Abstract
Background
Malaria remains a major health problem worldwide especially in sub-Saharan Africa. In Kenya, 80% of the population is at risk of contracting the disease. Pregnant mothers and children under five years are the most affected by this disease. Antimalarial drug resistance poses a major threat in the fight against malaria necessitating continuous search for new antimalarial drugs. Due to inadequate and inaccessible health facilities, majority of people living in rural communities heavily depend on traditional medicine which involves the use of medicinal plants for the management of malaria. Most of these indigenous knowledge is undocumented and risks being lost yet such information could be useful in the search of new antimalarial agents.
Aim of study
An ethnobotanical survey was carried out among the Luhya community of Kakamega East sub-County, a malaria epidemic region, with the aim of documenting the plants used in the management of malaria.
Materials and methods
Semi-structured questionnaires were used to collect information from 21 informants who included traditional medicine practitioners and other caregivers who had experience in use of plants in management of malaria. These were drawn from 4 villages located in Kakamega East sub-county, within Kakamega County based on their differences in topography. Information recorded included plant names, parts used, mode of preparation and administration and the sources of plant materials. A literature search was conducted using PubMed and google scholar to identify the reported traditional uses of these plants and studied antiplasmodial activities.
Results
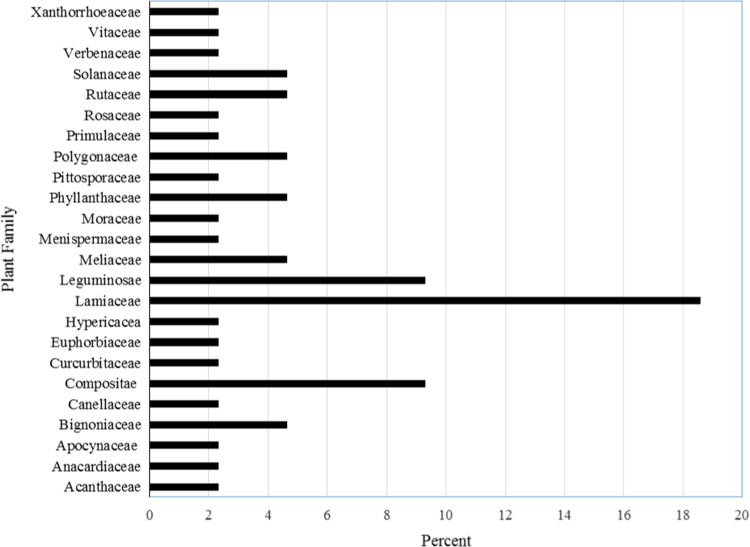
In this study, 57% of the informants were aged above 50 years and a total of 61% had either no formal education or had only attained primary school education. A total of 42 plant species belonging to 24 families were identified. Most plants used in the management of malaria in this community belonged to Lamiaceae (18%), Leguminosae (9%) and Compositae (9%) plant families. Plants mostly used included Melia azedarach L, Aloe spp, Ajuga integrifolia Buch. Ham, Vernonia amygdalina Del., Rotheca myricoides (Hochst.) Steane and Mabb, Fuerstia africana T.C.E.Fr., Zanthoxylum gilletii (De Wild.) P.G.Waterman and Leucas calostachys Oliv. Rumex steudelii Hochst.ex A. Rich and Phyllanthus sepialis Müll. Arg are reported for the first time in the management of malaria. Although Clerodendrum johnstonii Oliv. (Jeruto et al., 2011) and Physalis peruviana L.(Ramadan et al., 2015) are reported in other studies for management of malaria, no studies have been carried out to demonstrate their antiplasmodial activity.
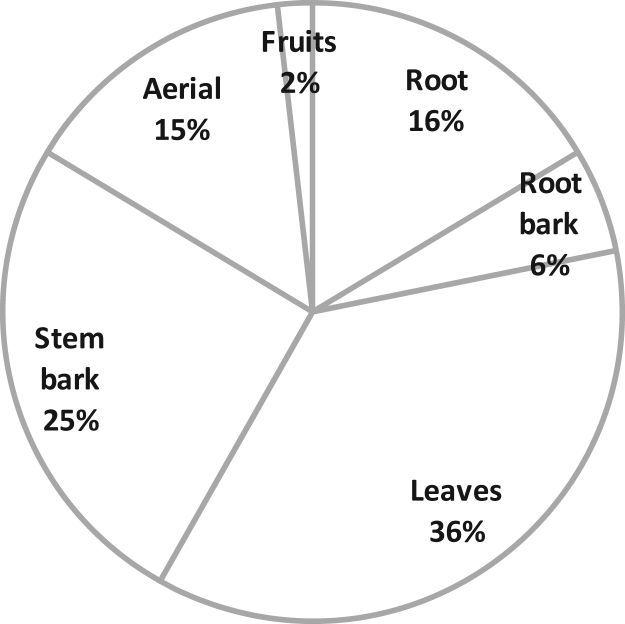
The plant parts mostly used were the leaves (36%) and stem barks (26%). Majority of these plants were prepared as decoctions by boiling and allowed to cool before administration (66%) while infusions accounted for 28% of the preparations. The literature mined supports the use of these plants for the management of malaria since most of them have demonstrated in-vitro and in-vivo antiplasmodial activities.
Conclusion
Most of the reported plant species in this study have been investigated for antiplasmodial activity and are in agreement with the ethnomedical use. Two (2) plants are reported for the first time in the management of malaria. There is need for documentation and preservation of the rich ethnomedical knowledge within this community given that most of the practitioners are advanced in age and less educated. There is also the danger of over-exploitation of plant species as most of them are obtained from the wild, mainly Kakamega forest. Therefore, there is need for determining the economically and medicinally important plants in this community and planning for their preservation.
Abbreviations: AIDS, Acquired Immune Deficiency Syndrome; APHRC, African Population and Health Research Center; CARTA, Consortium for Advanced Research Training in Africa; DelPHE, Development Partnerships in Higher Education; DfID, Department for International Development; HIV, Human Immunodeficiency Virus; IC50, Half Maximal Inhibitory Coefficient; KIPPRA, Kenya Institute for Public Policy Research and Analysis; KNBS, Kenya National Bureau of Statistics; NCAPD, National Coordinating Agency for Population and Development; PMI, President's Malaria Initiative; UK, United Kingdom; USAID, United States Agency for International Development; WHO, World Health Organization
Keywords: Ethnopharmacology; Malaria; Medicinal plants; Kakamega East, Luhya
Graphical abstract
1. Introduction
The World Health Organization (WHO) estimates that 3.2 billion people are at risk of malaria infection globally. In the year 2015, a total of 214 million cases and 438,000 deaths due to malaria were reported globally. The burden was highest in WHO-Africa region where 90% of all malaria death occurred (WHO, 2015). In Kenya malaria, still remains a major health problem with 80% of the population at risk of contracting the disease (PMI, 2015). Malaria affects the poor and marginalized populations. In most cases such populations live in rural areas and lack access to adequate healthcare facilities (Yusuf et al., 2010). Therefore, such people largely depend on herbal medicines for the management of malaria and other diseases.
Kakamega county lies within the western highlands malaria epidemic region in Kenya (KNBS and ICF Macro, 2011 and USAID, 2013). This region experiences seasonal malaria outbreaks. The epidemics are favored by the high temperature of above 18 °C during the long rainy seasons which is optimal for breeding of mosquitoes. The poverty level in this county is estimated at 57% (NCAPD, 2005). The high malaria incidences, high poverty levels and the proximity to the tropical forest, Kakamega forest, promotes the use of plants in management of various ailments including malaria.
Most of the African societies have a long history of indigenous healing practices. This knowledge is often passed from generation to generation by word of mouth. The Luhya community in Kakamega has a rich culture of herbalism. The practitioners of herbal medicine are well known within the community and are sought after for their skills in management of diseases. However other members of the community who are not herbal practitioners too have some knowledge on use of plants in management off common diseases and only employ within family context such as mothers in management of children illnesses without consulting the recognized herbalists (Wane, 2011). This knowledge is likely to be lost if not documented. This study therefore sought to document the ethnopharmacological knowledge in the management of malaria in Kakamega County.
2. Methods
2.1. Study area
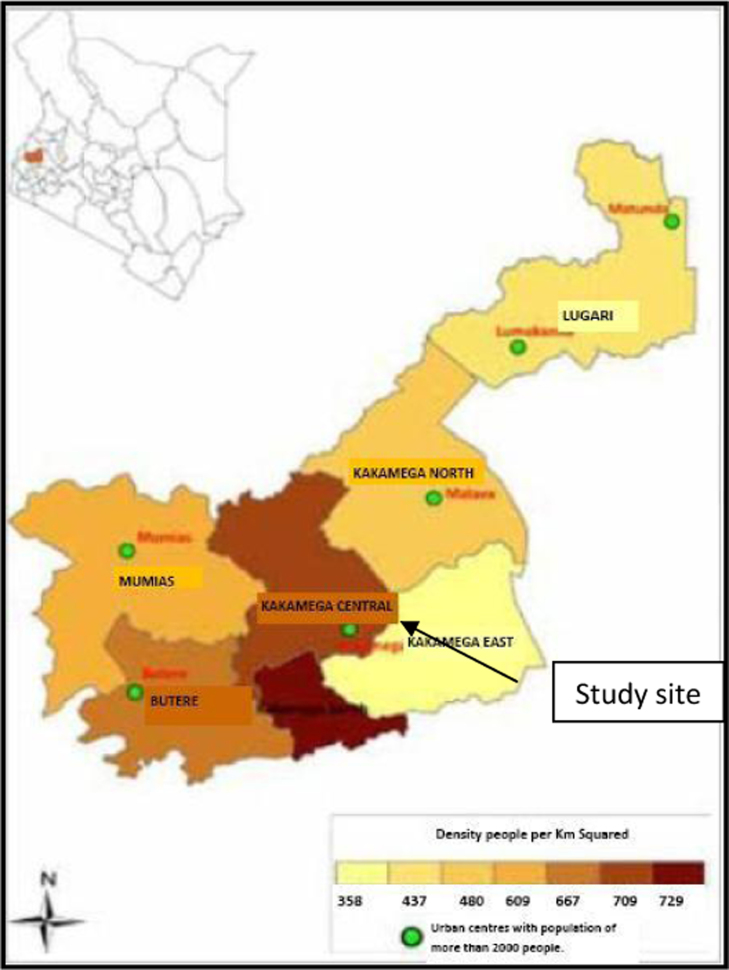
The ethnopharmacological survey was carried out in Kakamega sub-county in Kakamega County. Kakamega County is located in Western Kenya (Fig. 1). The county lies within the longitudes 34° 20′ 35.29″ E- 35° 09′ 27.04″ E and latitudes 0° 05′ 19.12″N- 0° 53′ 53.81″ N. It boarders several other counties, Bungoma to the North, Trans Nzoia to the North East, Uasin-Gishu and Nandi Counties to the East, Vihiga to the South, Siaya to the South West and Busia to the West. According to the 2009 census, the county has a total population of 1,660,651 people with population density of 515 people per km2. The poverty level in the county is estimated to be 57% (KIPPRA, 2013).
Fig. 1.
Map of Kakamega county showing study site. (Source: CRA, 2013)
Kakamega East District, the focus of this study is one of the 6 districts in Kakamega County. The district is mainly a rural set-up with no single urban center. The entire district is served with only dispensaries and health centers (NCAPD, 2005). The nearest high level health facility, Kakamega County hospital, 20 km away.
Kakamega East District is the home to the Kakamega forest, the only tropical rain forest in Kenya. This forest is the main source of herbal medicines for rural communities in the region (Nyunja et al., 2009). The poor health facilities and proximity to the forest are promote the use of herbal medicines in this community.
2.2. Ethical approval for the study
Ethical approval for the ethnobotanical survey was obtained from the Kenyatta National Hospital/University of Nairobi Ethics and Research Committee (P186/03/2015). The community gate keepers, who included village elders and church leaders were consulted and subsequently approved the study to be conducted within the local villages. The participants in this study were provided with information on the nature of study, benefits and risks involved. Those who agreed to participate signed a written consent at the beginning of the study.
2.3. Ethnobotanical survey
An ethnobotanical survey was carried out between the August and October 2015 in Kakamega East Sub-county of Kakamega County. The region was subdivided into 4 villages based on the differences in topography. Interviews were conducted using semi-structured questionnaires. The study was concluded when no more new information was realized. A total of 21 respondents, both male and female, who utilized antimalarial plants either for self-medication or for treating others were interviewed. A research assistant known to the locals accompanied the researchers during the interviews. Voucher specimens were prepared for all plants collected and deposited at the Department of Botany, University of Nairobi.
3. Results and discussion
3.1. Socio-economic characteristics of respondents
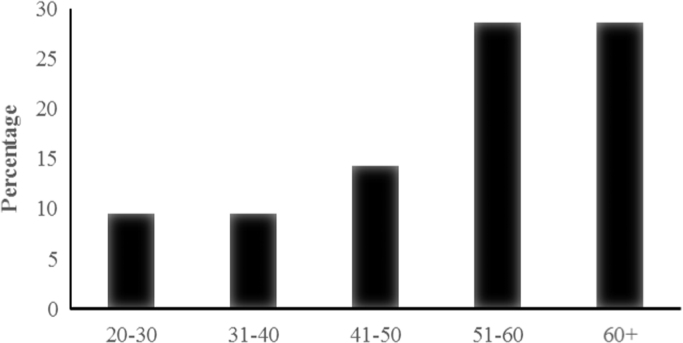
The majority (57%) of respondents in this survey were male aged >50 years of age (Fig. 2). The age ranged between 21 and 85 years. Usually the older members of the society have experience in the practice of traditional medicine and pass it on to the younger generation. The younger generation are also not readily accepted by the community as traditional practitioners as they are considered inexperienced (Lambert et al., 2011). In this study, the older practitioners were more recognized by the community than the younger ones. This explained why more than half the respondents were advanced in years.
Fig. 2.
Age Groups of Study Respondents.
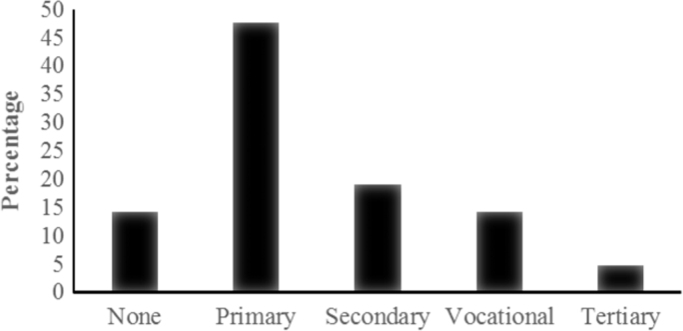
Most of the respondents had a primary school level of education (Fig. 3). The practice of traditional medicine has been for a long time been restricted to the less educated since the most educated people view it as ancient form of medicine that is primitive and inappropriate. Most of the practitioners only charge a small fee or no fee at all in managing the common diseases such as malaria since the plants are obtained locally making it not a lucrative business.
Fig. 3.
Education Level of Respondents.
3.2. Source of Ethnomedical Knowledge
Majority of the respondents (48%) had acquired the knowledge of the practice of traditional medicine from the older members of their families such as parents and grandparents. However, a relatively large proportion (43%) had acquired the knowledge though other means such as apprenticeship under practicing herbalists or by reading books about traditional medicine. Only 1 person had acquired the knowledge through formal training. This is similar to findings in other studies where apprenticeship is the commonest means of learning traditional practices (Lambert et al., 2011). In the recent years, there has been a global increase in demand and acceptability of traditional medicine (Abdullahi, 2011). In view of this, there is increase in commercialization of herbal medicines and more people learning about herbal medicine as a source of income. This may be the reason for the higher number (52%) of people in this study who practice herbalism even though they did not inherit the practice.
3.3. Antimalarial plants diversity
Most of the respondents in this study identified fever as the main symptom associated with malaria. Other symptoms mentioned included headache, vomiting, diarrhea and joint pains. They were also aware of severe form of malaria (cerebral malaria). Only one respondent claimed he could treat cerebral malaria. The rest indicated that such cases should be referred to hospital.
A total of 42 plant species belonging to 39 genera within 24 families were identified (Table 1). A large proportion of these plants were from the Lamiaceae (18%), Leguminosae (9%), and Compositae (9%) families (Fig. 4). Most of these plants were shrubs (42%) and trees (27%) followed by climbers (24%) and herbs (7%). Plants mostly cited included Melia azedarach L, Ajuga integrifolia Buch.-Ham and Aloe spp. Rumex steudelii Hochst.ex A. Rich and Phyllanthus sepialis Müll. Arg are reported for the first time in the management of malaria.
Table 1.
Plants used in the management of malaria among the Luhya community, Kakamega East sub-County.
| Voucher number | Family | Plant name | Local name | Growth form | Plant part used | Frequency of citation (%) | Mode of preparation |
|---|---|---|---|---|---|---|---|
| NMA2015/01 | Acanthaceae | Justicia betonica L. | – | Herb | Aerial | 14.3 | Pound, add cold water/Boil |
| NMA2015/02 | Anacardiaceae | Searsia natalensis (Bernh.ex C. Krauss) | Busangula | Herb | Leaves, Stem Bark | 4.8 | Boil in water |
| NMA2015/03 | Apocynaceae | Carissa edulis L. | Shikata/Achoka | Shrub | Root Bark | 4.8 | Boil in water/Inhale steam |
| NMA2015/04 | Compositae | Acmella caulirhiza Del. | Shituti | Herb | Aerial Part | 9.5 | Pound, add cold water |
| NMA2015/05 | Compositae | Microglossa pyrifolia (Lam.) Kuntze | Ing’oi | Shrub | Root, Leaves | 9.5 | Boil in water |
| NMA2015/06 | Compositae | Tithonia diversifolia (Hemsl.) A. Gray | Masambu malulu/libinzo | Shrub | Leaves | 9.5 | Pound, add cold water |
| NMA2015/07 | Compositae | Vernonia amygdalina Del. | Musulilitsa | Shrub | Leaves | 19.0 | Pound in cold water/ Boil |
| NMA2015/08 | Bignoniaceae | Markhamia lutea (Benth.) K.Schum. | Lusiola | Tree | Stem Bark | 9.5 | Boil in water |
| NMA2015/09 | Bignoniaceae | Spathodea campanulata P.Beauv. | Mutsulio | Tree | Stem Bark | 14.3 | Boil without crushing |
| NMA2015/10 | Canellaceae | Warbugia ugandensis Sprague | Apachi | Tree | Leaves, Stem Bark | 14.3 | Boil in water |
| NMA2015/11 | Curcurbitaceae | Cucumis aculeatus Cogn | – | Climber | Leaves | 4.8 | Pound, add cold water |
| NMA2015/12 | Euphorbiaceae | Crotom macrostachuys Hochst. ex Del. | Musutsu | Tree | Stem Bark | 4.8 | Boil in water |
| NMA2015/13 | Leguminosae | Albizia gummifera (J.F.Gmel.) C.A.Sm. | Musenzeli | Tree | Stem Bark | 9.5 | Boil without crushing |
| NMA2015/14 | Leguminosae | Erythrina abyssinica DC. | Murembe | Tree | Stem Bark | 4.8 | Boil in water |
| NMA2015/15 | Leguminosae | Senna didmobotrya (Fresen.) H.S.Irwin and Barneby | Lubinu | Shrub | Leaves | 4.8 | Boil in water |
| NMA2015/16 | Leguminosae | Senna occidentalis (L.) Link | Imbindi | Shrub | Root | 4.8 | Pound, add cold water |
| NMA2015/17 | Hypericaceae | Harungana madagascariensis Lam. ex Poir. | Musila | Tree | Stem Bark | 4.8 | Boil in water |
| NMA2015/18 | Lamiaceae | Ajuga integrifolia Buch.-Ham. | Imbuli yu mtakha | Herb | Aerial | 23.8 | Pound, add cold water |
| NMA2015/19 | Lamiaceae | Clerodendrum johnstonii Oliv. | Shiteng’oteng’o | Shrub | Leaves | 4.8 | Pounded in cold water/Boil |
| NMA2015/20 | Lamiaceae | Rotheca myricoides (Hochst.) Steane and Mabb. | Shisilangokho | Shrub | Rootbark, Leaves | 19.0 | Boil in water/Roast |
| NMA2015/21 | Lamiaceae | Fuerstia africana T.C.E.Fr. | Muvesemu | Herb | Aerial | 19.0 | Boiled or roasted |
| NMA2015/22 | Lamiaceae | Leucas calostachys Oliv. | Lumetsani | Herb | Aerial | 19.0 | Pound, add cold water/Boil/Steam |
| NMA2015/23 | Lamiaceae | Ocimun kilimandscharicum Gürke | M’monyi | Herb | Aerial | 4.8 | Inhale steam |
| NMA2015/24 | Lamiaceae | Plectranthus barbatus Andrews | Shilokha | Shrub | Leaves | 9.5 | Chew bud/boil in water |
| NMA2015/25 | Meliaceae | Mellia azedarach L | Muarubaini | Tree | Leaves, Stem Bark | 47.6 | Boil in water |
| NMA2015/26 | Meliaceae | Trichilia emetica Vahl | Munyama | Tree | Stem Bark | 4.8 | Boil in water |
| NMA2015/27 | Menispermaceae | Cissampelos mucronata A.Rich. | Mukoye | Climber | Root | 4.8 | Chewing |
| NMA2015/28 | Moraceae | Ficus thonningii Blume | Mutoto | Tree | Stem Bark | 9.5 | Boil in water |
| NMA2015/29 | Primulaceae | Maesa lanceolata Forssk. | Mushevesheve | Tree | Root Bark, Stem Bark | 4.8 | Boil in water |
| NMA2015/30 | Phyllanthaceae | Flueggea virosa (Roxb. ex Willd.) Royle | – | Shrub | Aerial | 4.8 | Boil in water |
| NMA2015/31 | Phyllanthaceae | Phyllanthus sepialis Müll. Arg. | – | Shrub | Leaves | 4.8 | Boil in water |
| NMA2015/32 | Pittosporaceae | Pittosporumviridiflorum Sims | M’monyo/Mkungune | Shrub | Leaves, Stem Bark | 4.8 | Boil in water |
| NMA2015/33 | Polygonaceae | Rumex abyssinicus Jacq. | Shikachi | Herb | Leaves | 9.5 | Pound, add cold water |
| NMA2015/34 | Polygonaceae | Rumex steudelii Hochst.ex A. Rich | Alukhava | Herb | Root | 9.5 | Pound, add cold water |
| NMA2015/35 | Rosaceae | Rubus pinnatus Wild. | Butunduli | Shrub | Leaves, Fruits | 4.8 | Pounded in cold water/chew |
| NMA2015/36 | Rutaceae | Clausena anisata (Willd.) Hook.f. ex Benth. | Shihunya bukundu | Shrub | Leaves | 4.8 | Boil in water |
| NMA2015/37 | Rutaceae | Zanthoxylum gilletii (De Wild.) P.G.Waterman | Shikuma | Tree | Stem Bark | 19.0 | Boil in water |
| NMA2015/38 | Solanaceae | Physalis peruviana L. | Mayengo | Shrub | Leaves | 4.8 | Inhale steam |
| NMA2015/39 | Solanaceae | Solanum incanum L. | Indalandalu | Shrub | Root | 4.8 | Pound, add cold water |
| NMA2015/40 | Verbenaceae | Lantana trifolia L. | Shimenenwa | Shrub | Leaves | 9.5 | Boil in water/steam |
| NMA2015/41 | Vitaceae | Rhoicissus tridentata (L.f.) Wild and R.B.Drumm. | Muhoko | Climber | Root | 4.8 | Boil in water |
| NMA2015/42 | Xanthorrhoeaceae | Aloe species | Shikakha | Herb | Leaves | 43.0 | Boil in water |
Fig. 4.
Frequency of use of plant families in management of malaria in Kakamega East sub-County.
More than 90% of the plants were referred to by their local names. However four of the plants could not be identified by their local names indicating that they may have been introduced into the region. Justicia betonica L. was referred to as the dark “Imbuli yu mutakha” which refers to Ajuga integrifolia Buch.-Ham that is commonly known in this region. The association of this plant with known plant could be due to the use for malaria or due to the bitter taste associated with both plants. However, the 2 plants have very different morphological characteristics. Cucumis aculeatus was referred to as “Khaseveve” because of having leaves with morphological similarity to a pumpkin plant's leaves (Liseveve).
The part of plant used as medicine plays an important role in sustainability of herbal medicine. The most commonly used plant parts were the leaves (36%) and stem barks (25%) (Fig. 5).
Fig. 5.
Plant parts used in malaria management.
The use of roots and root bark is not sustainable for medicinal plants since the plant is uprooted in most cases to obtain these parts. In this study, roots and root barks plant parts accounted for 22% of all the plants identified. Conservationists warn of over-exploitation of medicinal plants which are valued for their root parts and stem barks (Maroyi, 2013). In this study a total of 47% of the plants were valued for the root parts or stem barks therefore threatened by over-exploitation. Leaves and fruits are the most preferred parts for sustainable plant use since they are the least destructive to the plant and they accounted for 38% in this study.
3.4. Preparation of antimalarial medicines
Various methods are used in preparation of herbal medicine among the community. The most common preparation were decoctions (70%), which were made by boiling plant material before use. Other methods included cold maceration (30%), steaming (12%), roasting (8%) to obtain ash or chewing (8%). In most of the cases, the plant material was harvested and prepared just before use. Nevertheless, where the plants are not easily accessible, material was preserved by air-drying under shade and stored for future use.
3.5. Sources of plant material
The herbal medicines used for malaria were mainly obtained from the wild (77%) with only 23% cultivated. The cultivation of medicinal plants was mainly done for those plants not easily available in the community, the introduced plants or those that face extinction. In this study, Justicia betonica L. an introduced herb, was mainly planted along river beds. Ajuga integrifolia Buch.-Ham which almost faces extinction was also planted by the herbalists. Previous studies carried out in Kenya show that most of the herbal products are exclusively obtained from the wild. This strongly indicates the unsustainability of herbal practice in Kenya (McMullin et al., 2012).
3.6. Reported traditional uses and antiplasmodial activity
The identified plants in this study have been used in many communities for the management of various ailments including malaria and other febrile illnesses. A total of 38 out of the 42 identified plants have been tested in the laboratory for in-vivo and/or in-vitro antiplasmodial activities as summarized in Table 2. In vitro antiplasmodial activity is classified as high (IC50<5 μg/ml), promising (5<IC50<15 μg/ml), moderate (15<IC50<50 μg/ml) and inactive (IC50>50 μg/ml) (Lekana-Douki et al., 2011). Based on this criteria, 14 of the plants from our study are classified as possessing high antiplasmodial activity with Albizia gummifera (J.F.Gmel.) C.A.Sm., Leucas calostachys Oliv., Tithonia diversifolia and Harungana madagascariensis Lam. ex Poir. having the highest antiplasmodial activity of <1 μg/ml. Although Tithonia diversifolia (Hemsl.) A. Gray has promising antiplasmodial activity, study by Elufioye et al. (2009) showed that it is toxic to the liver and kidney therefore limiting its widespread use in the management of malaria.
Table 2.
Literature review of the plants used for management of malaria among the Luhya community of Kakamega East sub-County.
| Plant name | Traditional uses | In vitro and in vivo antimalarial activities | Antimalarial compounds isolated |
|---|---|---|---|
| Justicia betonica L. | Lower cholesterol, paralysis, earaches, headaches, bruises diarrhea, vomiting, constipation, pain and inflammation and Malaria (Gangabhavani and Ravishankar, 2013), | Ether aerial parts extract had IC50 of 13.36 µg/ml (Bbosa et al., 2013) | No reference |
| Searsia natalensis (Bernh.ex C. Krauss) | Malaria (Gathirwa et al., 2011), diarrhea, HIV (Mugisha et al., 2014) | CHCl3 leaf extract had IC50 of 1.8 μg/ml (Katuura, et al., 2007b). | No reference |
| Carissa edulis L | Sickle cell anemia, fever, epilepsy, pain (Ya'u et al., 2013), malaria (Orwa et al., 2007) | DCM, stems extract had IC50 of 33 μg/ml (Clarkson et al., 2004) | No reference |
| Spathodea campanulata P.Beauv. | Malaria, herpes, fever, diabetes, dysentery, ulcers, HIV (Lim, 2013) | Ethanolic leaf extract had IC50 >68 μg/ml (Rangasamy et al., 2008) | Lapachol (Ntie-Kang et al., 2014) |
| Markhamia lutea (Benth.) K.Schum. | Malaria (Lacroix et al., 2009)Anemia, diarrhea, microbial and parasitic infections (Ali et al., 2015) | EtOAc, Leaf extract exhibited 70% parasite suppression (Lacroix et al., 2011) | musambins A-C and musambiosides A-C(Lacroix et al., 2009) |
| Warbugia ugandensis Sprague | Worms, fever, gonorrhea, syphilis (Lacroix et al., 2011) (Were et al., 2010) | DCM, stem bark extract of IC50 of 8 μg/ml (Wube et al., 2008) with 69% parasite inhibition (Were et al., 2010) | coloratane sesquiterpenes.(Onguén et al., 2013) |
| Vernonia amygdalina Del. | Febrifuge, vermifuge, laxative, malaria, wounds and as appetizer (Ifeoma and Chukwunonso, 2011) | Ethanolic leaf extract IC50 of 9.83 µg/ml (Omoregie et al., 2011) | Vernolide, vernodalin, vernodalol and hydroxyvernolide (Onguén et al., 2013) |
| In-vivo parasite suppression of between 57.2–72.7% in combination with CQ (Challand and Willcox, 2009) | |||
| Tithonia diversifolia (Hemsl.) A. Gray | Diabetes mellitus, sore throat, menstrual pain, malaria, wounds (Owoyele et al., 2004) | ether extract of aerial parts had IC50 of 0.75 µg/ ml(Goffin et al., 2002) whereas the methanolic extract had 74% parasitemia suppression (Oyewole et al., 2008) | Tagitinin C (Onguén et al., 2013) |
| Acmella caulirhiza Del. | Toothache, throat and gum infections, dysentery, rheumatism, immune booster and malaria (Grubben, 2004) | DCM extract had IC50<10 μg/ml (Owuor et al., 2012) | No reference |
| Microglossa pyrifolia (Lam.) Kuntze | Malaria (Jeruto et al., 2011)Headache, cough, flu, cleansing airway (Moshi et al., 2012) | DCM, Leaf extract had IC50 of <15 μg/ml (Muganga et al., 2014) | diterpeness (Kohler et al., 2002) |
| Cucumis aculeatus Cogn | Diarrhea, leprosy, migraines, wounds, gonorrhea (Engels et al., 1991), malaria (Njoroge and Bussmann, 2006) | Aqueous fruit extract had IC50 of >30 μg/ml (Gakunju et al., 1995) | No reference |
| Crotom macrostachuys Hochst. ex Del. | Diabetes, dysentery, wounds, malaria, purgative, stomachache (Gelaw et al., 2012) | DCM, leaf extract IC50 of 2 μg/ml (Owuor et al., 2012) | No reference |
| Harungana madagascariensis Lam. ex Poir. | Anemia, malaria (Iwalewa et al., 2008), fever, nephrosis, jaundice, gastrointestinal disorders (Okoli et al., 2002) | Ethanolic stem bark extract had IC50 of <0.5 μg/ml and showed between 28.6–44.8% Parasite suppression (Iwalewa et al., 2008) | Bazouanthrone, feruginin A, harunganin, harunganol A and B (Ntie-Kang et al., 2014) |
| Rotheca myricoides (Hochst.) Steane and Mabb. | Measles, malaria, asthma, wounds, gonorrhea, rabies and eye disease (Hayelom et al., 2012) | Methanolic leaf extract, IC50 of 9.51–10.56 µg/ml and 82% parasite suppression at 600 mg/kg (Deressa et al., 2010) | No reference |
| Leucas calostachys Oliv. | Colds, headache (Okello et al., 2010) malaria (Nyambati et al., 2013) | Aqueous whole plant extract had IC50 of 0.8 µg/ml with parasite inhibition of 3.5–5.2% (Nyambati et al., 2013) | No reference |
| Ocimun kilimandscharicum Gürke | Colds, cough, analgesic, sedative, diarrhea, measles (Soni et al., 2012), malaria (Owuor et al., 2012) | DCM, Leaves and twigs had IC50 of < 1.5 (Owuor et al., 2012) | No reference |
| Fuerstia africana T.C.E.Fr. | Eye ailments, toothache (Kipkore et al., 2014) malaria (Muganga et al., 2010) | Pet-ether extract of aerial parts had IC50 of 1.5 μg/ml (Kigondu et al., 2011) | Ferruginol (Onguén et al., 2013) |
| Methanolic leaf extract had IC50 <15 μg/ml with > 70% parasite suppression (Muganga et al., 2014) | |||
| Clerodendrum johnstonii Oliv. | Abscess, hernia (Quattrocchi, 2012), malaria (Jeruto et al., 2011) | No reference | No reference |
| Plectranthus barbatus Andrews | Gastritis, respiratory disorders, cough, analgesic, hypertension, stomachache, epilepsy (Fernandes et al., 2012) malaria, break fevers (Al-Musayeib et al., 2012) | Methanolic extract had IC50 of 6.5 μg/ml, (Al-Musayeib et al., 2012) | No reference |
| Ajuga integrifolia Buch.-Ham | Vermifuge, toothache, hypertension, stomachache fever (Hailu and Engidawork, 2014) malaria (Gitua et al., 2012) | Aqueous leaf extract exhibited 90% parasite suppression (Gitua et al., 2012) | Ajugarin−1(Onguén et al., 2013) ergosterol−5,8-endoperoxide (Ntie-Kang et al., 2014) |
| Albizia gummifera (J.F.Gmel.) C.A.Sm. | Malaria, bacterial infections, skin diseases, stomachache (Kokila et al., 2013) | The alkaloidal fraction had IC50 of 0.06 µg/ml while spermine alkaloid exhibited parasite suppression of 43–72% (Rukunga et al., 2007) | spermine alkaloids (Rukunga et al., 2007) |
| Senna occidentalis (L.) Link | Malaria, vermifuge, analgesic, laxative, hepatoprotective, diuretic and febrifuge (Silva et al., 2011) | EtOH root bark extracts had IC50<3 μg/ml whereas 200 mg/kg of EtOH and DCM extracts exhibited >60% parasitaemia suppression. (Tona et al., 2001) | Quinones (Kayembe et al., 2010) |
| Senna didmobotrya (Fresen.) H.S.Irwin and Barneby | Intestinal worms, skin diseases, jaundice, veneral diseases, malaria, fever (Nagappan, 2012) | DCM/MeOH. Twigs extract had IC50 of 9.5 μg/ml (Clarkson et al., 2004) | No reference |
| Erythrina abyssinica DC. | Abortion, cough, malaria (Lacroix et al., 2011) | EtOAc, Bark extract showed 83% parasite suppression (Lacroix et al., 2011) | 5- deoxyabyssinin II and homobutein (Ntie-Kang et al., 2014) |
| Trichilia emetica Vahl | Diabetes, hypertension (Konaté et al., 2014), malaria (Diarra et al., 2015) | DCM/MeOH (1:1), leaves and twigs extract had of IC50 of 3.5 μg/ml (Clarkson et al., 2004) | Kurubasch aldehyde (Bero et al., 2009) |
| Mellia azedarach L. | Hepatoprotective, malaria, skin diseases, ulcers, fever, vermifuge, asthma (Qureshi et al., 2016) | DCM, Leaf extract had IC50 of 28 μg/ml (Lusakibanza et al., 2010) | No reference |
| Cissampelos mucronata A.Rich | Antisnake venom, veneral diseases, malaria, menstrual disorders, wounds, febrifuge (Nondo et al., 2011) | EtOAc root extract had IC50 <3.91 with active compound, curine IC50 of 0.24 (Omole, 2012) | curine (Ndiege, 2011) |
| Ficus thonningii Blume | Malaria (Falade et al., 2014), Diabetes, diarrhea, mental illness, gonorrhea, urinary tract infections (Dangarembizi et al., 2013) | Hexane, leaf extract IC50 of 10.4 μg/ml (Falade et al., 2014) | No reference |
| Flueggea virosa (Roxb. ex Willd.) Royle | Fever, stomachache, rheumatism, pneumonia, epilepsy, body pains (Magaji et al., 2007) malaria (Al-Rehaily et al., 2015) | MeOH/H2O leaves extract had IC50 of 7.8 (Willcox et al., 2011a) | securinine and viroallosecurinine (Al-Rehaily et al., 2015) |
| Alkaloids: securinine and viroallosecurinine had IC50 values of 2.7 and 2.9 μg/ml respectively (Al-Rehaily et al., 2015) | |||
| Phyllanthus sepialis Müll. Arg. | Tonic in pregnancy (PROTA, 2008) dental hygiene (Bussmann et al., 2006) | No reference | No reference |
| Pittosporum viridiflorum Sims | Chest complains, purgative, male impotence, asthma, coughs (Dold and Cocks, 2012) malaria (Nyongbela et al., 2013) | DCM whole plant extract had = IC50 of 3 μg/ml (Clarkson et al., 2004) | triterpenoid estersaponin, active (Nyongbela et al., 2013) |
| pittoviridoside (saponins) (Seo et al., 2002) | |||
| Rumex abyssinicus Jacq. | Wounds, liver diseases, malaria, gonorrhea (Mulisa et al., 2015) | DCM, root extract had IC50 <15 μg/ml, (Muganga et al., 2014) | No reference |
| Rumex steudelii Hochst.ex A. Rich | Antifertility, tonsillitis, wounds, eczema, hemorrhoids, leprosy (Solomon et al., 2010) (Gebrie et al., 2005) | No reference | No reference |
| Maesa lanceolata Forssk | Malaria (Katuura, et al., 2007a) | Chloroform leaf extract IC50 of 1.6 µg/ml (Katuura, et al., 2007b) | No reference |
| DCM/MeOH (1:1) twigs extract IC50 of 5.9 µg/ml (Clarkson et al., 2004) | |||
| Rubus pinnatus Wild | Bleeding gums, expectorant, demulcent, diarrhea (Quattrocchi, 2012), malaria (Lacroix et al., 2011) | EtOAc, leaf extract exhibited 20% parasite suppression (Lacroix et al., 2011) | No reference |
| Zanthoxylum gilletii (De Wild.) P.G.Waterman | Stomachache, gonorrhea, back pain, urinogenital infections (Gaya et al., 2013).malaria (Masinde, 2014) | DCM/MeOH (1:1) stem bark extract had IC50 of 2.52, 1.48 and 1.43 µg/ml against W2, D6 and 3D7 strains. (Masinde, 2014) | Nitidine (Muganga et al., 2014) Seasamine |
| 8-acetonyldihydrochelerythrine (Masinde, 2014) | |||
| Clausena anisata (Willd.) Hook.f. ex Benth. | vermifuge, febrifuge, measles, hypertension, malaria, analgesic, rheumatism (Okokon et al., 2012) | DCM, root extract had IC50 of 10 μg/ml (Clarkson et al., 2004) | No reference |
| Physalis peruviana L. | malaria, rheumatism, hepatitis, dermatitis, diuretic (Ramadan et al., 2015) | No reference | No reference |
| Solanum incanum L. | Pneumonia, liver pain, headache, toothache, stomachache, sore throat (PROTA, 2008), malaria (Nguta et al., 2010) | CHCl3/MeOH, leaf extract showed 31% parasite suppression (Murithi et al., 2014) | No reference |
| Lantana trifolia L. | Common cold, asthma, epilepsy, madness, childhood cerebral malaria, sickle cell anemia.(Nalubega et al., 2013) | Pet-ether aerial parts extract had IC50 of 13.2 μg/ml (Katuura et al., 2007b) | No reference |
| Rhoicissus tridentata (L.f.) Wild and R.B.Drumm. | Dysmenorrhea, uterotonic, indigestion, pregnancy and childbirth. (Mukundi et al., 2015), malaria (Gakunju et al., 1995) | Aqueous root extract had IC50 > 40 μg/ml (Gakunju et al., 1995) | No reference |
| Aloe species | Colds, malaria, stomachache, anemia (PROTA, 2008) | Ether leaf extract of A.dawei, IC50 of 7.9 μg/ml (Bbosa et al., 2013) | No reference |
CHCl3=Chloroform, DCM=Dichloromethane, EtOAc=Ethyl Acetate, MeOH=methanol, Pet-ether=Petroleum ether
Although Clerodendrum johnstonii Oliv. (Jeruto et al., 2011) and Physalis peruviana L. (Ramadan et al., 2015) are reported in other studies for management of malaria, no studies have been carried out to demonstrate their antiplasmodial activity. Less than half of these plants have antiplasmodial compounds isolated as shown in Table 2.
Several ethnobotanical studies to identify antimalarial plants have been carried out in Kenya. These studies identify a variety of plants used by different ethnic communities or regions in Kenya. They include the Nandi (Jeruto et al., 2011), Digo in Kwale (Muthaura et al., 2007), Msambweni (Nguta et al., 2010), Kisumu (Orwa et al., 2007), Central Kenya communities (Njoroge and Bussmann, 2006) and Kilifi (Gathirwa et al., 2011). This is the first study to document antimalarial plants used among the Luhya community in Kenya.
The different Kenyan communities utilize plants within the local communities in the management of malaria. The differences in geographical and climatic conditions which determine the flora available in a given region. However some plants have a wider distribution and therefore most likely used by most communities. For instance, Aloe species and Melia azedarach L. are plants utilized in all these studied communities. Very few similarities were observed in the utilization of plants among the Luhya community and the Coast Kenya regions communities (Msambweni and Kilifi). For instance, only 3 plants were used in by both the Luhya and Kilifi communities.
Several similarities were observed in the antimalarial plants used in Central Kenya, Nandi and the Luhya community. For instance, 30% of the plants used by the Luhya community were also utilized by the Nandi community. The Nandi and Luhya communities boarder each other. The similarities in the use of the plants could be either due to similarities in vegetation as a result or similar climatic conditions. It could also result from the interchange of knowledge within communities. It is therefore important to consider factors such as ecology, culture and even religious context in the protection of indigenous knowledge and not just a matter of ethnic group. To address this, the concept of ‘Collective Biocultural Heritage’ (BCH) was developed to consider cases where different communities share traditional knowledge within given shared territories and resources. This concept has been utilized in Peru where Inter-Community Agreement for equitable benefit sharing between 6 communities (Swiderska, 2007).
4. Conclusion
This study provides a documentation of plants used in the management of malaria in the Luhya community of Kakamega East sub-county. Most of the plants cited in this study have been reported elsewhere for management of malaria and have demonstrated antiplasmodial activities. This indicative of the rich nature of ethnomedical knowledge in this community. Rumex steudelii Hochst.ex A. Rich and Phyllanthus sepialis Müll. Arg are reported for the first time in the management of malaria. There is therefore need to preserve the ethnomedicine knowledge from this community given that most of the practitioners of traditional medicine are older generation with less education.
Conservation methods need to be put in place to secure the future of traditional medicine practice in this community. The current status of harvesting from the wild and use of roots and barks should be done in a sustainable manner. Members of the community should be educated on sustainable harvesting and cultivation of medicinal plants.
Funding
This research was supported by the Consortium for Advanced Research Training in Africa (CARTA). CARTA is jointly led by the African Population and Health Research Center (APHRC) and the University of the Witwatersrand and funded by the Wellcome Trust (UK) (Grant No: 087547/Z/08/Z), the Department for International Development (DfID) under the Development Partnerships in Higher Education (DelPHE), the Carnegie Corporation of New York (Grant No: B 8606), the Ford Foundation (Grant No: 1100-0399), Google. Org (Grant No: 191994), Sida (Grant No: 54100029), MacArthur Foundation (Grant No: 10-95915-000-INP) and British Council.
Acknowledgement
The authors thank CARTA Africa for funding the research. We are grateful to all the traditional herbal practitioners for their participation in the study. We thank Mr. Patrick Mutiso of the University of Nairobi, herbarium for identifying the plant species.
Footnotes
Supplementary data associated with this article can be found in the online version at 10.1016/j.jep.2016.08.050.
Contributor Information
Nillian Mukungu, Email: nmukungu@uonbi.ac.ke.
Kennedy Abuga, Email: koabuga@uonbi.ac.ke.
Faith Okalebo, Email: faith.okalebo@uonbi.ac.ke.
Raphael Ingwela, Email: ralphingwela@uonbi.ac.ke.
Julius Mwangi, Email: julius.mwangi@uonbi.ac.ke.
Appendix A. Supplementary material
Supplementary material
.
References
- Abdullahi A.A. Trends and challenges of traditional medicine in Africa. Afr. J. Tradit. Complement. Altern. Med. 2011;8:115–123. doi: 10.4314/ajtcam.v8i5S.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Musayeib N.M., Mothana R.A., Matheeussen A., Cos P., Maes L. In vitro antiplasmodial, antileishmanial and antitrypanosomal activities of selected medicinal plants used in the traditional Arabian Peninsular region. BMC Complement. Altern. Med. 2012;12:1–7. doi: 10.1186/1472-6882-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rehaily A., Yousaf M., Ahmad M., Samoylenko V., Li X.-C., Muhammad I., El Tahir K.H. Chemical and biological study of Flueggea virosa native to Saudi Arabia. Chem. Nat. Compd. 2015;51:187–188. [Google Scholar]
- Ali S., El-Ahmady S., Ayoub N., Singab A.N. Phytochemicals of Markhamia species (Bignoniaceae) and their therapeutic value: a review. Eur. J. Med. Plants. 2015;6:124–142. [Google Scholar]
- Bbosa G.S., Kyegombe D.B., Lubega A., Musisi N., Ogwal-Okeng J., Odyek O. Anti-plasmodium falciparum activity of Aloe dawei and Justicia betonica. Afr. J. Pharm. Pharmacol. 2013;7:2258–2263. [Google Scholar]
- Bero J., Frederich M., Quetin-Leclercq J. Antimalarial compounds isolated from plants used in traditional medicine. J. Pharm. Pharmacol. 2009;61:1401–1433. doi: 10.1211/jpp/61.11.0001. [DOI] [PubMed] [Google Scholar]
- Bussmann R.W., Gilbreath G.G., Solio J., Lutura M., Lutuluo R., Kunguru K., Wood N., Mathenge S.G. Plant use of the Maasai of Sekenani Valley, Maasai Mara, Kenya. J. Ethnobiol. Ethnomed. 2006;2 doi: 10.1186/1746-4269-2-22. 22-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challand S., Willcox M. A clinical trial of the traditional medicine Vernonia amygdalina in the treatment of uncomplicated malaria. J. Altern. Complement. Med. 2009;15:1231–1237. doi: 10.1089/acm.2009.0098. [DOI] [PubMed] [Google Scholar]
- Clarkson C., Maharaj V.J., Crouch N.R., Grace O.M., Pillay P., Matsabisa M.G., Bhagwandin N., Smith P.J., Folb P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004;92:177–191. doi: 10.1016/j.jep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- CRA – Commission for Revenue Allocation, 2013. Kenya County Fact Sheets, Kakamega County, Second ed., Kenya.
- Dangarembizi R., Erlwanger K.H., Moyo D., Chivandi E. Phytochemistry, pharmacology and ethnomedicinal uses of Ficus thonningii (Blume Moraceae): a review. Afr. J. Tradit., Complement., Altern. Med. 2013;10:203–212. doi: 10.4314/ajtcam.v10i2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deressa T., Mekonnen Y., Animut A. In-vivo anti-malarial activities of Clerodendrum myricoides,D odonea angustifolia and Aloe debrana against Plasmodium berghei. Ethiop. J. Health Dev. 2010;24:25–29. [Google Scholar]
- Diarra N., Klooster C.V., Togola A., Diallo D., Willcox M., Jong J. Ethnobotanical study of plants used against malaria in Selingue subdistrict, Mali. J. Ethnopharmacol. 2015;166:352–360. doi: 10.1016/j.jep.2015.02.054. [DOI] [PubMed] [Google Scholar]
- Dold T., Cocks M. Jacana Media Publisher; Aukland park 2092, South Africa: 2012. Voices From the Forest: Celebrating Nature and Culture in Xhosaland. [Google Scholar]
- Elufioye T.O., Alatise O.I., Fakoya F.A., Agbedahunsi J.M., Houghton P.J. Toxicity studies of Tithonia diversifolia A. Gray (Asteraceae) in rats. J. Ethnopharmacol. 2009;122:410–415. doi: 10.1016/j.jep.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Engels J.M.M., Hawkes J.G., Worede M. Plant Genetic Resources of Ethiopia. Cambridge University Press, New York, USA; 1991. [Google Scholar]
- Falade M.O., Akinboye D.O., Gbotosho G.O., Ajaiyeoba E.O., Happi T.C., Abiodun O.O., Oduola A.M.J. In vitro and in vivo antimalarial activity of Ficus thonningii Blume (Moraceae) and Lophira alata Banks (Ochnaceae), identified from the ethnomedicine of the Nigerian Middle Belt. J. Parasitol. Res. 2014;2014:972853. doi: 10.1155/2014/972853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes B.L.C., Câmara C.C., Soto-Blanco B. Anticonvulsant activity of extracts of Plectranthus barbatus leaves in mice. Evid. Based Complement. Altern. Med. 2012;2012 doi: 10.1155/2012/860153. Article ID 860153 (4 pp.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakunju D.M., Mberu E.K., Dossaji S.F., Gray A.I., Waigh R.D., Waterman P.G., Watkins W.M. Potent antimalarial activity of the alkaloid nitidine, isolated from a Kenyan herbal remedy. Antimicrob. Agents Chemother. 1995;39:2606–2609. doi: 10.1128/aac.39.12.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangabhavani K., Ravishankar K. Evaluation of analgesic and antiinflammatory activities of ethanolic extract of whole plant Justicia betonica. World J. Pharm. Pharm. Sci. 2013;2:5218–5228. [Google Scholar]
- Gathirwa J.W., Rukunga G.M., Mwitari P.G., Mwikwabe N.M., Kimani C.W., Muthaura C.N., Kiboi D.M., Nyangacha R.M., Omar S.A. Traditional herbal antimalarial therapy in Kilifi district, Kenya. J. Ethnopharmacol. 2011;134:434–442. doi: 10.1016/j.jep.2010.12.043. [DOI] [PubMed] [Google Scholar]
- Gaya C.H., Kawaka J.F., Muchugi A., Ngeranwa J.J. Variation of alkaloids in the Kenyan Zanthoxylum gilletii (De Wild Waterman) Afr. J. Plant Sci. 2013;7:438–444. [Google Scholar]
- Gebrie E., Makonnen E., Debella A., Zerihun L. Phytochemical screening and pharmacological evaluations for the antifertility effect of the methanolic root extract of Rumex steudelii. J. Ethnopharmacol. 2005;96:139–143. doi: 10.1016/j.jep.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Gelaw H., Adane L., Tariku Y., Hailu A. Isolation of crotepoxide from berries of Croton macrostachyus and evaluation of its anti-Leishmanial activity. J. Pharmacogn. Phytochem. 2012:1. [Google Scholar]
- Gitua J.N., Muchiri D.R., Nguyen X.A. In vivo antimalarial activity of Ajuga remota water extracts against Plasmodium berghei in mice. Southeast Asian J. Trop. Med. Public Health. 2012;43:545–548. [PubMed] [Google Scholar]
- Goffin E., Ziemons E., De Mol P., de Madureira Md.C., Martins A.P., da Cunha A.P., Philippe G., Tits M., Angenot L., Frederich M. In vitro antiplasmodial activity of Tithonia diversifolia and identification of its main active constituent: tagitinin C. Planta Med. 2002;68:543–545. doi: 10.1055/s-2002-32552. [DOI] [PubMed] [Google Scholar]
- Grubben, G.J.H., Denton, O.A., 2004. Plant Resources of Tropical Africa 2: Vegetables. PROTA Foundation, Wageningen, Netherlands
- Hailu W., Engidawork E. Evaluation of the diuretic activity of the aqueous and 80% methanol extracts of Ajuga remota Benth (Lamiaceae) leaves in mice. BMC Complement. Altern. Med. 2014;14 doi: 10.1186/1472-6882-14-135. 135–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayelom K., Mekbeb A., Eyasu M., Wondwossen E., Kelbesa U. Methanolic effect of Clerodendrum myricoides root extract on blood, liver and kidney tissues of mice. Afr. Health Sci. 2012;12:489–497. doi: 10.4314/ahs.v12i4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifeoma I.I., Chukwunonso E.C.C.E. Current perspectives on the medicinal potentials of Vernonia amygdalina Del. J. Med. Plants Res. 2011;5:1051–1061. [Google Scholar]
- Iwalewa E.O., Omisore N.O., Adewunmi C.O., Gbolade A.A., Ademowo O.G., Nneji C., Agboola O.I., Daniyan O.M. Anti-protozoan activities of Harungana madagascariensis stem bark extract on trichomonads and malaria. J. Ethnopharmacol. 2008;117:507–511. doi: 10.1016/j.jep.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Jeruto P., Mutai C., Ouma G., Lukhoba C. An inventory of medicinal plants that the people of Nandi use to treat malaria. J. Anim. Plant Sci. 2011;9:1192–1200. [Google Scholar]
- Katuura E., Waako P., Ogwal-Okeng J., Bukenya-Ziraba R. Traditional treatment of malaria in Mbarara District, western Uganda. Afr. J. Ecol. 2007;45:48–51. [Google Scholar]
- Katuura E., Waako P., Tabuti J.R.S., Bukenya-Ziraba R., Ogwal-Okeng J. Antiplasmodial activity of extracts of selected medicinal plants used by local communities in western Uganda for treatment of malaria. Afr. J. Ecol. 2007;45:94–98. [Google Scholar]
- Kayembe J.S., Taba K.M., Ntumba K., Tshiongo M.T.C., Kazadi T.K. In vitro anti-malarial activity of 20 quinones isolated from four plants used by traditional healers in the Democratic Republic of Congo. J. Med. Plants Res. 2010;4:991–994. [Google Scholar]
- Kigondu E.V.M., Matu E.N., Gathirwa J.W., Irungu B.N., Mwikwabe N.M., Amalemba G.M., Omar S.A., Amugune B., Kirira P.G. Medicinal properties of Fuerstia africana T.C.E. Friers (Lamiaceae) Afr. J. Health Sci. 2011;19 [Google Scholar]
- Kipkore W., Wanjohi B., Rono H., Kigen G. A study of the medicinal plants used by the Marakwet Community in Kenya. J. Ethnobiol. Ethnomed. 10. 2014:24. doi: 10.1186/1746-4269-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIPPRA – Kenya Institute for Public Policy Research and Analysis, 2013. Poverty, Inequality and Population Growth, Kenya Economic Report 2013. GoK, pp. 19–28.
- KNBS – Kenya National Bureau of Statistics, ICF Macro, 2011. Kenya Malaria Survey Indicator 2010. Ministry of Public Health and Sanitation.
- Kohler I., Jenett-Siems K., Kraft C., Siems K., Abbiw D., Bienzle U., Eich E. Herbal remedies traditionally used against malaria in Ghana: bioassay-guided fractionation of Microglossa pyrifolia (Asteraceae) Z. Nat. C J. Biosci. 2002;57:1022–1027. doi: 10.1515/znc-2002-11-1212. [DOI] [PubMed] [Google Scholar]
- Kokila K., Priyadharshini S.D., Sujatha V. Phytopharmacological properties of Albizia species: a review. Int. J. Pharm. Pharm. Sci. 2013;5:70–73. [Google Scholar]
- Konaté K., Yomalan K., Sytar O., Zerbo P., Brestic M., Patrick V.D., Gagniuc P., Barro N. Free radicals scavenging capacity, antidiabetic and antihypertensive activities of flavonoid-rich fractions from leaves of Trichilia emetica and Opilia amentacea in an animal model of Type 2 Diabetes mellitus. Evid. Based Complement. Altern. Med. eCAM. 2014;2014:867075. doi: 10.1155/2014/867075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix D., Prado S., Deville A., Krief S., Dumontet V., Kasenene J., Mouray E., Bories C., Bodo B. Hydroperoxy-cycloartane triterpenoids from the leaves of Markhamia lutea, a plant ingested by wild chimpanzees. Phytochemistry. 2009;70:1239–1245. doi: 10.1016/j.phytochem.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Lacroix D., Prado S., Kamoga D., Kasenene J., Namukobe J., Krief S., Dumontet V., Mouray E., Bodo B., Brunois F. Antiplasmodial and cytotoxic activities of medicinal plants traditionally used in the village of Kiohima, Uganda. J. Ethnopharmacol. 2011;133:850–855. doi: 10.1016/j.jep.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Lambert, J., Leonard, K., Mungai, G., Omindi-Ogaja, E., Gatheru, G., Mirangi, T., Owara, J., Herbst, C.H., Ramana, G., Lemiere, C., 2011. The contribution of traditional herbal medicine practitioners to Kenyan health care delivery: results from community health-seeking behavior vignettes and a traditional herbal medicine practitioner survey. Health, Nutrition and Population (HNP) discussion paper. World Bank, Washington, DC.
- Lekana-Douki J.B., Oyegue Liabagui S.L., Bongui J.B., Zatra R., Lebibi J., Toure-Ndouo F.S. In vitro antiplasmodial activity of crude extracts of Tetrapleura tetraptera and Copaifera religiosa. BMC Res. Notes. 2011;4:506. doi: 10.1186/1756-0500-4-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T.K. Springer; Netherlands: 2013. Edible Medicinal and Non-Medicinal Plants. [Google Scholar]
- Lusakibanza M., Mesia G., Tona G., Karemere S., Lukuka A., Tits M., Angenot L., Frederich M. In vitro and in vivo antimalarial and cytotoxic activity of five plants used in Congolese Traditional Medicine. J. Ethnopharmacol. 2010;129:398–402. doi: 10.1016/j.jep.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Magaji M., Anuka J., Abdu-Aguye I., Yaro A., Hussaini I. Preliminary studies on anti-inflammatory and analgesic activities of Securinega virosa (Euphorbiaceae) in experimental animal models. J. Med. Plants Res. 2007;2:039–044. [Google Scholar]
- Maroyi A. Traditional use of medicinal plants in south-central Zimbabwe: review and perspectives. J. Ethnobiol. Ethnomed. 2013;9:1–18. doi: 10.1186/1746-4269-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masinde, W.R.G., 2014. Phytochemical investigation of Zanthoxylum gilletii (Rutaceae) for antiplasmodial biomolecules Chemistry. University of Nairobi, Nairobi, Kenya
- McMullin S., Phelan J., Jamnadass R., Iiyama M., Franzel S., Nieuwenhuis M. Trade in medicinal tree and shrub products in three urban centres in Kenya. For. Trees Livelihoods. 2012;21:188–206. [Google Scholar]
- Moshi M.J., Otieno D.F., Weisheit A. Ethnomedicine of the Kagera Region, north western Tanzania. Part 3: plants used in traditional medicine in Kikuku village, Muleba District. J. Ethnobiol. Ethnomed. 2012;8 doi: 10.1186/1746-4269-8-14. 14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muganga R., Angenot L., Tits M., Frederich M. In vitro and in vivo antiplasmodial activity of three Rwandan medicinal plants and identification of their active compounds. Planta Med. 2014;80:482–489. doi: 10.1055/s-0034-1368322. [DOI] [PubMed] [Google Scholar]
- Muganga R., Angenot L., Tits M., Frédérich M. Antiplasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malaria. J. Ethnopharmacol. 2010:128. doi: 10.1016/j.jep.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Mugisha M.K., Asiimwe S., Namutebi A., Borg-Karlson A.K., Kakudidi E.K. Ethnobotanical study of indigenous knowledge on medicinal and nutritious plants used to manage opportunistic infections associated with HIV/AIDS in Western Uganda. J. Ethnopharmacol. 2014;155:194–202. doi: 10.1016/j.jep.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Mukundi M., Mwaniki N., Piero N., Murugi N., Daniel A. In vivo anti-diabetic effects of aqueous leaf extracts of Rhoicissus tridentata in alloxan induced diabetic mice. J. Dev. Drugs. 2015;4:2. [Google Scholar]
- Mulisa E., Asres K., Engidawork E. Evaluation of wound healing and anti-inflammatory activity of the rhizomes of Rumex abyssinicus J. (Polygonaceae) in mice. BMC Complement. Altern. Med. 2015;15:1–10. doi: 10.1186/s12906-015-0878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murithi C., Fidahusein D., Nguta J., Lukhoba C. Antimalarial activity and in vivo toxicity of selected medicinal plants naturalised in Kenya. Int. J. Educ. Res. 2014;2:395–406. [Google Scholar]
- Muthaura C.N., Rukunga G.M., Chhabra S.C., Mungai G.M., Njagi E.N. Traditional antimalarial phytotherapy remedies used by the Kwale community of the Kenyan Coast. J. Ethnopharmacol. 2007;114:377–386. doi: 10.1016/j.jep.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Nagappan R. Evaluation of aqueous and ethanol extract of bioactive medicinal plant, Cassia didymobotrya (Fresenius) Irwin & Barneby against immature stages of filarial vector, Culex quinquefasciatus Say (Diptera: Culicidae) Asian Pac. J. Trop. Biomed. 2012;2:707–711. doi: 10.1016/S2221-1691(12)60214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalubega R., Nyanzi S.A., Nakavuma J.L., Kamatenesi-Mugisha M. Ethnobotanical uses of Lantana trifolia L. and Sida cuneifolia Roxb. In Mukungwe and Wabinyonyi sub-counties of Central Uganda. J. Intercult. Ethnopharmacol. 2013;2:155–164. [Google Scholar]
- NCAPD – National Coordinating Agency for Population and Development . Kakamega District Strategic Plan 2005–2010. Government of Kenya; Nairobi: 2005. [Google Scholar]
- Ndiege I.O. Department of Chemistry, Kenyatta University, Nairobi; 2011. Anti-malarial Activity and Phytochemical Studies of Cissampelos mucronata and Stephania abyssinica. [Google Scholar]
- Nguta J.M., Mbaria J.M., Gakuya D.W., Gathumbi P.K., Kiama S.G. Antimalarial herbal remedies of Msambweni, Kenya. J. Ethnopharmacol. 2010;128:424–432. doi: 10.1016/j.jep.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Njoroge G.N., Bussmann R.W. Diversity and utilization of antimalarial ethnophytotherapeutic remedies among the Kikuyus (Central Kenya) J. Ethnobiol. Ethnomed. 2. 2006:8. doi: 10.1186/1746-4269-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nondo O.R.S., Mbwambo Z.H., Kidukuli A.W., Innocent E.M., Mihale M.J., Erasto P., Moshi M.J. Larvicidal, antimicrobial and brine shrimp activities of extracts from Cissampelos mucronata and Tephrosia villosa from coast region, Tanzania. BMC Complement. Altern. Med. 2011;11:1–7. doi: 10.1186/1472-6882-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntie-Kang F., Onguene P.A., Lifongo L.L., Ndom J.C., Sippl W., Mbaze L.M. The potential of anti-malarial compounds derived from African medicinal plants, Part II: a pharmacological evaluation of non-alkaloids and non-terpenoids. Malar. J. 2014;13:81. doi: 10.1186/1475-2875-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyambati G.K., Lagat Z.O., Maranga R.O., Samuel M., Ozwara H. In vitro Anti-plasmodial Activity of Rubia cordifolia, Harrizonia abyssinica, Leucas calostachys Olive and Sanchus schweinfurthii Medicinal Plants. J. Appl. Pharm. Sci. 2013;3:57–62. [Google Scholar]
- Nyongbela K.D., Lannang A.M., Ayimele G.A., Ngemenya M.N., Bickle Q., Efange S. Isolation and identification of an antiparasitic triterpenoid estersaponin from the stem bark of Pittosporum mannii (Pittosporaceae) Asian Pac. J. Trop. Dis. 2013;3:389–392. [Google Scholar]
- Nyunja A., Onyango J., Erwin B. The Kakamega forest medicinal plant resources and their utilization by the adjacent Luhya community. Int. J. Trop. Med. 2009;4:82–90. [Google Scholar]
- Okello S.V., Nyunja R.O., Netondo G.W., Onyango J.C. Ethnobotanical study of medicinal plants used by Sabaots of Mt. Elgon Kenya. Afr. J. Tradit. Complement. Altern. Med. 2010;7:1–10. doi: 10.4314/ajtcam.v7i1.57223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okokon J.E., Etebong E.O., Udobang J.A., Essien G.E. Antiplasmodial and analgesic activities of Clausena anisata. Asian Pac. J. Trop. Med. 2012;5:214–219. doi: 10.1016/S1995-7645(12)60027-3. [DOI] [PubMed] [Google Scholar]
- Okoli A.S., Okeke M.I., Iroegbu C.U., Ebo P.U. Antibacterial activity of Harungana madagascariensis leaf extracts. Phytother. Res. 2002;16:174–179. doi: 10.1002/ptr.991. [DOI] [PubMed] [Google Scholar]
- Omole R.A. Department of Chemistry, Kenyatta University; Kenya: 2012. Anti-malarial Activity and Phytochemical Studies of Cissampelos mucronata and Stephania abyssinica. [Google Scholar]
- Omoregie E.S., Pal A., Sisodia B. In vitro antimalarial and cytotoxic activities of leaf extracts of Vernonia amygdalina (Del.) Niger. J. Basic Appl. Sci. 2011;19:121–126. [Google Scholar]
- Onguén É.P.A., Ntie-Kang F., Lifongo L.L., Ndom J.C., Sippl W., Mbaze L.M. The potential of anti-malarial compounds derived from African medicinal plants. Part I: a pharmacological evaluation of alkaloids and terpenoids. Malar. J. 2013;12:449. doi: 10.1186/1475-2875-12-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwa J., Mwitari P., Matu E., Rukunga G. Traditional healers and the management of malaria in Kisumu District, Kenya. East Afr. Med. J. 2007;84:51–55. doi: 10.4314/eamj.v84i2.9504. [DOI] [PubMed] [Google Scholar]
- Owoyele V.B., Wuraola C.O., Soladoye A.O., Olaleye S.B. Studies on the anti-inflammatory and analgesic properties of Tithonia diversifolia leaf extract. J. Ethnopharmacol. 2004;90:317–321. doi: 10.1016/j.jep.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Owuor B.O., Ochanda J.O., Kokwaro J.O., Cheruiyot A.C., Yeda R.A., Okudo C.A., Akala H.M. In vitro antiplasmodial activity of selected Luo and Kuria medicinal plants. J. Ethnopharmacol. 2012;144:779–781. doi: 10.1016/j.jep.2012.09.045. [DOI] [PubMed] [Google Scholar]
- Oyewole I.O., Ibidapo C.A., Moronkola D.O., Oduola A.O., Adeoye G.O., Anyasor G.N., Obansa J.A. Anti-malarial and repellent activities of Tithonia diversifolia (Hemsl.) leaf extracts. J. Med. Plants Res. 2008;2:171–175. [Google Scholar]
- PMI – President's Malaria Initiative, 2015. Kenya. Malaria Operational Plan-PMI.
- PROTA, 2008. Medicinal Plants, Vol. 1, Plant Resources of Tropical Africa. PROTA Foundation/Backhuys, Wageningen, Netherlands.
- Quattrocchi, U., 2012. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology (5 Volume Set). CRC Press, Oxfordshire, UK.
- Qureshi H., Arshad M., Akram A., Raja N.I., Fatima S., Amjad M.S. Ethnopharmacological and phytochemical account of paradise tree (Melia azedarach L.: Meliaceae) Pure Appl. Biol. 2016;5:5–14. [Google Scholar]
- Ramadan M., El-Ghorab A., Ghanem K. Volatile compounds, antioxidants, and anticancer activities of Cape gooseberry fruit (Physalis peruviana. L.): an in-vitro study. J. Arab Soc. Med. Res. 2015;10:56–64. [Google Scholar]
- Rangasamy D., Asirvatham D., Muthusamy J., Rajamanickam K., Muthusamy P., Jeyaraman A. Preliminary phytochemical screening and antimalarial studies of Spathodea campanulatum P. Beauv leaf extracts. Ethnobot. Leafl. 2008;12:811–819. [Google Scholar]
- Rukunga G.M., Muregi F.W., Tolo F.M., Omar S.A., Mwitari P., Muthaura C.N., Omlin F., Lwande W., Hassanali A., Githure J., Iraqi F.W., Mungai G.M., Kraus W., Kofi-Tsekpo W.M. The antiplasmodial activity of spermine alkaloids isolated from Albizia gummifera. Fitoterapia. 2007;78:455–459. doi: 10.1016/j.fitote.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Seo Y., Berger J.M., Hoch J., Neddermann K.M., Bursuker I., Mamber S.W., Kingston D.G.I. A new triterpene saponin from Pittosporum viridiflorum from the Madagascar Rainforest. J. Nat. Prod. 2002;65(1):65–68. doi: 10.1021/np010327t. [DOI] [PubMed] [Google Scholar]
- Silva M.G.B., Aragão T.P., Vasconcelos C.F.B., Ferreira P.A., Andrade B.A., Costa I.M.A., Costa-Silva J.H., Wanderley A.G., Lafayette S.S.L. Acute and subacute toxicity of Cassia occidentalis L. stem and leaf in Wistar rats. J. Ethnopharmacol. 2011;136:341–346. doi: 10.1016/j.jep.2011.04.070. [DOI] [PubMed] [Google Scholar]
- Solomon T., Largesse Z., Mekbeb A., Eyasu M., Asfaw D. Effect of Rumex steudelii methanolic root extract on ovarian folliculogenesis and uterine histology in female albino rats. Afr. Health Sci. 2010;10:353–361. [PMC free article] [PubMed] [Google Scholar]
- Soni N., Gill D., Sagar B.S., Raheja S., Agrawal S. Ocimum kilimandscharicum: A systematic review. J. Drug Deliv. Ther. 2012;2 [Google Scholar]
- Swiderska, K., 2007. Protecting Community Rights Over Traditional Knowledge: Implications of Customary Laws and Practices, Research Partners’ Workshop Panama.
- Tona L., Mesia K., Ngimbi N.P., Chrimwami B., Okond'Ahoka, Cimanga K., de Bruyne T., Apers S., Hermans N., Totte J., Pieters L., Vlietinck A.J. In-vivo antimalarial activity of Cassia occidentalis, Morinda morindoides and Phyllanthus niruri. Ann. Trop. Med. Parasitol. 2001;95:47–57. [PubMed] [Google Scholar]
- USAID – United States Agency for International Development . Center for Disease Control and Prevention; Washington DC: 2013. President's Malaria Initiative, Kenya: Malaria Operational Plan FY 2013. [Google Scholar]
- Wane N. The Kenyan herbalist ruptures the status quo in health and healing. Counterpoints. 2011;379:280–298. [Google Scholar]
- Were P.S., Kinyanjui P., Gicheru M.M., Mwangi E., Ozwara H.S. Prophylactic and curative activities of extracts from Warburgia ugandensis Sprague (Canellaceae) and Zanthoxylum usambarense (Engl.) Kokwaro (Rutaceae) against Plasmodium knowlesi and Plasmodium berghei. J. Ethnopharmacol. 2010;130:158–162. doi: 10.1016/j.jep.2010.04.034. [DOI] [PubMed] [Google Scholar]
- WHO – World Health Organization, 2015. Malaria Report 2015. World Health Organisation, Geneva.
- Willcox M.L., Graz B., Falquet J., Diakite C., Giani S., Diallo D. A “reverse pharmacology” approach for developing an anti-malarial phytomedicine. Malar. J. 2011;10:S8. doi: 10.1186/1475-2875-10-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wube A.A., Bucar F., Gibbons S., Asres K., Rattray L., Croft S.L. Antiprotozoal activity of sesquiterpenes from Warburgia ugandensis towards Trypanosoma brucei rhodesiense and Plasmodium falciparum in vitro. Planta Med. 2008;74:PA222. [Google Scholar]
- Ya'u J., Chindo B.A., Yaro A.H., Okhale S.E., Anuka J.A., Hussaini I.M. Safety assessment of the standardized extract of Carissa edulis root bark in rats. J. Ethnopharmacol. 2013;147:653–661. doi: 10.1016/j.jep.2013.03.064. [DOI] [PubMed] [Google Scholar]
- Yusuf O.B., Adeoye B.W., Oladepo O.O., Peters D.H., Bishai D. Poverty and fever vulnerability in Nigeria: a multilevel analysis. Malar. J. 2010;9:1–6. doi: 10.1186/1475-2875-9-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material