark focal areas) that are seen in the spine, pelvis, sternal bone, and left femur. Note that the dark signal of the brain, spleen, spinal cord, and testicles is a normal finding, as are the small but prominent lymph nodes in the neck, axilla, and groin. Given the presence of extensive bone metastases, there is no need for dedicated local restaging prostate MRI.
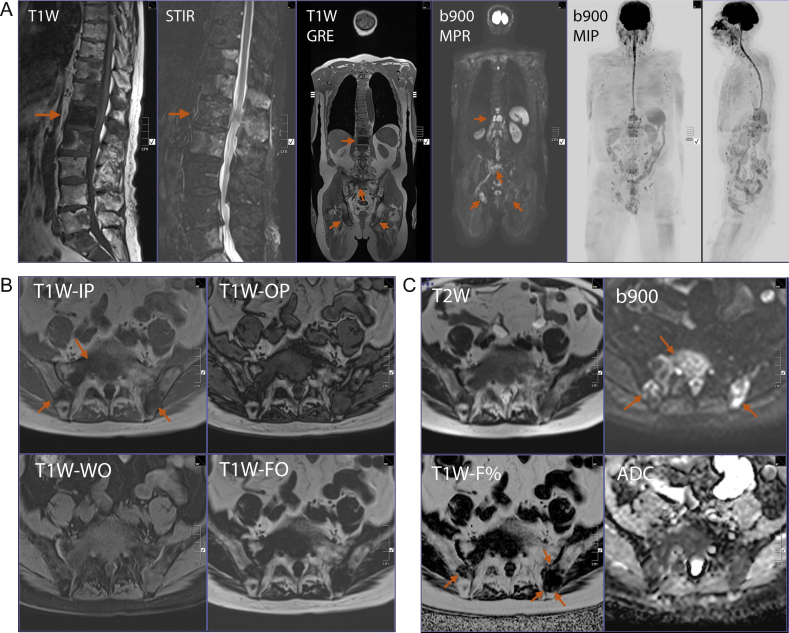
Fig. 2.
Typical comprehensive whole-body magnetic resonance imaging protocol depicting extensive metastatic bone disease. Clinical details: 73-yr-old man with known prostate cancer recurrence, bone and nodal metastases on abiraterone treatment, and rising prostate-specific antigen (49.9 ng/ml). Restaging examination. Typical comprehensive whole-body magnetic resonance imaging examination undertaken using a 3.0T scanner (45min). (A) Panels 1 and 2: zoomed sagittal T1 weighted (W) turbo spin-echo and sagittal short tau inversion recovery (STIR) images of the spine showing multiple metastases with proximal caudal equina impingement at L1. General narrowing of the lumbar spinal canal due to disc degeneration. Panel 3: coronal T1W gradient-recalled echo (GRE) sequence shows the metastasis at L1 (horizontal arrow) but the deposits in the adjacent vertebrae are less conspicuous. Deposits are visible in the sacrum and in both ischia (slanting arrows). Panel 4: coronal b900 diffusion weighted image (multiplanar reconstruction [MPR]) showing multiple hyperintense foci at the bone metastatic sites (except the left ischium). Panels 5 and 6: b900 three dimensional MIP images with coronal and sagittal projections confirm multiple bone metastases (as dark regions). Note that the dark signal of the brain, spleen, spinal cord, and testicles is a normal finding, as are the small lymph nodes in the neck, axillae, and groin. (B) Zoomed T1W images obtained using the Dixon technique with in-phase (IP), opposed-phase (OP), water only (WO), and fat only (FO) reconstructions at the level of the sacrum. Multiple sacral and iliac bone metastases are seen (best depicted on the T1W-IP and FO images; arrows). (C) Zoomed T2W and b900 images in the top row at the same level as B. Increased conspicuity of the metastases on the diffusion weighted b900 image (arrows) due to suppression of the background fat signal. The T1W-fat% (F%) image is calculated using the T1W-wWOand T1W-FO images from B. Small amounts of F are visible with the deposits in the iliac bones on the F% image (arrows on F% image).
The core WB-MRI protocol when used alone is designed for bone and lymph node metastasis detection (should be completed within 30 min of table time; Fig. 1). More comprehensive assessments (core plus extensions) should be used for patients with established metastatic including visceral disease. Depending on the sequences used, comprehensive assessments can be completed within 45–50 min of table time (Fig. 2).
The core WB-MRI protocol is adequate for the detection of metastatic disease in the setting of biochemical recurrence after primary therapy, or in the setting of M0-CRPC, when the aim is to detect the onset of metastatic disease. Comprehensive assessments are recommended for known metastatic disease and for those patients in whom serial tumour response assessments (including clinical trials) are planned (see Supplementary data for clinical indications for WB-MRI and respective protocol suggestions).
3.2. Clinical information
The following information should be available to radiologists at the time of reporting: tumour histology (and reclassifications of histology) and clinical indication(s) for performing the study.
In patients with known relapsed prostate cancer, the relevant clinical state of patients including whether castrate naive or castrate resistant, symptom sites, and descriptions of the known anatomic distribution of the disease (local, nodal, or distant) should be indicated.
Prostate-specific antigen (PSA) levels should be known and in the setting of M0-CRPC, the PSA-doubling time (PSADT) should be indicated as needed, because short PSADT is strongly associated with a higher likelihood of metastasis development and progression.
Prior and ongoing therapies received including details of the treatment for the primary tumour, radiotherapy locations, and surgical interventions, should be known. All current hormonal and chemotherapy medications should also be specified (eg, radiologists may not be aware that antiandrogens are almost never stopped when treatments are changed, or that steroid supplementation and steroid switching is part of abiraterone therapy, which alters bone marrow fat composition and causes osteoporosis). Supportive medications including steroids, blood transfusion, and the use of bone marrow growth factors, should also be indicated as they too affect image appearances.
3.3. Assessing WB-MRI images
Multi-sequence evaluations should be undertaken of all diffusion weighted (DW)-MR images (low b-value, intermediate [if obtained] and high b-values, and apparent diffusion coefficient [ADC] maps) in conjunction with the anatomical and relative F% images, using image linking and scrolling workstation facilities and coregistration tools as diagnostic aids (Fig. 2).
Radial maximum intensity projections of high b-value images displayed using the inverted scale are useful for global tumour volume assessments and for localising regional tumour distribution. These images are able to display disease within the soft tissues of the pelvis, lymph-nodes, and distant bone metastases. Maximum intensity projections images should not be used alone for disease assessments because false positive and false negative can occur (vide infra).
The evaluation of source b800–1000 value images of DW-MRI sequences is based on comparing high b-value image intensity to adjacent muscle signal intensity, but assessments of ADC maps is numeric (unit: × 10−3mm2/s or μm2/s).
The definitions for hypointense and hyperintense signal on b800–1000 value images remains subjective but can be gauged by using adjacent muscle as the reference background tissue [18], [19].
It must be borne in mind that not all hyperintense bone lesions on high b-value images are malignant in nature. Causes for apparent high b-value false-positive focal bone lesions include bone marrow oedema caused by fractures, osteoarthritis, infection, bone infarcts, vertebral haemangiomas, and isolated bone marrow islands [20].
Strategies for mitigating against these false-positive hyperintensities include direct correlations with morphologic appearances including T1 weighted (W)-fast spin echo, opposed-phase gradient-recalled echo, relative fat (F)% images [21], [22], and ADC values (ADC values of normal bone marrow are generally below 600–700 um2/s and viable tumour lies between 700 um2/s and 1400 um2/s; tumour ADC values ≥ 1400 um2/s is usually observed in treated/necrotic disease). Every suspicious lesion on high b-value images should be evaluated using coregistration tools (Fig. 2).
It must be noted that not all bone metastases are hyperintense on b800–1000 value images. This may occur because of sparse tumour cell infiltration, dense matrix mineralisation (de-novo or related to bisphosphonate/denosumab usage), when there is significant necrosis (de-novo or after therapies), or when metastases are healed. The potential for error in detecting metastases can be mitigated by the evaluations of all the images obtained including anatomic findings, intermediate b-value images (if obtained), and relative F% images [12].
The presence of metastases can also be obscured when background bone marrow hyperplasia occurs with the use of bone marrow growth factors (Supplementary Fig. 2). The detection of skeletal metastases on WB-diffusion weighted imaging (DWI) may also be impaired in areas of body movement such as the ribs and occasionally in the sternum. Skull vault metastasis evaluations are best undertaken by evaluating the axial source high b-value images. The visibility of skull base disease is additionally impaired because of susceptibility effects.
3.3.1. Obtaining ADC values
ADC measurements should only be obtained from metastases when water is detectable on DWI (all b-value images should be examined); otherwise the ADC values will be erroneous, reflecting only the noise in the images. Thus, dense sclerotic metastases without signal intensities detectable on DWI should not have their ADC measured.
The absence of tissue signal intensity on very high (b800–1000) b-value images does not invalidate a tissue from ADC measurements, provided that signal intensity is detectable on low and intermediate b-value images (see Supplementary Fig. 3 for the importance of such measurements).
The authors recognise that there are limitations of the ADC cut-off values referred to above, which are partly related to the fact that ADC values depend on the choice of b-values of DWI used for calculations (hence the constraints on the b-values choices recommended). The authors also recognise that ADC values may also depend on the diffusion time achievable on diffusion sequences (which is highly dependent on sequence waveforms and scanner specifications), on the method of fat suppression, and on a variety of other technical factors.
When there are deviations from the recommended b-values and fat suppression methods used due to machine/software or technical factors, then institutions can determine their own muscle normalised high b-value signal intensity and ADC cut-off values of normal marrow and for untreated bone marrow metastases [18].
3.4. Structured reporting
Relevant prior/concurrent imaging studies should be available at the time of image assessments including bone scans, CT studies, and PET/CT examinations. Prior WB-MRI examinations and their reports should also be available.
Radiologists should be familiar with the normal range of appearances on their equipment as these can vary slightly between MRI scanners. They should also be aware of the range of imaging artefacts that may be encountered.
It should be understood that exclusion of metastasis can never be absolute; it is important that all those involved in patient management recognise the limitations of WB-MRI investigations. Radiologists working in multidisciplinary teams are best placed to educate other caregivers on the potential advantages and limitations for specific patient indications.
Structured clinical and tabulated template reporting should be undertaken for each examination. The structured, textural WB-MRI report should comprise the following components:
3.4.1. Indication/clinical details
A statement regarding the patient's clinical state, prior treatments including local and pelvic disease, current pathological status, PSA/PSADT and the specific clinical question being posed.
3.4.2. Technique
Details of the technique (core or comprehensive protocol), including contrast medium administration, and whether or not dedicated regional images were obtained.
Important deviations in techniques and artefacts should be noted with their causes (eg, metal implant artefacts, patient movement from pain, reception coil nonusage or failure etc.), and their likely effect on imaging interpretation should be specifically stated. If patient specific solutions for improving image quality have already been noted, then these should additionally be documented, so the same image adjustments are performed at follow-up.
When comparisons are made with previous examinations, the dates and regions scanned of prior studies should be indicated.
3.4.3. Findings
WB-MRI reports are usually structured to initial evaluations of the spine (and pelvic bones) and then head to thighs in descending order. As a viable alternative, structuring of reports under headings of primary site, lymph nodes (regional and nonregional), bone, and visceral metastases following the TNM format can also be undertaken [23].
Imaging findings should include free text but should also include measurements of lesions. Unusual sites of suspected disease should be mentioned. Clear identification of marker lesions by anatomic location, size measurement, and by sequence/slice number(s) as necessary (tabulated as outlined in the Supplementary data).
Reportable elements are dependent on the protocol employed for the WB-MRI examination. Bone, nodal disease, visceral, and soft tissue assessments maybe undertaken with the appropriate caveats; inability to depict microscopic metastases in normal sized nodes, inability to consistently visualise lung metastases <1 cm in size, inability to definitively exclude brain metastases in the absence of contrast medium administration, etc.
Other pertinent, including negative, findings can be included. The presence of complications such as renal obstruction and likelihood of bone compromise that could lead to a significant skeletal event should be stated.
Follow-up studies should mirror baseline assessments. Changes in the measurements of marker lesions are an essential part of the objective assessments of response, often determining clinical decisions regarding therapy continuation. This places extra responsibility on radiologists to provide accurate and objective reports that enable oncologic clinicians to use the scan information appropriately.
3.4.4. Impression or conclusion
Whenever possible, a clear, brief summary statement of the overall assessment of the disease status indicating any changes over time should be presented. Recommended terms for overall patient response should encompass the range of potential observations that incorporate on a scale, the likely disease status (highly likely to be responding, likely to be responding, stable, likely to be progressing, highly likely to be progressing, and discordant).
The summary should specifically mention assessments of the primary tumour, nodal disease (local and distant), and visceral sites of metastatic disease. Comments on the general trend of change, intermediate lesions, uncertainties, and differential responses should be specifically noted.
When progression is observed, distinguish between the emergence of new disease and growth of previously noted disease.
The presence of complications such as renal obstruction and likelihood of bone compromise that could lead to a significant skeletal event should be highlighted.
Recommendations regarding follow-up duration, biopsy, and alternative radiological studies to clarify the nature of equivocal findings should also be made, as required.
3.5. Baseline lesion identification, mapping and measurements
At baseline, all unequivocal cancer lesions should be assigned to their regional location using the baseline reporting template form (Supplementary data). Fourteen regions have been selected for assessment at baseline and on follow-up studies: primary disease, seven skeletal regions, five nodal and visceral, and other sites. Detailed instruction can be found in baseline assessments methods (Supplementary data).
Measurements of bone lesions should be undertaken on high quality T1 W images. Lymph nodes and soft tissue assessment should also be undertaken using the measurement template form (Supplementary data). Up to five discrete bone lesions (≥ 1.5 cm longest diameter) should be chosen with at least one lesion in the appendicular skeleton (≥ 1.5 cm) if present. Up to five discrete lymph nodes (≥ 1.5 cm short axis) and up to five soft tissue lesions (≥ 1.5 cm; long axis) measurements can be obtained (15 lesions maximum). Thus, the 1.5-cm threshold applies to all measured lesions.
The 1.5-cm size threshold has been deliberately set to account for the poorer spatial resolution of WB-MRI images compared with whole body CT scans. Pixel sizes using a 256 × 256 mm matrix at a whole body field of view of 400–440 mm, can be 1.5–2 mm for T1 W and T2 W sequences. For DWI pixel sizes are in the order of 3–4 mm. The 5–7-mm thick slice of images further compounds partial volume averaging effects. This resolution is much less than what is achievable by routine CT scans (0.8–1 mm), meaning that lesion size measurement accuracy is lower for MRI. However, when spatial resolution allows (three dimensional, fast spin echo sequences), the 1.5-cm threshold can be relaxed to 1.0 cm. Active medical physics engagement may be necessary to clarify what can reasonably be achieved locally.
Potential target lesion(s) should be chosen for measurement recording. Nontarget lesions should be recorded but not measured. The presence of nonmeasurable disease should be recorded (Response Evaluation Criteria in Solid Tumours [RECIST] v1.1 guidance) [24]. Thus, lymph nodes that are ≥1.0 cm but less than 1.5 cm in short axis are considered pathologic but nonmeasureable (note that nonmeasurable in this context refers to a specific RECIST defined term). Visceral disease ≥1.5 cm in long axis is considered as measurable but when unequivocally malignant but less than <1.5 cm they are considered pathologic but nonmeasurable. Further detailed instructions can be found in baseline assessments methods (Supplementary data).
In rare instances, when there are unequivocal malignant lesions <1.5 cm and clinical trial entry mandates the presence of measurable disease, a relaxation of the ≥1.5-cm threshold may be applied (minimum > 1 cm, ≥ 1.5 cm preferred) in the knowledge of the caveats on image resolution discussed above.
3.6. Follow-up and response assessments
3.6.1. Frequency of follow-up
In clinical trials, we suggest that WB-MRI be performed at fixed-time intervals to better understand when and for how long antitumour effects occur. To minimise patient exposure to ineffective treatments, particularly for treatments for which the optimal timing is unknown, periodic assessments every 8–9 wk should be employed in clinical trials for 6 mo, and every 12 wk thereafter (PGWG compliant) [8].
For patients receiving clinically approved treatments, routine 12 weekly assessments will suffice, unless there are clinical indications for earlier re-evaluations. This recommendation is consistent with recent European and International consensus guidelines for monitoring the progress of APC [4], [5].
For patients with M0-CRPC who are undergoing surveillance to detect the emergence of metastatic disease, 16-wk interval scanning can be undertaken (PCWG compliant) [8].
3.6.2. Response categories
Response assessments are undertaken by noting measurements and observation changes at a regional level, and on a whole patient basis, at each follow-up examination.
At each follow-up study, measurements should be repeated, following the lesions identified on baseline measurements (Supplementary data).
At each follow-up study, changes in the metastatic patterns should be assessed and recorded at a regional level using regional response assessment template forms in the Supplementary data.
Regional level response assessments use a scale of 1–5 indicating the likely response category: (1) highly likely to be responding, (2) likely to be responding, (3) stable, (4) likely to be progressing, and (5) highly likely to be progressing, using the criteria defined in Table 3.
Table 3.
METastasis Reporting and Data System for Prostate Cancer regional response assessment categories
| RAC | Classification | Region | Descriptionsa |
|---|---|---|---|
| 1 | Highly likely to be responding | Local, nodal, and visceral | • Consistent with RECIST v1.1/PCWG criteria for unequivocal response (partial/complete; see below) |
| Bone | Return of normal marrow in areas previously infiltrated by focal/diffuse metastatic infiltration • Decrease in number/size of focal lesions sufficient to indicate high likelihood response • Evolution of diffuse neoplastic pattern to focal lesions Decreasing soft tissue associated with bone disease • Dense lesion sclerosis (edge to edge), sharply defined, very thin/disappearance of hyperintense rim on T2W-FS images • The emergence of intra/peritumoural fat within/around lesions (fat dot/halo signs) • Previously evident lesion shows increase in ADC from ≤1400 μm2/s to >1400 μm2/sb • ≥40% increase in ADC from baseline with corresponding decrease in high b-value SI; and morphological findings consistent with stable or responding diseasec |
||
| 2 | Likely to be responding | Local, nodal, and visceral | • Changes depicting tumour response that do not meet RECIST v1.1/PCWG criteria for partial or complete response (see below) |
| Bone | Evidence of improvement, but not enough to fulfil criteria for RAC 1. For example: • Previously evident lesions showing increases in ADC from ≤1000 μm2/s to <1400 μm2/sb • >25% but <40% increase in ADC from baseline with corresponding decrease in high b-value SI; and morphological findings consistent with stable or responding diseasec |
||
| 3 | No change | All | • No observable change |
| 4 | Likely to be progressing | Local, nodal, and visceral | • Changes depicting tumour progression that do not meet RECIST v1.1/PCWG criteria for progression (see below) |
| Bone | • Evidence of worsening disease, but not enough to fulfil criteria for RAC 5 • Equivocal appearance of new lesion(s) • No change in size but increasing SI on high b-value images (with ADC values < 1400 μm2/s) consistent with possible disease progressionb • Relapse disease: re-emergence of lesion(s) that previously disappeared or enlargement of lesion(s) lesions that had partially regressed/stabilized with prior treatments Imaging depicted bone lesions that might be clinically significant (therefore excludes asymptomatic fractures in noncritical bones) • Soft tissue in spinal canal causing narrowing not associated with neurological findings and not requiring radiotherapy |
||
| 5 | Highly likely to be progressing | Local, nodal, and visceral | • Changes depicting tumour progression that meet RECIST v1.1/PCWG criteria for unequivocal progression (see below) |
| Bone | • New critical fracture(s)/cord compression requiring radiotherapy/surgical intervention → only if confirmed as malignant by MRI signal characteristics • Unequivocal new focal/diffuse area(s) of metastatic infiltration in regions of prior normal marrow • Unequivocal increase in number/size of focal lesions Evolution of focal lesions to diffuse neoplastic pattern • Appearance/increasing soft tissue associated with bone disease • New lesions/regions of high signal intensity on high b-value images with ADC value between 600 μm2/s and 1000 μm2/s |
||
| RECIST v1.1 categories [24] • Complete response: disappearance of all target lesions • Partial response: at least a 30% decrease in the sum of the longest diameter (LD) of target lesions, taking as reference the baseline sum LD • Stable disease: neither sufficient shrinkage to qualify for partial response nor sufficient increase to qualify for progressive disease, taking as reference the smallest sum LD since the treatment started • Progressive disease: at least a 20% increase in the sum of the LD of target lesions, taking as reference the smallest sum LD recorded since the treatment started or the appearance of one or more new lesions Progression of local prostate disease • Use RECIST v1.1 for progression criteria above applied to local disease Progression of nodes • <1.0 cm nodes have to have grown by at least 5 mm in short axis from baseline or treatment nadir and be ≥1 cm to be considered to have progressed • For nodes that are 1.0–1.5 cm that have grown by at least 5 mm in short axis from baseline or treatment nadir and are ≥1.5 cm in short axis can be considered to have progressed • For nodes ≥1.5 cm short axis use RECIST v1.1 progression criteria Progression of visceral disease • Use RECIST v1.1 progression criteria above applied to visceral disease | |||
ADC = apparent diffusion coefficient; FS = fast spin; MRI = magnetic resonance imaging; PCWG = Prostate Cancer Clinical Trials Working Group; RAC = response assessment category; RECIST = Response Evaluation Criteria in Solid tumours; SI = signal intensity; W = weighted.
Multiple criteria need to be met to category response.
These response assessment categories (RAC) have been designed to capture regional responses for both bone and soft tissue assessments; further details on use of RAC categories are found in the Supplementary data.
3.6.3. Discordant response assessments
MET-RADS-P incorporates a scoring system that enables documentation of heterogeneity of responses (mixed/discordant responses) at the regional level using RACs.
A three pattern scoring system that records responses should be used (primary [dominant]/secondary/tertiary). The primary/dominant pattern is the response (on the RAC 1–5 scale) seen in the majority of lesions within regions. The secondary pattern records the second most frequent RAC pattern of response within the region (Supplementary Table 2).
When there are three patterns in a region, and the tertiary is RAC 1–3, this tertiary pattern can/should be ignored as a minor response/stable disease is unlikely to be prognostically important. Thus, the tertiary pattern should only be used to document progressing/progressive disease (RAC patterns 4 and 5) occurring in a minority of lesions in a region (when not already recorded on the primary or secondary assessments).
3.7. Final response assessment
The status of the primary disease, nodes, viscera, and bone disease should be recorded separately using the overall response assessment template form (Supplementary data). Note that there is no separate recording of overall patient response on the form, which should be indicated in the text report (see section 3.4 above) taking into account the overall imaging impressions.
3.7.1. Soft tissue disease
Unlike regional response assessments, overall response for the primary tumour, nodal, and visceral disease should be categorical, thus following established guidelines (PCWG modifications of RECIST v1.1) [8]. The following categories should be assigned: complete response, partial response, stable disease, progressive disease, and discordant.
Any progression assignment in soft tissues based on measurements should be from baseline or treatment induced nadir whichever is lower. Overall progression based on measurements is the only time when treatment induced nadir is used. Other progression assignments are as per RECIST v1.1 (including new disease).
3.7.2. Bone disease
Unlike soft tissue assessments, the overall response of bone disease should be on a scale of 1–5 indicating the likely overall response category: (1) highly likely to be responding, (2) likely to be responding, (3) stable, (4) likely to be progressing, (5) highly likely to be progressing, and (6) discordant. This overall bone assessment should be based on the regional assessments using the criteria in Table 3.
3.7.3. Discordant response
Discordance indicates the presence of progressing bone/soft tissue disease, not meeting definitive progression criteria in the primary category, that is, when the majority of disease is stable or responding.
The discordant category for soft tissue disease should only be applied for stable and partial response categories to reflect its prognostic importance. Similarly, for bone disease, the discordant category should only be applied for stable and responding categories.
Discordance should be separately reported for primary, nodal, viscera, and bone; evaluations of regional discordant responses on forms in the Supplementary data will enable the specific identification of the anatomic sites of mixed responses.
In each discordant case, indicate whether discordance is a secondary (ie, major discordance) or tertiary (ie, minor discordance) assessment (Supplementary Table 2).
4. Conclusions
The MET-RADS-P system provides comprehensive characterisation of APC state, not only at the start of treatments, but also over time as the disease evolves. MET-RADS-P is suitable for guiding patient care (using the regional and overall assessment criteria), but can also be incorporated into clinical trials when lesion measurements become more important. MET-RADS-P allows the categorisation of patients with specific patterns of disease for clinical trials stratification. MET-RADS-P enables the evaluation of the benefits of continuing therapy, when there are signs that the disease is progressing (discordant responses).
MET-RAD-P requires validation within clinical trials. We suggest that MET-RADS-P be evaluated in studies that assess the effects of treatments known to kill tumour cells such as those targeting the androgen axis and cytotoxic chemotherapy. In these studies, MET-RADS-P should be compared with other response BMs, and correlated to quality of life measures, rates of skeletal events, and progression free survival. The latter are prerequisites for the introduction of WB-MRI into longer term follow-up studies that prospectively collect appropriate meta-data, which would allow objective assessments of whether WB-MRI is effective in supporting drug development. Clearly, MET-RADS-P is not at the point where it can support regulatory approvals of new therapeutic approaches. It is anticipated that, as evidence accrues from clinical trials, more specific recommendations and/or algorithms incorporating MET-RADS-P will emerge. Thus, we recommend that MET-RADS-P is now evaluated in clinical trials, to assess its impact on the clinical practice of APC.
Author contributions: Anwar R. Padhani had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Padhani, Lecouvet, Tunariu, Koh, De Keyzer, Collins, Sala, Schlemmer, Petralia, Vargas, Fanti, Tombal, de Bono.
Acquisition of data: Padhani, Lecouvet, Tunariu, Koh, De Keyzer, Collins.
Analysis and interpretation of data: Padhani, Lecouvet, Tunariu, Koh, De Keyzer, Collins, Sala, Schlemmer, Petralia, Vargas, Fanti, Tombal, de Bono.
Drafting of the manuscript: Padhani, Lecouvet, Tunariu, Koh, De Keyzer, Collins, Vargas, Fanti.
Critical revision of the manuscript for important intellectual content: Schlemmer, Petralia, Tombal, de Bono.
Statistical analysis: Padhani, Lecouvet.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Tombal, de Bono.
Other: None.
Financial disclosures: Padhani certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Padhani/Koh have research agreements and speakers’ bureau declarations for Siemens Healthcare.
Funding/Support and role of the sponsor: None.
Acknowledgments: We are grateful to FRS-FNRS Televie, Fondation Contre le Cancer, Fondation Saint Luc, Belgium and Memorial Sloane Kettering Cancer Centre Support Grant/Core Grant (P30 CA008748) for funding our work. We also acknowledge the support from Prostate Cancer UK and Movember to the London Movember Prostate Cancer Centre of Excellence at The Institute of Cancer Research and Royal Marsden, and through an Experimental Cancer Medical Centre grant from Cancer Research UK and the Department of Health (Reference C51/A7401). The authors acknowledge National Health Service funding to the National Institute for Health Research Biomedical Research Centre at the Royal Marsden, and The Institute of Cancer Research. The funders played no role in the decision to undertake this review, the preparation of the manuscript, or in the decision to submit the paper for publication. We thank Michael Kosmin for his input into the design of the heterogeneity assessment proforma. We also thank Aurelius Omlin and Silke Gillessen for their helpful clinical insights.
Associate Editor: Giacomo Novara
Footnotes
Please visit www.eu-acme.org/europeanurology to read and answer questions on-line. The EU-ACME credits will then be attributed automatically.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eururo.2016.05.033.
Appendix A. Supplementary data
References
- 1.Oudard S. Progress in emerging therapies for advanced prostate cancer. Cancer Treat Rev. 2013;39:275–289. doi: 10.1016/j.ctrv.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Body J.-J., Casimiro S., Costa L. Targeting bone metastases in prostate cancer: Improving clinical outcome. Nat Rev Urol. 2015;12:340–356. doi: 10.1038/nrurol.2015.90. [DOI] [PubMed] [Google Scholar]
- 3.Sridhar S.S., Freedland S.J., Gleave M.E. Castration-resistant prostate cancer: from new pathophysiology to new treatment. Eur Urol. 2014;65:289–299. doi: 10.1016/j.eururo.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Fitzpatrick J.M., Bellmunt J., Fizazi K. Optimal management of metastatic castration-resistant prostate cancer: Highlights from a European Expert Consensus Panel. Eur J Cancer. 2014;50:1617–1627. doi: 10.1016/j.ejca.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Gillessen S., Omlin A., Attard G. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol. 2015;26:1589–1604. doi: 10.1093/annonc/mdv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halabi S., Kelly W.K., Ma H. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;10:1652–1659. doi: 10.1200/JCO.2015.65.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans C.P., Higano C.S., Keane T. The PREVAIL study: Primary outcomes by site and extent of baseline disease for enzalutamide-treated men with chemotherapy-naïve metastatic castration-resistant prostate cancer. Eur Urol. 2016:1–9. doi: 10.1016/j.eururo.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Scher H.I., Morris M.J., Stadler W.M. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016:1–38. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padhani AR, Lecouvet FE, Tunariu N, et al. Rationale for modernizing imaging in advanced prostate cancer. Eur Urol Focus. In press. [DOI] [PubMed]
- 10.Lecouvet F.E., Talbot J.N., Messiou C. Monitoring the response of bone metastases to treatment with Magnetic Resonance Imaging and nuclear medicine techniques: A review and position statement by the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer. 2014;50:2519–2531. doi: 10.1016/j.ejca.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Shen G., Deng H., Hu S., Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol. 2014;43:1503–1513. doi: 10.1007/s00256-014-1903-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu L.-P., Cui L.-B., Zhang X.X. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bone malignancy. Medicine (Baltimore) 2015;94:e1998. doi: 10.1097/MD.0000000000001998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taouli B., Beer A.J., Chenevert T. Diffusion-weighted imaging outside the brain: Consensus statement from an ISMRM-sponsored workshop. J Magn Reson Imaging. 2016 doi: 10.1002/jmri.25196. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spritzer C.E., Afonso P.D., Vinson E.N. Bone marrow biopsy: RNA isolation with expression profiling in men with metastatic castration-resistant prostate cancer--factors affecting diagnostic success. Radiology. 2013;269:816–823. doi: 10.1148/radiol.13121782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson D., Van Allen E.M., Wu Y.M. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tombal B., Rezazadeh A., Therasse P., Van Cangh P.J., Vande Berg B., Lecouvet F.E. Magnetic resonance imaging of the axial skeleton enables objective measurement of tumor response on prostate cancer bone metastases. Prostate. 2005;65:178–187. doi: 10.1002/pros.20280. [DOI] [PubMed] [Google Scholar]
- 17.Lecouvet F.E. Springer-Verlag Berlin Heidelberg; Berlin, Germany: 2011. MRI For response assessment in oncologic bone marrow lesions. [Google Scholar]
- 18.Padhani A.R., van Ree K., Collins D.J., D'Sa S., Makris A. Assessing the relation between bone marrow signal intensity and apparent diffusion coefficient in diffusion-weighted MRI. AJR Am J Roentgenol. 2013;200:163–170. doi: 10.2214/AJR.11.8185. [DOI] [PubMed] [Google Scholar]
- 19.Amlani A., Ghosh-Ray S., van Ree K. Proc Int Soc Magn Reson Med. Salt Lake, UT. 2013. Relationships between diffusion weighted signal intensity, adc and water/fat content of malignant bone marrow; p. 3895. [Google Scholar]
- 20.Koh D.-M., Blackledge M., Padhani A.R. Whole-body diffusion-weighted MRI: tips, tricks, and pitfalls. AJR Am J Roentgenol. 2012;199:252–262. doi: 10.2214/AJR.11.7866. [DOI] [PubMed] [Google Scholar]
- 21.Moulopoulos L.A., Koutoulidis V. Springer Berlin Heidelberg; Berlin, Germany: 2014. Bone marrow MRI: a pattern based approach. [Google Scholar]
- 22.Vande Berg B.C., Malghem J., Lecouvet F.E., Maldague B. Magnetic resonance imaging of normal bone marrow. Eur Radiol. 1998;8:1327–1334. doi: 10.1007/s003300050547. [DOI] [PubMed] [Google Scholar]
- 23.Pasoglou V., Larbi A., Collette L. One-step TNM staging of high-risk prostate cancer using magnetic resonance imaging (MRI): Toward an upfront simplified ‘all-in-one’ imaging approach? Prostate. 2013;74:469–477. doi: 10.1002/pros.22764. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Cookson M.S., Roth B.J., Dahm P. Castration-resistant prostate cancer: AUA guideline. J Urol. 2013;190:429–438. doi: 10.1016/j.juro.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Halabi S., Lin C.-Y., Kelly W.K. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:671–677. doi: 10.1200/JCO.2013.52.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker C., Nilsson S., Heinrich D. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 28.de Bono J.S., Logothetis C.J., Molina A. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beer T.M., Armstrong A.J., Rathkopf D.E. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James N.D., Sydes M.R., Clarke N.W. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2015;6736:1–15. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan C.J., Smith M.R., Fizazi K. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 32.Dennis E.R., Jia X., Mezheritskiy I.S. Bone scan index: a quantitative treatment response biomarker for castration-resistant metastatic prostate cancer. J Clin Oncol. 2012;30:519–524. doi: 10.1200/JCO.2011.36.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweeney C.J., Chen Y.-H., Carducci M. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ceci F., Castellucci P., Graziani T. 11C-Choline PET/CT in castration-resistant prostate cancer patients treated with docetaxel. Eur J Nucl Med Mol Imaging. 2016;43:84–91. doi: 10.1007/s00259-015-3177-4. [DOI] [PubMed] [Google Scholar]
- 35.Tait C., Moore D., Hodgson C. Quantification of skeletal metastases in castrate-resistant prostate cancer predicts progression-free and overall survival. BJU Int. 2014;114:E70–E73. doi: 10.1111/bju.12717. [DOI] [PubMed] [Google Scholar]
- 36.Sabbatini P., Larson S.M., Kremer A. Prognostic significance of extent of disease in bone in patients with androgen-independent prostate cancer. J Clin Oncol. 1999;17:948–957. doi: 10.1200/JCO.1999.17.3.948. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Lopez R, Lorente D, Blackledge MD, et al. Volume of bone metastasis assessed with whole-body diffusion-weighted imaging is associated with overall survival in metastatic castration-resistant prostate cancer. Radiology. In-press. http://dx.doi.org/10.1148/radiol.2015150799 [DOI] [PubMed]
- 38.Aparicio A.M., Harzstark A.L., Corn P.G. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:3621–3630. doi: 10.1158/1078-0432.CCR-12-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris M.J., Molina A., Small E.J. Radiographic Progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 Results. J Clin Oncol. 2015;33:1356–1363. doi: 10.1200/JCO.2014.55.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonpavde G., Pond G.R., Templeton A.J. Association between RECIST changes and survival in patients with metastatic castration-resistant prostate cancer receiving docetaxel. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.10.008. pii: S0302-2838(15)00978-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Messiou C., Collins D.J., Morgan V.A., Bianchini D., de Bono J.S., de Souza N.M. Use of apparent diffusion coefficient as a response biomarker in bone: effect of developing sclerosis on quantified values. Skeletal Radiol. 2014;43:205–208. doi: 10.1007/s00256-013-1768-3. [DOI] [PubMed] [Google Scholar]
- 42.Messiou C., Collins D.J., Giles S. Assessing response in bone metastases in prostate cancer with diffusion weighted MRI. Eur Radiol. 2011;21:2169–2177. doi: 10.1007/s00330-011-2173-8. [DOI] [PubMed] [Google Scholar]
- 43.Koh D.M., Blackledge M., Collins D.J. Reproducibility and changes in the apparent diffusion coefficients of solid tumours treated with combretastatin A4 phosphate and bevacizumab in a two-centre phase I clinical trial. Eur Radiol. 2009;19:2728–2738. doi: 10.1007/s00330-009-1469-4. [DOI] [PubMed] [Google Scholar]
- 44.Messiou C., Collins D.J., Morgan V.A., Desouza N.M. Optimising diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur Radiol. 2011;21:1713–1718. doi: 10.1007/s00330-011-2116-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.