Abstract
Allergic aspergillosis is a Th2 T-lymphocyte-mediated pulmonary complication in patients with atopic asthma and cystic fibrosis. Therefore, any therapeutic strategy that selectively inhibits Th2 T-cell activation may be useful in downregulating allergic lung inflammation in asthma. In the present study, we developed a CpG oligodeoxynucleotide (ODN)-based immune intervention of allergic inflammation in a mouse model of allergic aspergillosis. Four different groups of mice were used in a short-term immunization protocol. Three experimental groups of animals (groups 1 to 3) were sensitized with Aspergillus fumigatus antigens. Animals in group 1 were immunized with A. fumigatus antigen alone, while those in group 2 were treated with CpG-ODN 1 day before the first antigen immunization, and the animals in group 3 received the first CpG-ODN administration between the antigen treatments. The animals in group 4 served as controls and were given phosphate-buffered saline. Allergen-specific serum immunoglobulins and total immunoglobulin E in different groups of animals were measured by enzyme-linked immunosorbent assay, while airway remodeling and cytokine production were studied by immunohistochemistry. The results demonstrated that CpG-ODN administration either before (group 2) or between (group 3) antigen treatments resulted in reduced total immunoglobulin E levels and peripheral blood eosinophil numbers compared to A. fumigatus allergen-sensitized group 1 animals. Similarly, treatment with CpG-ODN also downregulated inflammatory cell infiltration, goblet cell hyperplasia, and basement membrane thickening compared to A. fumigatus-sensitized mice. The distinct reduction in peripheral blood eosinophilia and airway remodeling in CpG-ODN-treated mice emphasized its usefulness as an immunomodulating agent for allergic fungal diseases.
Allergic asthma is characterized by airway eosinophilia, bronchial hyperresponsiveness, and airway remodeling, with an underlying disease mechanism mainly orchestrated by CD4+ Th2 T lymphocytes. The rising incidence of asthma, with more than 130 million people affected in Western countries alone, creates an urgent need for effective treatment and therapeutic strategies (17).
Allergen-specific immunoglobulin E (IgE) plays an important role in eosinophil recruitment during the late-phase reaction. Interleukin-4 (IL-4), the major Th2 cytokine involved in differentiation and activation, is also secreted by several non-T-helper cells, including mast cells and NK cells. The early production of IL-4 from these cellular sources may trigger Th2 cell differentiation, resulting in a predominantly allergic phenotype. In contrast, Th1 cells produce the cytokine gamma interferon (IFN-γ), which downregulates IL-4 and inhibits allergic responses (6). Considerable evidence accumulated in the literature also indicates that eosinophils play a key role in mediating injury to the bronchial mucosa (3).
Recent studies have shown that IL-12 and IFN-γ inhibit allergen-induced Th2 cytokine expression, IgE synthesis, eosinophil recruitment, and airway remodeling in murine models of asthma (20, 21). Among various strategies that have been attempted for immunomodulation of allergic reactions, immunization with nonmethylated CpG oligodeoxynucleotides (ODN) holds promise to induce the innate immune system and produce a variety of Th1-associated cytokines, predominantly IFN-γ and IL-12 from monocytes and NK cells (4). In animal models of asthma induced by ovalbumin, Schistosoma mansoni, and ragweed, CpG-ODN administration reduce the allergen responses with a significant reduction in lung and blood eosinophilia and allergen-specific serum IgE levels (2, 4, 22).
Allergic aspergillosis is caused by exposure to A. fumigatus antigens from the environment and the proteins and toxins from inhaled fungal spores complicate asthma and result in immunologic lung destruction (11, 12). The disease is characterized by high serum total IgE, A. fumigatus-specific IgE, and peripheral blood eosinophilia and central bronchiectasis (3, 8, 11). In children, allergic aspergillosis could be the cause of recurrent pneumonia and may increase the severity of asthma, and in cystic fibrosis patients, fungal colonization may worsen the course of disease and prognosis (3, 18).
The murine models of allergic aspergillosis provide valuable information on the immunopathogenesis of fungal allergy with major involvement of Th2 cytokines, IgE, and eosinophils in the development of the disease (9, 14). Hence, in the search for an alternative immunomodulation strategy, we attempted to develop a CpG-ODN-based immune intervention in a mouse model of allergic aspergillosis.
MATERIALS AND METHODS
Synthesis of ODN.
CpG-ODN GCTAGACGTTAGCGT, containing unmethylated CpG, oligodeoxynucleotides (ODNs), and methylated oligonucleotide without the CpG motif (M-ODN) (5′-TGACTGTGAAGGTTAGAGATCA-3′) were synthesized by TriLink Biotechnologies (San Diego, Calif.). The phosphate backbone of the oligonucleotide was modified to a phosphorothioate group to impart nuclease resistance and hence prevent degradation when used in the in vivo studies. The modified oligonucleotide was purified by high-pressure liquid chromatography (2). These CpG-ODNs had undetectable lipopolysaccharides for their endotoxin levels when tested by the Limulus amebocyte assay (BioWhittaker, Walkersville, Md.).
Induction of allergic airway inflammation.
Six- to 8-week-old female BALB/c mice were purchased from Charles River laboratories (Wilmington, Mass.). Four different groups of animals with five mice in each group were used in this study. The immunization protocol is given in Table 1. Three intraperitoneal injections were given at 3-day intervals with doses of 100 μg of A. fumigatus culture filtrate extract in phosphate-buffered saline (PBS) mixed with 1 mg of alum (Sigma). Following the intraperitoneal injections, the animals in all three groups were given three intranasal challenges with 50 μg of A. fumigatus antigen in PBS. As shown in Table 1, the animals belonging to groups 2 and 3 also received injections of CpG-ODN intraperitoneally or intranazsally (50 μg in PBS/mouse). The control mice in group 4 received only PBS instead of antigen or CpG.
TABLE 1.
Immunization schedule for CpG-mediated immune intervention in mice sensitized with A. fumigatus antigen
| Day and routea | Immunization
|
|||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | |
| −1 (IP) | CpG | |||
| 1 (IP) | Antigen | Antigen | Antigen | PBS |
| 4 (IP) | Antigen | Antigen | Antigen | PBS |
| 7 (IP) | Antigen | Antigen | Antigen | PBS |
| 8 (IP) | CpG | CpG | ||
| 10 (IN) | Antigen | Antigen | Antigen | PBS |
| 11 (IN) | CpG | CpG | ||
| 13 (IN) | Antigen | Antigen | Antigen | PBS |
| 14 (IN) | CpG | CpG | ||
| 17 (IN) | Antigen | Antigen | Antigen | PBS |
| 18 | Sacrificed | Sacrificed | Sacrificed | Sacrificed |
IP, intraperitoneal; IN, intranasal.
Determination of A. fumigatus-specific antibodies in serum and bronchoalveolar lavage fluid samples.
A. fumigatus-specific IgG1, IgG2a, and IgG2b levels in the sera and bronchoalveolar lavage fluids of mice were measured by enzyme-linked immunosorbent assay (9). Bronchoalveolar lavage fluid was collected from each animal by injecting 1 ml of cold Ca2+-, Mg2+-, and pyrogen-free PBS with 0.1% EDTA through the trachea with an 18-gauge catheter into the lung, and the fluid was slowly withdrawn with an attached syringe. The process was repeated one more time, and the total fluid was pooled. The collected lavage samples were centrifuged at 1,200 rpm for 5 min, and the protein concentration in the supernatants was estimated by the bicinchoninic acid method. The supernatants were aliquoted and kept at −20°C for future use. Microtiter plates were coated with A. fumigatus antigens (5 μg/ml) and kept overnight at 4°C. Serum (1:50, vol/vol) and bronchoalveolar lavage fluid (undiluted) were added to the plate and incubated for 3 h at room temperature. The addition of secondary antibody and color development were carried out as described earlier (14).
Estimation of total IgE in serum samples.
The total IgE in the serum samples was measured by enzyme-linked immunosorbent assay with a rat anti-mouse monoclonal antibody as previously reported (10, 14). The serum IgE levels were expressed as nanograms per milliliter of serum with a standard curve.
Lung histology.
The lungs were infused with 1 ml of sterile PBS and fixed in 10% neutral buffered formalin. The tissues were processed and embedded in paraffin, and sections were cut at a thickness of 5 μm and stained with hematoxylin and eosin, periodic acid Schiff base, and Trichrome Masson stains and evaluated microscopically (10). Morphological changes evaluated included perivascular and peribronchial inflammation and infiltration of eosinophils, lymphocytes, macrophages, neutrophils, and plasma cells. Perivascular and peribronchial eosinophil infiltration was graded as none to severe on a 0 to 4 scale as described previously (9, 10, 23).
Eosinophil staining of the blood.
Eosinophils were evaluated in blood samples collected from the heart at the time of sacrifice as described earlier (23).
EPO staining of lung sections.
Lung tissues embedded in tissue freezing medium (Polysciences, Inc.) were cryosectioned at 10 μm and fixed onto poly-L-lysine-coated slides. Lung eosinophil infiltration was studied by microscopic examination after staining for eosinophil peroxidase with DAB (3,3-diaminobenzidine tetrahydrochloride; Sigma). The sections were incubated initially with 10 mM cyanide buffer and rinsed in PBS, followed by incubation with peroxidase substrate and DAB for 10 min at room temperature. The slides were rinsed thoroughly with water and examined under a light microscope. The number of eosinophil peroxidase (EPO)-positive cells in each lung section was counted from five different microscopic fields. The average total cells from each of five mice belonging to groups 1 and 2 were enumerated and compared.
IL-4, IL-5, and IFN-γ staining of the lung sections.
Lung sections were fixed with 4% paraformaldehyde after rinsing with PBS. The slides were then incubated in 0.3% H2O2 in PBS for 15 min. After washing three times with PBS for 5 min each, sections were blocked with PBS containing 5% bovine serum albumin for 3 h. The sections were then incubated with a 1:20 dilution of biotinylated anticytokine antibodies (Pierce Chemical; Rockford, Ill.) in PBS containing 3% bovine serum albumin for 2 h at room temperature. Streptavidin-peroxidase (1:50) was added, and the slides were incubated for 30 min at room temperature. The color was developed with DAB substrate as mentioned above. The number of cytokine-positive cells in each lung section was counted as described above for EPO.
Statistics.
The mean serum antibody levels, total serum IgE, and eosinophil numbers from different groups of mice were compared by one-way analysis of variance. The differences between group 1 and the other groups were compared with Dunnett's post hoc test, and a P of <0.05 was considered statistically significant.
RESULTS
Effects of CpG-ODN administration on A. fumigatus-specific immunoglobulin levels in sera and bronchoalveolar lavage fluids.
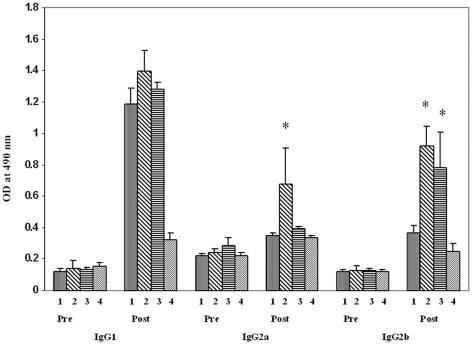
In an attempt to develop an immunomodulatory intervention for Th2 cytokine-mediated hypersensitivity responses, we examined CpG-ODN administration at different time points during A. fumigatus-induced development of allergic lung inflammation (Table 1). The antibody response in the different groups of mice is shown in Fig. 1. A. fumigatus-specific IgG2a antibody levels in serum samples from group 2 mice who had received CpG-ODN before antigen sensitization were higher compared with A. fumigatus-immunized mice in group 1, and the post hoc test revealed that the difference was statistically significant (P = 0.022). The IgG2b values for groups 2 and 3 were significantly higher in comparison with the values for group 1 animals, with P < 0.02. On the other hand, CpG-ODN administration either before (group 2) or between (group 3) allergen administrations failed to show statistically significant differences in A. fumigatus-specific IgG1 levels among groups 1, 2, and 3. All control animals showed only normal levels of IgG antibodies. There was a trend towards an increase in A. fumigatus-specific IgG2a in bronchoalveolar lavage fluids of group 2 animals (data not shown) following two intranasal CpG-ODN instillations between the allergen challenges, although the difference was not statistically significant.
FIG. 1.
Effect of CpG-ODN on A. fumigatus-specific IgG1, IgG 2a, and IgG2b in the sera of different groups of animals. Bars represent mean optical density values (mean + standard error) from five mice in each group studied by enzyme-linked immunosorbent assay. The immunoglobulin levels in pre- and postimmunization serum samples are presented for group 1, group 2, group 3, and group 4 from left to right within each grouping. The asterisk (*) indicates a statistically significant difference in immunoglobulin levels between A. fumigatus-sensitized group 1 and CpG-treated group 2 and group 3 mice (P < 0.05).
CpG-ODN downregulates total IgE in the sera of CpG-treated mice.
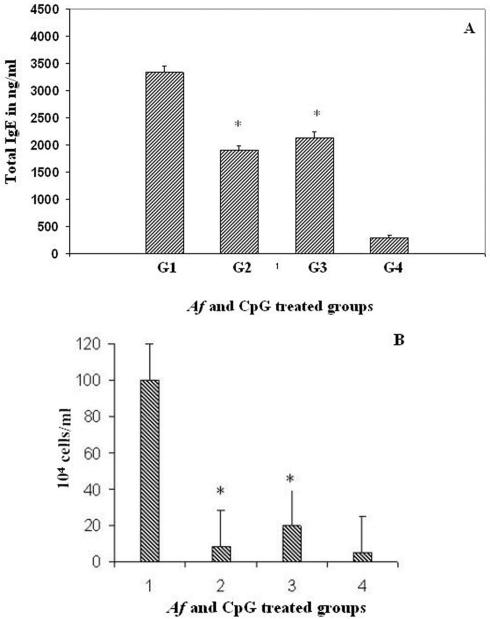
There was a 7- to 12-fold increase in total IgE levels in sera from A. fumigatus-sensitized (group 1) and CpG-ODN-treated (groups 2 and 3) animals compared to PBS-treated control group 4 (Fig. 2A). However, CpG-treated groups 2 and 3 exhibited a significant reduction in total IgE in sera compared to A. fumigatus-sensitized group 1, and post hoc analysis showed that the differences were statistically significant at P < 0.05 for both groups compared to group 1.
FIG. 2.
(A) Total serum IgE in A. fumigatus-sensitized and CpG-ODN-treated mice. Bars represent mean optical values (mean + standard error). The asterisk (*) indicates a statistically significant difference in total IgE levels among A. fumigatus-sensitized group 1 and CpG-treated group 2 and group 3 mice (P < 0.05). (B) Eosinophils in the peripheral blood of A. fumigatus-sensitized and CpG-ODN-treated mice. The bars represent mean cell counts (mean + standard error) in samples from five mice in each group. The asterisk (*) represents a statistically significant difference between A. fumigatus-sensitized group 1 and CpG-treated group 2 and group 3 (P < 0.001).
Effect of CpG-ODN on peripheral blood eosinophil numbers.
Peripheral blood eosinophil counts among all groups were significantly different (P < 0.001), with blood eosinophil counts for group 1 to be significantly higher than group 2, group 3, and group 4, with mean differences of 92 ± 12, 80 ± 12, and 98 ± 14, respectively (Fig. 2B).
CpG-ODN downregulate the expression of IL-4 and IL-5 in airways of A. fumigatus-sensitized and -challenged mice.
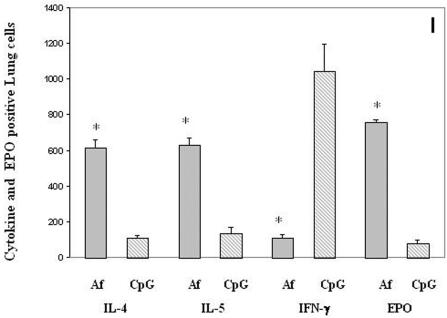
To investigate the potential immunomodulatory mechanism responsible for the CpG-ODN-induced Th1 responses over the existing Th2 responses in A. fumigatus-sensitized mice, we examined the expression of IFN-γ-, IL-4-, and IL-5-secreting cells in lung sections from A. fumigatus-sensitized (group 1) and CpG-ODN-treated (group 2) animals (Fig. 3). Immunostaining of the lung sections with anticytokine antibodies revealed a significant reduction in IL-4- and IL-5-producing cells (>90%) in CpG-ODN-treated animals (Fig. 3A and C) compared with an A. fumigatus-sensitized animals (Fig. 3B and D). A predominantly Th1 type immune response in vaccinated animals was also evidenced by an increase in the number of IFN-γ-producing cells (Fig. 3E) compared with A. fumigatus-treated animals in group 1 (Fig. 3F). Cells-expressing IL-4, IL-5, and IFN-γ were enumerated and compared among the different groups and are shown in Fig. 3I. There was a marked reduction of cells-expressing IL-4 and IL-5 but an increase in IFN-γ-secreting cells in the CpG-treated group compared to the group treated with A. fumigatus alone. In all these cases, P was less than 0.001.
FIG. 3.
Immunomodulatory effects of CpG-ODN treatment in lung sections of A. fumigatus-sensitized and CpG-ODN-treated mice (magnifications, ×60). (A) IL-4-expressing cells in the lung section of a CpG-treated (group 2) mouse. (B) IL-4-expressing cells in the lung section of an A. fumigatus-sensitized mouse with no CpG treatment (group 1). The arrow indicates the cytokine-expressing cells. (C) IL-5-expressing cells in the lung section of a group 2 animal. (D) IL-5-expressing cells in the lung section of a group 1 animal. The arrow indicates IL-5-expressing cells. (E) IFN-γ-expressing cells on the lung section of a group 2 animal. The arrow indicates IFN-γ-producing cells. (F) IFN-γ staining of the lung section from a group 1 mouse. (G) Eosinophil peroxidase staining of the lung section from a CpG-treated group 2 animal. (H) Eosinophil peroxidase staining of the lung section from a group 1 animal. (I) The number of IL-4-, IL-5-, IFN-γ-, and EPO-positive cells in lung sections enumerated quantitatively. Results are represented as mean + standard error (for details, see Materials and Methods). The asterisk (*) represents a statistically significant difference in cytokine- and EPO-positive cells in lung sections from A. fumigatus-sensitized and CpG-treated mice (P < 0.001).
CpG-ODN inhibit A. fumigatus-induced airway and lung eosinophilia.
Examination of eosinophil infiltration in the lungs by eosinophil peroxidase staining of lung sections indicates a significant increase in eosinophil numbers in A. fumigatus-sensitized and A. fumigatus-challenged mice (group 1) compared with DNA-treated mice (Fig. 3G). Even with the high doses of the A. fumigatus sensitization protocol (100 μg/immunization) used in this study, CpG-ODN significantly inhibited eosinophil recruitment in the lung and peripheral blood of treated groups (Fig. 3H). EPO-positive cells in the lung showed significant reductions (P < 0.001) in their numbers in CpG-treated mice compared to mice treated with Aspergillus organisms alone (Fig. 3I).
CpG-ODN restricts inflammatory cell infiltration in airways.
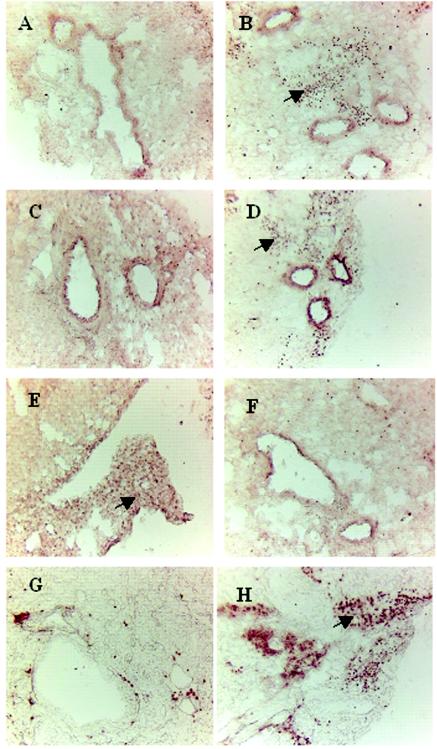
Histopathological analysis of the airways of mice sensitized and challenged with A. fumigatus demonstrated chronic interstitial infiltration with lymphocytes, a small number of plasma cells, epithelioid histiocytes, neutrophils,and a large number of eosinophils (Fig. 4B). The bronchioles were surrounded by chronic inflammatory infiltrates and were frequently lined with a hyperplastic epithelium. Under higher magnification, the majority of the infiltrating cells were identified as eosinophils (a grade of 4), and a severe bronchial epithelial hyperplasia was noticed throughout the lung section (Fig. 4D). Although there was cellular infiltration in the lung section from CpG-treated mice, we noticed a marked reduction in eosinophils from grade 4 to grade 1 around the small bronchi and blood vessels and also less damage to the bronchial epithelial lining (Fig. 4A and C).
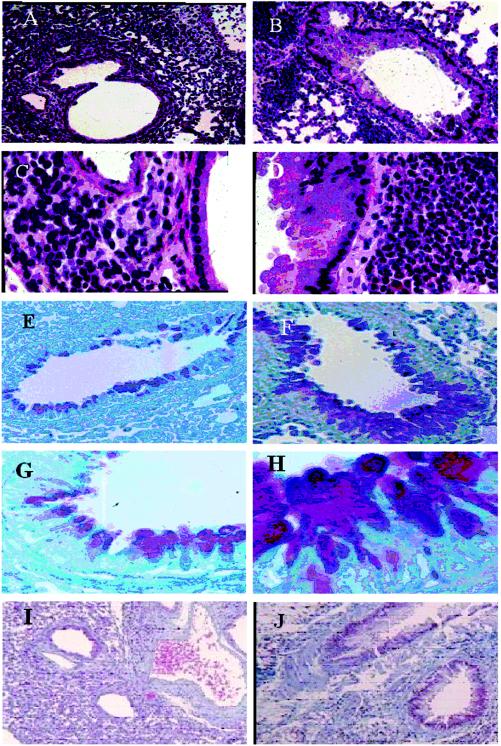
FIG. 4.
Lungs from A. fumigatus-sensitized (group 1) and CpG-ODN -treated (group 2) mice were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin, periodic acid-Schiff base, and Trichrome. (A) Hematoxylin and eosin staining of group 2 lung sections, showing moderate inflammatory infiltrates adjacent to bronchioles and small blood vessels without eosinophil infiltrations (magnifications, ×60), (B) Hematoxylin and eosin staining of a lung section from a group 1 animal showing extensive peribronchaeolar and perivascular inflammation. The bronchial epithelium is hyperplastic (×60). (C) Higher magnification of A (×400). (D) Higher magnification of B (×400), showing the large number of eosinophils and distinct epithelial hyperplasia. (E) Periodic acid-Schiff staining of the lung section from a group 2 mouse, showing very few goblet cells around bronchial mucosa. (F) Periodic acid-Schiff staining of the lung section from a group 1 mouse, showing the large number of mucous glycoconjugate-producing goblet cells in bronchial mucosa. (G and H) Higher magnifications (×400) of E and F, respectively. (I) Trichrome staining of a section from a group 2 mouse exhibiting less peribronchial collagen deposition (×60). (J) There was significantly more collagen deposition around the airways of group 1 compared to CpG-ODN-treated animals.
CpG-ODN downregulates mucus glycoprotein production and basement membrane thickening in A. fumigatus-sensitized mice.
Airway remodeling in chronic asthma is also characterized by significant mucopolysaccharide secretion and airway goblet cell hyperplasia. CpG-ODN administration, 24 h before the A. fumigatus sensitization and challenge (group 2) exhibited a significant reduction in the secretion of mucous glycoprotein (Fig. 4E, G) compared to A. fumigatus-sensitized mice (Fig. 4F, H). The thickness of the subepithelial collagen layer was evaluated in A. fumigatus immunized and CpG-ODN-vaccinated mice with Trichrome Masson stain. Immunomodulation in CpG-ODN-vaccinated mice was evident by a decrease in submucosal collagen deposition in airways (Fig. 4I) compared to that in the airways from A. fumigatus-immunized mice (Fig. 4J).
DISCUSSION
Allergic aspergillosis is caused by exposure to A. fumigatus spores from the environment and the immunologic lung destruction is largely due to fungal colonization of the respiratory tree and the result of immunologic inflammatory response of the host to the fungal antigens (11). Inflammatory responses of the airways are characterized by cellular infiltration of eosinophils and T lymphocytes expressing Th2 cytokines as well as extensive airway remodeling (9, 14, 15). Recent studies have shown that bacterial DNA carrying non methylated CpG motifs can be used as vaccine adjuvant to selectively induce Th1-dominated immune responses and therefore might be useful as an immunomodulatory agents to treat or prevent undesired Th2-dominant immune responses, such as in allergic disorders (5, 7).
This short-term immunization protocol was designed to investigate various parameters such as the effect of allergen exposure on overall immune response in mice under the cytokine milieu of Th1 type (group 2). The immunization protocol applied to group 3 was mainly to evaluate whether an ongoing Th2 response (as seen in allergen-sensitized patients) could be converted to a Th1 response with CpG intervention.
Earlier reports have shown that the effect of CpG-ODN on reducing the number of tissue eosinophils is immediate and sustained for about a week (2). Both groups 2 and 3 demonstrated significant reduction in total IgE in serum and peripheral blood eosinophils, indicating a deviation of the response towards a Th1 type. However, this response was pronounced in group 2 mice receiving systemic CpG-ODN 1 day before the beginning of allergen sensitization. Similarly, in the present study, a twofold increase in A. fumigatus-specific IgG2a and IgG2b as well as significant reduction in IgE levels in the serum and bronchoalveolar lavage fluid samples of CpG-ODN-treated mice compared with A. fumigatus-sensitized animals suggest an antigen-specific Th1 cell recruitment. However, A. fumigatus-sensitized group 1 and CpG-ODN-treated groups 2 and 3 exhibited comparable allergen-specific IgG1. This finding is in line with our previous results, where anti-IL-4-treated and IL-4 knockout BALB/c mice produced specific IgG1 antibody when exposed to Aspergillus antigen, indicating that IgG1 is not predominantly regulated by IL-4 (13, 15).
Experimental evidence has shown that CpG-induced immunomodulation begins with the activation of the innate immune response that could initially prevent the development of asthmatic symptoms by inhibiting early eosinophil accumulation and later even bias the ongoing immune response to produce an antigen-specific Th1 microenvironment in the host encountering subsequent antigen exposures (19, 22). In a murine model of invasive pulmonary aspergillosis, local delivery of CpG-ODN and a recombinant A. fumigatus allergen resulted in functional maturation and activation of airway dendritic cells towards a Th1 immune response and resistance to the fungus (1). Another recent study demonstrated that when mice primed with CpG-ODN-treated dendritic cells, a marked depletion of CD4+ CD25+ Treg cells resulting in enhancement of IFN-γ producing cells and downregulation of IL-4 production in lymph node cells (16). Indeed, in the present mouse model of allergic aspergillosis, CpG administration resulted in a significant reduction in eosinophil recruitment in the lung, indicating a persistent Th1 response.
In order to investigate the effect of intranasal CpG administration in airways, we carried out immunostaining for IL-4-, IL-5-, and IFN-γ-secreting cells in the A. fumigatus-sensitized (group 1) and CpG-vaccinated (group 2) animals. CpG-vaccinated animals had a significant reduction in IL-4-secreting cells in the airways compared to airways from A. fumigatus-sensitized mice. The presence of IFN-γ-producing cells in the CpG-treated airways may be instrumental in augmenting a predominant Th1 response associated with the corresponding reduction of IL-4-producing cells and a reduced Th2 response (6). The ability of CpG to reduce IL-5 cytokine secretion in the lung compartment can be correlated with a marked reduction in airway eosinophilic infiltration in CpG-treated mice. Taken together, the overall immune responses in CpG-ODN-treated mice in this study show results comparable to those of other studies, where administration of anti-IL-4, anti-IL-5, IFN-γ, and IL-12 inhibited allergic lung inflammation in mouse models of asthma (19). In this context, the use of CpG-ODN for the treatment of respiratory complication due to fungal allergens may be more advantageous than administration of exogenous cytokines because endogenously produced cytokines by CpG-ODN are likely to follow a regulatory mechanism and thereby may cause less toxicity than exogenously administered cytokines.
In allergic aspergillosis, the inhaled fungal spores are trapped in the tenacious mucus in patients' large segmental bronchi, germinate, and form hyphae, and the immune reactions and proteolytic enzymes generated by A. fumigatus cause pulmonary infiltrates, tissue damage, and eventually central bronchiectasis (3, 12). In this study, the A. fumigatus-sensitized animals in group 1 showed similar airway remodeling with peribronchial and perivascular infiltration and accumulation of a large number of eosinophils, goblet cell hyperplasia, and basement membrane thickening. A significant reduction in airway eosinophilia, mucus secretion, and collagen deposition in the airways of CpG-treated mice strongly suggest a possible immune intervention by DNA vaccination in combating the airway remodeling in patients with complication of allergic aspergillosis.
In conclusion, we report CpG-ODN-mediated immune intervention via the systemic and mucosal routes in a murine model of allergic aspergillosis. A significant increase in A. fumigatus-specific Th1 responses and distinct reduction in pulmonary eosinophilia and airway remodeling in animals receiving CpG-ODN prophylactically compared with the A. fumigatus-sensitized mice emphasize the usefulness of DNA-mediated intervention in allergic diseases. Hence, CpG-ODN may be explored as an immunomodulating agent for fungus-induced inflammation in patients with allergic aspergillosis.
Acknowledgments
This investigation was supported by U.S. Veterans Affairs Medical Research and by a grant from Children's Hospital of Wisconsin.
We gratefully acknowledge the technical assistance of Laura Castillo and Nancy Elms.
Editor: T. R. Kozel
REFERENCES
- 1.Bozza, S., R. Gaziano, G. B. Lipford, C. Montagnoli, A. Bacci, P. Di Francesco, V. P. Kurup, H. Wagner, and L. Romani. 2002. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect. 4:1281-1290. [DOI] [PubMed] [Google Scholar]
- 2.Broide, D., J. Schwarze, H. Tighe, T. Gifford, M. D. Nguyen, S. Malek, J. Van Uden, E. Martin-Orozco, and E. W. Gelfand. 1999. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J. Immunol. 161:7054-7062. [PubMed] [Google Scholar]
- 3.Greenberger, P. A. 2003. Allergic bronchopulmonary aspergillosis, p. 1353-1371. In N. F. Adkinson, Jr., J. W. Yunginger, W. W. Busse, B. S. Bochner, S. T. Holgate, and F. E. R. Simons (ed.), Middleton's allergy principles and practice. Mosby, Philadelphia, Pa.
- 4.Kline, J. N., T. J. Waldschmidt, T. R. Businga, J. E. Lemish, J. V. Weinstock, P. S. Thorne, and A. M. Krieg. 1998. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J. Immunol. 160:2555-2559. [PubMed] [Google Scholar]
- 5.Krieg, A. M. 2002. From A to Z on CpG. Trends Immunol. 23:64-65. [DOI] [PubMed] [Google Scholar]
- 6.Krieg, A. M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 7.Krieg, A. M., G. Hartmann, and A. K. Yi. 2000. Mechanism of action of CpG DNA. Curr. Top. Microbiol. Immunol. 247:1-21. [DOI] [PubMed] [Google Scholar]
- 8.Kurup, V. P., B. Banerjee, S. Hemmann, P. A. Greenberger, K. Blaser, and R. Crameri. 2000. Selected recombinant Aspergillus fumigatus allergens bind specifically to IgE in ABPA. Clin. Exp. Allergy 30:988-993. [DOI] [PubMed] [Google Scholar]
- 9.Kurup, V. P., H. Choi, P. S. Murali, and R. L. Coffman. 1994. IgE and eosinophil regulation in a murine model of allergic aspergillosis. J. Leukoc. Biol. 56:593-598. [DOI] [PubMed] [Google Scholar]
- 10.Kurup, V. P., J. Guo, P. S. Murali, H. Y. Choi, and J. N. Fink. 1997. Immunopathologic responses to Aspergillus antigen in interleukin-4 knockout mice. J. Lab. Clin. Med. 130:567-575. [DOI] [PubMed] [Google Scholar]
- 11.Kurup, V. P., and A. Kumar. 1991. Immunodiagnosis of aspergillosis. Clin. Microbiol. Rev. 4:439-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurup, V. P., A. Kumar, W. R. Kenealy, and P. A. Greenberger. 1994. Aspergillus ribotoxins react with IgE and IgG antibodies of patients with allergic bronchopulmonary aspergillosis. J. Lab. Clin. Med. 123:749-756. [PubMed] [Google Scholar]
- 13.Kurup, V. P., P. S. Murali, J. Guo, H. Choi, B. Banerjee, J. N. Fink, and R. L. Coffman. 1997. Anti-interleukin (IL)-4 and -IL-5 antibodies downregulate IgE and eosinophilia in mice exposed to Aspergillus antigens. Allergy 52:1215-1221. [DOI] [PubMed] [Google Scholar]
- 14.Kurup, V. P., J. Q. Xia, R. Crameri, D. A. Rickaby, H. Y. Choi, S. Fluckiger, K. Blaser, C. A. Dawson, and K. J. Kelly. 2001. Purified recombinant A. fumigatus allergens induce different responses in mice. Clin. Immunol. 98:327-336. [DOI] [PubMed] [Google Scholar]
- 15.Kurup, V. P., J. Q. Xia, D. A. Rickaby, C. A. Dawson, H. Choi, and J. N. Fink. 1999. Aspergillus fumigatus antigen exposure results in pulmonary airway resistance in wild-type but not in IL-4 knockout mice. Clin. Immunol. 90:404-410. [DOI] [PubMed] [Google Scholar]
- 16.Oldenhove, G., M. de Heusch, G. Urbain-Vansanten, J. Ubin, C. Maliszewski, O. Leo, and M. Moser. 2003. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J. Exp. Med. 198:259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sears, M. R. 1997. Descriptive epidemiology of asthma. Lancet ii:SII1-SII4. [DOI] [PubMed] [Google Scholar]
- 18.Skov, M., T. Pressler, H. E. Jensen, N. Hoiby, and C. Koch. 1999. Specific IgG subclass antibody pattern to Aspergillus fumigatus in patients with cystic fibrosis with allergic bronchopulmonary aspergillosis (ABPA). Thorax 54:44-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiegelberg, H. L., and E. Raz. 2002. DNA-based approaches to the treatment of allergies. Curr. Opin. Mol. Ther. 4:64-71. [PubMed] [Google Scholar]
- 20.Sur, S., P. Bouchard, D. Holbert, and M. R. Van Scott. 2000. Mucosal IL-12 inhibits airway reactivity to methacholine and respiratory failure in murine asthma. Exp. Lung Res. 26:477-489. [DOI] [PubMed] [Google Scholar]
- 21.Sur, S., J. Lam, P. Bouchard, A. Sigounas, D. Holbert, and W. J. Metzger. 1996. Immunomodulatory effects of IL-12 on allergic lung inflammation depend on timing of doses. J. Immunol. 157:4173-4180. [PubMed] [Google Scholar]
- 22.Sur, S., J. S. Wild, B. K. Choudhury, N. Sur, R. Alam, and D. M. Klinman. 1999. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J. Immunol. 162:6284-6293. [PubMed] [Google Scholar]
- 23.Xia, J. Q., D. A. Rickaby, K. J. Kelly, H. Choi, C. A. Dawson, and V. P. Kurup. 1999. Immune response and airway reactivity in wild and IL-4 knockout mice exposed to latex allergens. Int. Arch. Allergy Immunol. 118:23-29. [DOI] [PubMed] [Google Scholar]