Introduction
Radioimmunotherapy (RIT) has provided a valuable tool in the treatment of patients with indolent B cell non-Hodgkin lymphoma (NHL). The two RIT agents that are currently Food and Drug Administration (FDA)-approved in NHL, 131I-tositumomab and 90Y-ibritumomab tiuxetan, target the CD20 antigen, which is present on the surface of most normal and malignant B cells, thereby delivering a radiation dose directly to the tumor cells. 131I-tositumomab shows response rates in relapsed/refractory indolent NHL of approximately 65–70%, with approximately half being complete responses (CRs) [1–3]. While response rates are high in indolent B-NHL, duration of response is limited in most patients, lasting 1–2 years. Experience with aggressive lymphomas is more limited than that with indolent lymphomas, and results have been mixed. In a phase 2 study involving multiple histologies, none of the patients with diffuse large B-cell lymphoma (DLBCL) achieved a complete response [1]. A subsequent study in second-line treatment of patients with DLBCL not eligible for transplant showed that about half of patients responded, but those who had received prior rituximab had a much lower response rate (19%) [4]. A study of 90Y-ibritumomab tiuxetan in mantle cell lymphoma (MCL) [5] showed a response rate of 67%, but event-free survival (EFS) was 6 months.
One mechanism by which lymphoma cells could show resistance to RIT is a lack of target antigen. CD20, however is nearly universally expressed on newly diagnosed B cell lymphomas, and is not commonly lost with disease progression [6,7]. Therefore, resistance to radiation may play a more relevant role in lack of responsiveness to RIT than inadequate expression of the target antigen.
Bortezomib, through inhibition of the chymotrypsin- like activity of the 26S proteasome in mammalian cells [8], disrupts normal regulation of critical cellular path- ways including cellular protective responses to radiation. Bortezomib is active as a single agent in MCL, inducing responses in approximately one-third of relapsed patients [9], while reports of activity in other NHL subtypes are mixed [10–12]. Since bortezomib has only modest hematologic toxicity, consisting mainly of short-lived thrombocytopenia, combination of this drug with RIT, for which hematologic toxicity is the major adverse effect, should show limited overlapping toxicity. We hypothesized that administration of bortezomib during the time of maximal radiation exposure with 131I-tositumomab would optimize responsiveness of lymphoma to RIT and therefore improve therapeutic outcomes in patients with NHL, including those with aggressive subtypes.
Materials and methods
Patients
Patients with relapsed and refractory B-cell NHL were eligible for the study. Key inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status 0–2, adequate organ function, including absolute neutrophil count (ANC) > 1500/μL and platelet count > 150 X 109/L, creatinine clearance > 20 mL/min, bilirubin < 1.5 Xupper limit of normal (ULN) and no symptomatic heart disease. Patients were excluded from study therapy if they had bone marrow involvement by lymphoma of greater than 25%, prior stem cell transplant, grade > 2 peripheral neuropathy, human immunodeficiency virus (HIV) or active brain metastases. Patients who had received prior RIT were excluded, but prior bortezomib was permitted. All patients provided written informed consent. The study was reviewed and approved by the Institutional Review Boards of Weill Cornell Medical College and the University of Michigan and was conducted in accordance with the Declaration of Helsinki. The study was registered with ClinicalTrials.gov as NCT00777114.
Treatment
This study was a phase 1 dose-escalation trial designed to evaluate the maximum tolerated dose (MTD) of bortezomib in combination with 131I-tositumomab. Secondary objectives included assessment of response rates and progression-free survival (PFS). The schedule of bortezomib treatment was planned to optimize proteasome inhibition during the time of maximal radiation exposure [1], and therefore patients received one dose of bortezomib prior to the therapeutic dose of 131I-tositumomab, and then four additional twice- weekly doses through day 12 (approximately five times the mean effective half-life of 131I-tositumomab).
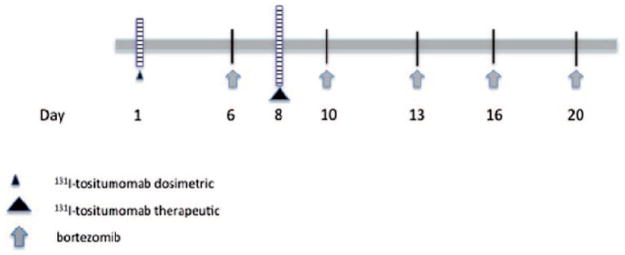
A dosimetric dose of 131I-tositumomab was administered on day 1, followed by three whole-body gamma camera scans for purposes of dose calculation and evaluation of biodistribution. A therapeutic dose was delivered on day 8. After an initial cohort received a reduced whole-body dose of 50 cGy 131I-tositumomab to mitigate for the possible risk of unexpected hematologic toxicity, all subsequent cohorts received RIT at the standard dose of 75 cGy. Patients were treated with escalating doses of bortezomib (0.3 to 1.2 mg/ m2) on days 6, 10, 13, 16 and 20 (schema shown in Figure 1). Dose levels are shown in Table I.
Figure 1.
Treatment schema. The dosimetric dose of 131I-tositumomab was 5 mCi in all patients. Therapeutic dosing of 131I-tositumomab was as per the dose escalation scheme.
Table I.
Dose levels.
| Dose level | 131I-tositumomab | Bortezomib | Number treated | DLT |
|---|---|---|---|---|
| 1 | 50 cGy | 0.3 mg/m2 | 2 | 0 |
| 2 | 75 cGy | 0.3 mg/m2 | 2 | 0 |
| 3 | 75 cGy | 0.6 mg/m2 | 2 | 0 |
| 4 | 75 cGy | 0.9 mg/m2 | 8 | 0 |
| 5 | 75 cGy | 1.2 mg/m2 | 11 | 4 |
DLT, dose limiting toxicity.
Patients were followed with weekly complete blood counts for 12 weeks, or until recovery from neutrophil and platelet nadir. After one patient experienced grade 3 disseminated herpes zoster, the protocol was amended to mandate prophylaxis for varicella zoster.
Statistical analysis
The primary endpoint was identification of the MTD of bortezomib in combination with 131I-tositumomab. Dose escalation was undertaken via the Time to Event-Continuous Reassessment Model (TiTE-CRM) [13,14]. The TiTE-CRM method assumes a simple model for the probability of a dose limiting toxicity (DLT) as a function of the combination, and uses the occurrence of toxicities in the patients previously enrolled in the trial to subsequently determine which dose to allocate to the next patient. New subjects are continuously enrolled without pausing for complete follow-up of current subjects. DLT was defined as any grade 3 or 4 non- hematologic toxicity or any grade 4 hematologic toxicity as defined by the Common Terminology Criteria for Adverse Events (CTCAE) version 3.
Secondary endpoints included response rate and PFS. Response rate was defined using the Revised Response Criteria for Malignant Lymphoma [15]. An exact 95% confidence interval (CI) was calculated to assess the precision of the observed response rate. PFS was calculated by the Kaplan-Meier method and defined as time from initiation of treatment to documentation of disease progression or death from any cause. Greenwood’s formula was used to calculate 95% Cis for the Kaplan Meier estimates. All analyses were performed in Stata Version 12.0 (StataCorp, College Station, TX).
Results
Thirty-one patients were enrolled in the study between April 2007 and June 2012 (Table II). Six patients did not receive treatment due to ineligibility (three patients), lack of availability of drug (one patient), early progression (one patient) or abnormal biodistribution of 131I-tositumomab (one patient). Of the 25 patients who did receive study treatment, the median age was 68 (range 40–81). Eleven patients (44%) had follicular lymphoma (FL), eight (32%) had DLBCL, five (20%) had MCL and one had marginal zone lymphoma (MZL). The median number of prior therapies was 2 (range 1–4), and all but one patient had received prior rituximab.
Table II.
Patient demographics.
| Age, median (range) | 68 (40–81) |
| Gender | n (%) |
| Male | 18 (72) |
| Female | 7 (28) |
| Histology | |
| FL | 11 (44) |
| DLBCL | 8 (32) |
| MCL | 5 (20) |
| MZL | 1 (4) |
| Prior therapies | |
| <3 | 17 (68) |
| ≥3 | 8 (32) |
FL, follicular lymphoma; DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma.
Of the 25 treated patients, all but one patient completed study treatment. In the patient who did not complete treatment, the last bortezomib dose was held due to grade 3 hyponatremia. The therapeutic dose of 131I-tositumomab was delayed for 1 week in two patients due to fevers and chills experienced on the day the dose was due.
Twenty-four of the 25 patients were evaluable for DLT. One patient was not evaluable due to early disease progression; that patient proceeded to alternative therapy 4 weeks after initiation of study treatment. Two patients each were treated at dose levels 1, 2 and 3, and none experienced DLT. Eight patients were treated at dose level 4, again with no patients experiencing DLT. At dose level 5, DLT was observed in four of 11 patients. Two of these patients experienced grade 4 thrombocytopenia, one had grade 3 hyponatremia and one patient developed grade 3 herpes zoster. The MTD of the combination was determined to be dose level 4: bortezomib 0.9 mg/m2 in combination with 131I-tositumomab at 75 cGy. Treatment was generally well tolerated. Hematologic toxicity was common, mild to moderate in severity and reversible. Grade 3 and 4 hematologic toxicities included neutropenia in five patients (20%), anemia in one patient (4%) and thrombocytopenia in eight patients (33%) (Table III)
Table III.
Toxicity: grades 3 and 4.
| Toxicity | n (%) |
|---|---|
| Neutropenia | 5 (20) |
| Thrombocytopenia | 8 (33) |
| Anemia | 1 (4) |
| Hyponatremia | 1 (4) |
| Herpes zoster | 1 (4) |
All patients experiencing hematologic toxicities recovered blood counts to baseline during the time of follow-up. The most common grades 1 and 2 non-hematologic toxicities included fatigue and chills (Table IV).
Table IV.
Non-hematologic toxicity present in ≥5 patients.
| Toxicity | Grade 1–2 (%) |
|---|---|
| Fatigue | 6 (24) |
| Chills | 8 (32) |
| Dizziness | 5 (20) |
| Anorexia | 5 (20) |
| Fever | 8 (32) |
| Hypocalcemia | 9 (36) |
| Hyponatremia | 7 (28) |
| Hypoglycemia | 5 (20) |
Two patients developed new grade 1 peripheral neuropathy while on treatment. One patient developed human anti-mouse antibody (HAMA) 12 weeks after receiving 131I-tositumomab. No patient developed hypothyroidism during the follow-up period.
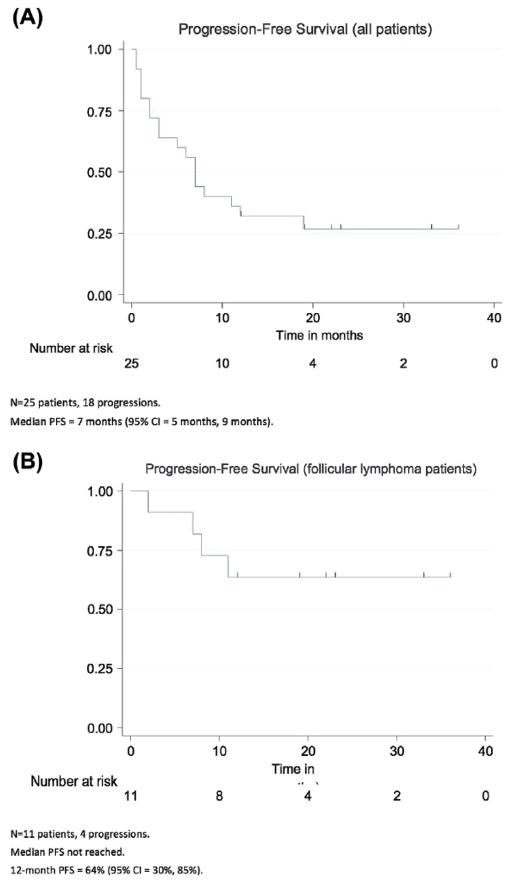
Sixteen of the 25 patients (64%; 95% CI = 43–82%) achieved an objective response to the study treatment, including 10 of 11 (91%) patients with FL, three of eight (36%) patients with DLBCL and three of five (60%) patients with MCL (Table V). CRs were observed in nine of 11 patients with FL, and one each with DLBCL and MCL. Nine patients did not respond to study treatment. At a median follow-up of 7 months (range 1–36 months), the median PFS for all patients was 7 months (95% CI = 5–9 months) [Figure 2(A)]. Median PFS for patients with FL was not reached; the 12-month PFS for patients with FL was 64% (95% CI = 30–85%) [Figure 2(B)]. Seven of 11 patients with FL remained in remission at a median follow-up of 22 months (range 12–36 months) [Figure 2(B)].
Table V.
Responses by histology.
| Histology | n | ORR (%) | CR (%) | PR (%) | SD/PD (%) |
|---|---|---|---|---|---|
| DLBCL | 8 | 3 (36) | 1 (12) | 2 (25) | 5 (63) |
| MCL | 5 | 3 (60) | 1 (20) | 2 (40) | 2 (40) |
| FL | 11 | 10 (91) | 9 (82) | 1 (9) | 1 (9) |
| MZL | 1 | 0 | 0 | 0 | 1 (100) |
| Total | 25 | 16 (64) | 11 (44) | 5 (20) | 9 (36) |
DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma; FL, follicular lymphoma; MZL, marginal zone lymphoma; ORR, overall response rate; CR, complete response; PR, partial response; SD/PD, stable disease/progressive disease.
Fig 2.
Progression-free survival in (A) all patients with follicular lymphoma.
Discussion
Although RIT is effective in relapsed indolent lymphomas, the fact that most responses are of limited duration combined with the suboptimal activity in aggressive NHL sub- types suggests that improvements can be made. Our strategy of radiosensitization using bortezomib is based on preclinical studies demonstrating improved responsiveness to radiation with exposure to bortezomib [16]. Bortezomib appears to exert this effect through suppression of nuclear factor-κB (NF-κB) activation, and by inhibiting up-regulation of anti- apoptotic proteins in response to radiation damage [17].
The present phase 1 study was designed to focus bortezomib dosing during the time of greatest radiation exposure to the tumor cells in order to maximize synergy. To achieve this, one dose of bortezomib was given prior to the treatment dose of 131I-tositumomab, and bortezomib dosing was continued for four additional doses during the 2 weeks after RIT, when tumor exposure to radiation is expected to be highest [1]. With this regimen, the combination of bortezomib with 131I-tositumomab was tolerable, with the MTD of bortezomib given in combination with standard dose 131I-tositumomab identified to be 0.9 mg/m2.
No unexpected toxicities were observed, with hematologic toxicity being most prominent. The rates of grade 3 and grade 4 hematologic toxicity were comparable to those observed with 131I-tositumomab alone [1, 2]. The observation of disseminated zoster is consistent with the baseline immunosuppression of pretreated patients with lymphoma, and is a known complication of bortezomib [18]; therefore, all patients treated with this regimen should undergo viral prophylaxis. Other toxicities were mostly mild and reversible. Peripheral neuropathy was rare and mild. Hypothyroidism, a known risk following 131I-tositumomab, was not observed in this study. This finding might be related to limited patients numbers and/or relatively short follow up.
The combination of bortezomib and 131I-tositumomab showed notable activity in this preliminary study, particularly in FL. Although numbers were small, the response rate in FL of 91% with 82% CR, is higher than expected with RIT, response rates in MCL and DLBCL were lower than in FL, but still notable. All the patients with aggressive lymphoma had received prior rituximab, which has previously been demonstrated to be associated with markedly inferior responses [4].
Two other trials have been published exploring the combination of bortezomib in combination with RIT, both using 90Y-ibritumomab tiuxetan administered on the day of the third dose. Patients subsequently received consolidation with bortezomib after count recovery. Both studies found the combination to be tolerable, with hematologic toxicity common. Both showed activity, although response rates were lower than those reported here. Beaven et al. showed an overall response rate of 50% in patients with mixed NHL histologies. In the study by Roy et al., 89% of a group with mostly FL responded, but with only a 22% CR rate. While the difference in activity could be due to small numbers enrolled in these phase 1 studies, another potential explanation is the difference in dosing schedule. The present study was designed to maximize exposure to the tumor. Both previous studies had relatively limited overlapping exposure, which may have moderated the synergy of the two drugs. Another potential explanation for differences in activity could be the difference in RIT agent. Although no differences in the clinical activity between 131I-tositumomab and 90Y-ibritumomab tiuxetan, it is conceivable that synergistic interactions between the two RIT agents and bortezomib could differ, possibly through mechanistic differences between the type II tositumomab and the type I ibritumomab [21].
In conclusion, the combination of bortezomib and 131I-tositumomab is tolerable in both indolent and aggressive relapased/refractory B-cell NHL. Although limited by the small cohort number in this phase 1 study, the response rates noted in this mixed population suggest improved activity over RIT alone, particularly with regard to response rats in FL. This combination merits study in trials designed to further explore its activity.
Acknowledgments
R.L.E. was supported in part by the Charles, Lillian and Betty Neuwirth Clinical Scholar Award. P.J.C. and M.M. were partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1-TR000457-06). J.O. was partially supported by a grant from the Center to Reduce Cancer Health Disparity (R21 CA153177-03).
References
- 1.Kaminski MS, Estes J, Zasadny KR, et al. Radioimmunotherapy with iodine (131) I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood. 2000;96:1259–1266. [PubMed] [Google Scholar]
- 2.Kaminski MS, Zelenetz AD, Press OW, et al. Pivotal study of Iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomas. J Clin Oncol. 2001;19:3918–3928. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 3.Vose JM, Wahl RL, Saleh M, et al. Multicenter phase II study of iodine-131 tositumomab for chemotherapy-relapsed/refractory low- grade and transformed low-grade B-cell non-Hodgkin’s lymphomas. J Clin Oncol. 2000;18(6):1316–1323. doi: 10.1200/JCO.2000.18.6.1316. [DOI] [PubMed] [Google Scholar]
- 4.Morschhauser F, Illidge T, Huglo D, et al. Efficacy and safety of yttrium-90 ibritumomab tiuxetan in patients with relapsed or refractory diffuse large B-cell lymphoma not appropriate for autologous stem-cell transplantation. Blood. 2007;110:54–5.8. doi: 10.1182/blood-2007-01-068056. [DOI] [PubMed] [Google Scholar]
- 5.Wang M, Oki Y, Pro B, et al. Phase II study of yttrium-90- ibritumomab tiuxetan in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:5213–5218. doi: 10.1200/JCO.2009.21.8545. [DOI] [PubMed] [Google Scholar]
- 6.Chu PG, Chen YY, Molina A, et al. Recurrent B-cell neoplasms after rituximab therapy: an immunophenotypic and genotypic study. Leuk Lymphoma. 2002;43:2335–2341. doi: 10.1080/1042819021000040044. [DOI] [PubMed] [Google Scholar]
- 7.Davis TA, Grillo-Lopez AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135–3143. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 8.Rajkumar SV, Richardson PJ, Hideshima T, et al. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23:630–639. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 10.Goy A, Younes A, McLaughlin P, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:667–675. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 11.Strauss SJ, Maharaj L, Hoare S, et al. Bortezomib therapy in patients with relapsed or refractory lymphoma: potential correlation of in vitro sensitivity and tumor necrosis factor alpha response with clinical activity. J Clin Oncol. 2006;24:2105–2112. doi: 10.1200/JCO.2005.04.6789. [DOI] [PubMed] [Google Scholar]
- 12.Di Bella N, Taetle R, Kolibaba K, et al. Results of a phase 2 study of bortezomib in patients with relapsed or refractory indolent lymphoma. Blood. 2010;115:475–480. doi: 10.1182/blood-2009-08-233155. [DOI] [PubMed] [Google Scholar]
- 13.Normolle D, Lawrence T. Designing dose-escalation trials with late-onset toxicities using the time-to-event continual reassessment method. J Clin Oncol. 2006;24:4426–4433. doi: 10.1200/JCO.2005.04.3844. [DOI] [PubMed] [Google Scholar]
- 14.Cheung YK, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000;56:1177–1182. doi: 10.1111/j.0006-341x.2000.01177.x. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 16.Goel A, Dispenzieri A, Greipp PR, et al. PS-341-mediated selective targeting of multiple myeloma cells by synergistic increase in ionizing radiation-induced apoptosis. Exp Hematol. 2005;33:784–795. doi: 10.1016/j.exphem.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Tamatani T, Tamakaru N, Hara K, et al. Bortezomib-enhanced radiosensitization through the suppression of radiation-induced nuclear factor-kappaB activity in human oral cancer cells. Int J Oncol. 2013;42:935–944. doi: 10.3892/ijo.2013.1786. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Kim K, Kim BS, et al. Bortezomib and the increased incidence of herpes zoster in patients with multiple myeloma. Clin Lymphoma Myeloma. 2008;8:237–240. doi: 10.3816/CLM.2008.n.031. [DOI] [PubMed] [Google Scholar]
- 19.Beaven AW, Shea TC, Moore DT, et al. A phase I study evaluating ibritumomab tiuxetan (Zevalin®) in combination with bortezomib (Velcade®) in relapsed/refractory mantle cell and low grade B-cell non-Hodgkin lymphoma. Leuk Lymphoma. 2012;53:254–258. doi: 10.3109/10428194.2011.608445. [DOI] [PubMed] [Google Scholar]
- 20.Roy R, Evens AM, Patton D, et al. Bortezomib may be safely combined with Y-90- ibritumomab tiuxetan in patients with relapsed/ refractory follicular non-Hodgkin lymphoma: a phase I trial of combined induction therapy and bortezomib consolidation. Leuk Lymphoma. 2013;54:497–502. doi: 10.3109/10428194.2012.722215. [DOI] [PubMed] [Google Scholar]
- 21.Beers SA, Chan CHT, James S, et al. Type II (tositumomab) anti- CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood. 2008;112:4170–4177. doi: 10.1182/blood-2008-08-172999. [DOI] [PMC free article] [PubMed] [Google Scholar]