Abstract
Persons with multiple sclerosis (PwMS) have high fall risk due to altered balance. To measure dynamic balance during walking, margin of stability (MoS) examines how the extrapolated center of mass moves relative to the base of support. This study investigates how MoS is affected in PwMS during walking at preferred, slow, and fast speeds, as well as the relationship between MoS and the Expanded Disability Severity Score (EDSS), fall history, and self-report balance confidence questionnaire. MoS was evaluated in PwMS without clinical gait impairment (MS1; n=20), PwMS with clinical gait impairment (MS2; n=20), and age-matched healthy controls (HC) (n=20), in the anterior/posterior (AP) and medial/lateral (ML) direction at heel strike and midstance. In the AP direction, MS2 had a higher MoS than HC (p<0.001) and MS1 (p<0.001) at heel strike and midstance. In the ML direction, MS2 had a higher MoS than HC (p<0.001) at heel strike only. At midstance, slow pace had a lower MoS than preferred pace (p<0.001) and fast pace (p=0.007). Compared to HC, PwMS walk slower which increases their AP MoS. In the ML direction, slow walking causes lower MoS at midstance, so PwMS increase their MoS by taking wider steps. AP MoS correlated with EDSS (p=0.008) and number of falls (p=0.001), and ML MoS correlated with number of falls (p=0.027). Walking slower, with shorter step length, and with wider step widths increases MoS for PwMS but may be a poor adaptive gait strategy since falls still occur.
Keywords: Multiple sclerosis, Margin of stability, Dynamic balance, Conservative gait strategy, Walking speed
1. Introduction
Multiple Sclerosis (MS) is an autoimmune disease which results in progressive demyelination of axons and causes symptoms including limb weakness, gait ataxia, spasticity, depression, and vertigo (Noseworthy et al., 2000). Approximately 400,000 people in the United States and 2.1 million worldwide have MS (Campbell et al., 2014). More than half of adults with MS experience falls, with about half of these falls resulting in injury requiring medical attention (Peterson et al., 2013). In a study comparing fall occurrences in over 350 persons with MS (PwMS) balance was one of the most common attributing factors (41.5%) (Peterson et al., 2013) and these falls lead to high healthcare costs and a low health-related quality-of-life (Campbell et al., 2014). In order to understand the movement characteristics which result in PwMS having increased fall risk, it is necessary to characterize their dynamic stability during walking.
While many studies have evaluated gait patterns in PwMS, most of these studies focus only on lower extremity motion or only on trunk motion. Compared to healthy controls, PwMS have gait alterations characterized by a decreased walking velocity, shorter stride length, prolonged double support time, and wider step width (Sosnoff et al., 2012). Along with altered stepping patterns, PwMS have a more regular and repeatable step width and length compared to controls, reflecting poor adaptability (Kaipust et al., 2012). Compared to healthy controls, PwMS also have altered trunk kinematics during gait, which is characterized by an increased range of motion in the lateral and transverse planes (Spain et al., 2012), and by both less periodic and more divergent variability, particularly in the medial/lateral direction (Huisinga et al., 2013). Trunk motion is a good approximation of center of mass (CoM) motion (Moe-Nilssen and Helbostad, 2002), suggesting that PwMS have poor control of their CoM during walking. Looking at both footfall information and trunk motion during gait in PwMS may help to identify dynamic stability features that indicate an individual’s fall risk.
Dynamic stability during gait can be measured by Margin of Stability (MoS) which evaluates the motion of the CoM relative to the base of support where the base is defined by foot placement (Hof et al., 2005). Stability is defined as maintaining the whole body’s CoM within the limits of the base of support. In dynamic situations such as gait, the velocity of the CoM must be taken into account (Pai and Patton, 1997). MoS is based on a model of a single degree of freedom inverted pendulum rotating about the ankle joint, with the ground reaction force acting at the AP or ML edge of the foot, and defining the system as stable when the CoM is kept within the limits of the base of support. Specifically, MoS is the distance between the ‘extrapolated center of mass’ and the edge of the base of support (Hof et al., 2005). The extrapolated CoM incorporates the CoM position and velocity which better represents the CoM’s kinematic state (Hof et al., 2005). Increasing or decreasing gait speed should affect MoS since the CoM velocity would change with altered walking speeds (Hak et al., 2013a). A positive MoS means that the CoM is stable inside the base of support, whereas a negative MoS means the CoM has left the base of support. An increased MoS means that the CoM is further inside the base of support, and a decreased MoS means that the CoM is closer to the limits of the base of support.
MoS has not been previously evaluated in PwMS but is uniquely suited to evaluate dynamic stability in this population because MoS combines footfall and CoM motion, which are both altered in PwMS (Sosnoff et al., 2012; Spain et al., 2012), into one measure. PwMS also experience falls at a high rate (Campbell et al., 2014), but the disease specific adaptations which contribute to those falls is unclear. MoS is unique as it can be independently quantified at any instant in time during the gait cycle and it has been used previously to evaluate perturbation responses (Hof et al., 2010), adaptations to destabilizing environments (McAndrew Young et al., 2012), and to understand gait adaptions in populations with musculoskeletal disorders (Hof et al., 2007). In PwMS, dynamic stability changes at specific points in the gait cycle can be highlighted by evaluating MoS at heel strike and midstance. These dynamic stability changes may indicate when PwMS are at higher risk for falls during gait. Therefore, the purpose of this study is to compare MoS between healthy controls and PwMS who have or don’t have clinical gait impairments. Because both footfall patterns and CoM motion are altered in PwMS relative to healthy controls, it was hypothesized that MoS would also be altered in PwMS. However, based on these previously reported MS-specific gait alterations including decreased walking speed, increased step width, increased trunk motion, it was unclear how these combined alterations would affect the direction of difference for MoS in PwMS compared to healthy controls. The secondary purpose of this study is to investigate the clinical relevance of MoS, by evaluating the relationship between MoS and fall history, Expanded Disability Severity Scale (EDSS), and self-reported balance confidence. We hypothesized that MoS would correlate with all three of these measures.
2. Methods
2.1 Subjects
Sixty subjects (Table 1) were compared in the present study. The University of Kansas Medical Center Human Research Committee approved this study and all participants gave informed written consent. Healthy controls (HC) were free of any known neurological or musculoskeletal pathology or disorder that would have an adverse effect on the participant’s balance or gait. All MS subjects were between the ages of 21–60, had relapsing remitting MS, had an EDSS score less than 5.5, and were able to walk 25 feet without an assistive mobility aid. To assess general walking disability, three timed 25-foot walk (T25FW) tests were performed and averaged for each subject. Based on their T25FW, PwMS were divided into two groups: 20 subjects with MS who performed the T25FW in <5 seconds were classified as the MS group 1 (MS1) who did not have clinical gait impairments, 20 subjects with MS with a T25FW >5 seconds were classified as the MS group 2 (MS2) who did have clinically impaired gait (Table 1). The T25FW was used to separate groups because this outcome measure is frequently used as a clinical assessment tool of mobility and as an outcome measure in clinical trials (Goodman et al., 2009; Huisinga et al., 2014). BMI was significantly different (p=0.038) between HC and MS2 only (Table 1).
Table 1.
Demographics mean +/− SD, for heathy controls and subjects with multiple sclerosis with (MS2) and without (MS1) clinical gait impairments.
| Healthy Control (n=20) |
MS1 (n=20) |
MS2 (n=20) |
|
|---|---|---|---|
| Age (years) | 47.55 (7.78) | 45.82 (8.62) | 45.91 (8.72) |
| Gender (F/M) | 14/6 | 14/6 | 14/6 |
| BMI | 25.62 (3.58) | 28.99 (6.05) | 30.67 (8.11) |
| 25FTW (sec) | 4.28 (0.57) | 4.30 (0.45) | 6.45 (1.42) |
| EDSS | – | 1.58 (0.57) | 2.60 (1.26) |
| Falls in previous 6 months | – | 0.15 (0.37) | 2.30 (3.62) |
| ABC | – | 83.75 (14.15) | 96.16 (20.99) |
| Years since diagnosis | – | 10.21 (4.40) | 11.15 (7.07) |
BMI – Body mass index
25FTW – timed 25 foot walk
EDSS – Kurtzke’s expanded disability status scale
ABC – Activity Specific Balance Confidence questionnaire.
2.2 Data Collection
Kinematic data was collected using retroreflective markers placed bilaterally on the anterior and posterior superior iliac spine, heel, lateral malleolus, top of the second metatarsal phalangeal joint (toe), and at the lateral metatarsal phalangeal (lateral MTP). Marker positions in 3D space were recorded with an 8-camera motion capture system (Motion Analysis, Santa Rosa, CA, USA) sampling at 60 Hz. Subjects walked across a 25 foot walkway at their self-selected 1) preferred pace, 2) slow pace, and 3) fast pace with these instructions: normal pace “walk at a comfortable pace”; slow pace “walk slower than is preferred”; fast pace “walk as fast as you can safely”. The motion capture collection volume was set to the middle 15 feet of the walkway so data was collected while subjects were walking at steady state. A person’s preferred walking speed is effectively their optimum walking speed since it indicates the lowest metabolic cost (Holt et al., 1991) and optimum gait variability (Dingwell and Marin, 2006). Walking slower or faster than preferred speed adds additional constraint to the neuromuscular system which may further reveal dynamic instability (Dingwell et al., 2007; Kang and Dingwell, 2008). The three different walking conditions were done in a randomized order. Each subject completed 5 trials for each condition.
Disease severity was assessed with the Kurke’s expanded disability status scale (EDSS) administered by a neurologist (author SL). The self-reported number of falls in the six months prior to data collection was recorded. Patients completed the Activity-specific Balance Confidence (ABC) questionnaire reporting their self-perceived confidence during daily life activities.
2.3 Data Analysis
Margin of stability and extrapolated center of mass calculations were adapted from Hof et al (2005), and shown below (Equation 1, Equation 2, Figure 1).
| (1) |
| (2) |
Where wo is the natural frequency of the pendulum used in the model (wo2=g/l), with g being the acceleration of gravity (g=9.81 m/s2), and l being the distance of the center of mass to the axis of rotation, which was defined using the ankle marker (McAndrew Young et al., 2012). The position of the CoM (XCoM) was estimated as the geometric center of the triangle formed by the two anterior superior iliac spine markers, and the midpoint between the two posterior superior iliac spine markers (Whittle, 1997). Velocity of the CoM (VCoM) was found using a first order central difference formula of the XCoM time series. UMax was defined using the toe marker in the anterior/posterior direction, which represents the forward MoS, and lateral MTP in the medial/lateral direction (McAndrew Young et al., 2012).
Figure 1.
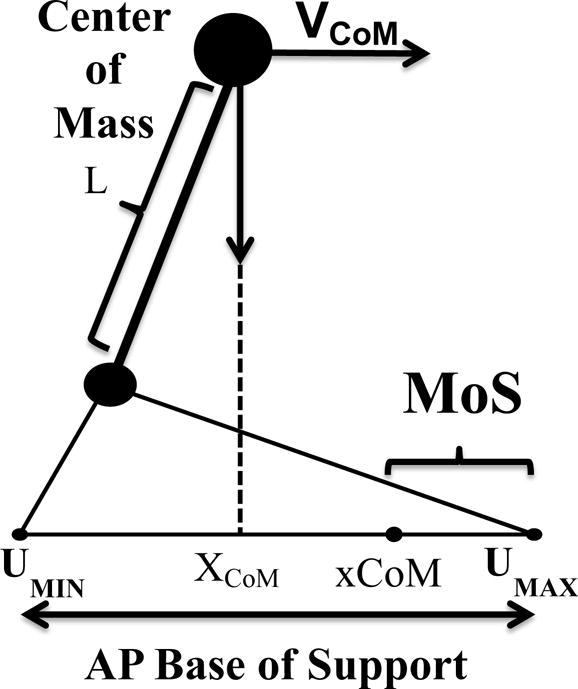
Visual representation of the AP MoS calculation.
MoS was calculated in both the anterior/posterior (AP) and medial/lateral (ML) directions, at both heel strike and midstance for each stance phase recorded. Previous studies have examined MoS at each heel strike (McAndrew Young et al., 2012; McCrum et al., 2014); however, in the gait cycle, midstance may be the point most susceptible to a ML perturbation, causing a fall or corrective stepping (Hof et al., 2010). Heel strike was defined as the instant in which the heel marker reached its local stride to stride minimum (Fellin et al., 2010). Mid stance phase was defined as the instant in time when the CoM passes the ankle marker (Lee and Farley, 1998). For each trial, heel strike and midstance indices were plotted and visually inspected to assure proper identification. In the current study, the left and right stance phases of each subject were treated as independent observations, as we were not comparing individual differences between right and left steps. Stride length, step width, and CoM velocity were calculated for each trial to aid the interpretation of results. Step width was defined as the distance between heel markers at each heel strike (Elble et al., 1991). Since the right and left leg were treated independently, stride length was the distance in the AP direction between two consecutive strikes of the same heel (Elble et al., 1991). CoM velocity was taken as the average AP CoM velocity. All data analysis was performed using custom software in Matlab R2013a (The Mathworks, Inc). 1–2 full stance phases from each trial were used for analysis in the present study, totaling 5–10 steps for each condition, which was averaged for each subject to calculate MoS, stride length, and step width.
Two way repeated measures ANOVA’s were performed to identify the main effect of walking speed condition (slow, normal, fast) and group (control, MS1, MS2) for each of the outcome variables. Post hoc tests were done to determine differences between groups and walking conditions. Pearson’s correlations compared the relationship between preferred pace AP and ML MoS and EDSS, number of falls, and ABC scores for all PwMS. Significance was set at α=0.05. All statistics were performed with SPSS software (SPSS version 22).
3. Results
3.1 AP Margin of Stability
There was a significant main effect of group at heel strike and midstance (Table 2). At both heel strike and midstance, the MS2 had a significantly higher MoS than HC (p<0.001) and the MS1 (p<0.001). There was also a significant main effect of walking speed at heel strike and midstance. Fast pace had a lower MoS than both preferred pace (p<0.001) and slow pace (p<0.001), and that preferred pace had a lower MoS than slow pace (p<0.001). There was a significant interaction between group and walking speed at heel strike and midstance. While MS2 had a higher MoS than both HC and MS1 at every walking speed, there was no difference in MoS between HC and MS1 at heel strike or midstance (Figure 2).
Table 2.
MoS compared across groups and walking conditions
| AP MoS (m) at Heel Strike mean (SD) |
Effect of Speed | Effect of Group | Interaction | |||
|---|---|---|---|---|---|---|
| Slow | Preferred | Fast |
F = 832.2 (p<0.001)* |
F = 36.11 (p<0.001)* |
F = 21.99 (p<0.001)* |
|
|
| ||||||
| HC | 0.215 (0.041) |
0.114 (0.046) |
0.001 (0.058) |
|||
| MS1 | 0.215 (0.04) |
0.123 (0.036) |
0.021 (0.05) |
|||
| MS2 | 0.227 (0.037) |
0.175 (0.033) |
0.107 (0.043) |
|||
|
| ||||||
|
AP MoS (m) at Midstance mean (SD) |
Effect of Speed | Effect of Group | Interaction | |||
|
| ||||||
| Slow | Preferred | Fast |
F = 805.7 (p<0.001)* |
F= 24.62 (p<0.001)* |
F = 16.08 (p<0.001)* |
|
|
| ||||||
| HC | 0.087 (0.025) |
0.028 (0.032) |
−0.025 (0.038) |
|||
| MS1 | 0.09 (0.029) |
0.036 (0.025) |
−0.019 (0.033) |
|||
| MS2 | 0.099 (0.017) |
0.065 (0.018) |
0.03 (0.027) |
|||
|
| ||||||
|
ML MoS (m) at Heel Strike mean (SD) |
Effect of Speed | Effect of Group | Interaction | |||
|
| ||||||
| Slow | Preferred | Fast |
F = 84.25 (p<0.001)* |
F = 4.971 (p=0.009)* |
F = 1.423 (p=0.227) |
|
|
| ||||||
| HC | 0.128 (0.020) |
0.118 (0.016) |
0.116 (0.022) |
|||
| MS1 | 0.141 (0.026) |
0.130 (0.022) |
0.121 (0.023) |
|||
| MS2 | 0.146 (0.033) |
0.134 (0.029) |
0.126 (0.024) |
|||
|
| ||||||
|
ML MoS (m) at Midstance mean (SD) |
Effect of Speed | Effect of Group | Interaction | |||
|
| ||||||
| Slow | Preferred | Fast |
F = 8.208 (p<0.001)* |
F = 0.724 (p=0.487) |
F = 1.064 (p=0.375) |
|
|
| ||||||
| HC | 0.098 (0.018) |
0.103 (0.018) |
0.104 (0.020) |
|||
| MS1 | 0.104 (0.021) |
0.109 (0.021) |
0.107 (0.021) |
|||
| MS2 | 0.106 (0.025) |
0.107 (0.023) |
0.107 (0.022) |
|||
HC – heathy controls; MS1– MS subjects without clinical gait impairment; MS2– MS subjects with clinical gait impairment.
Significant main effect (p < 0.05)
Figure 2.
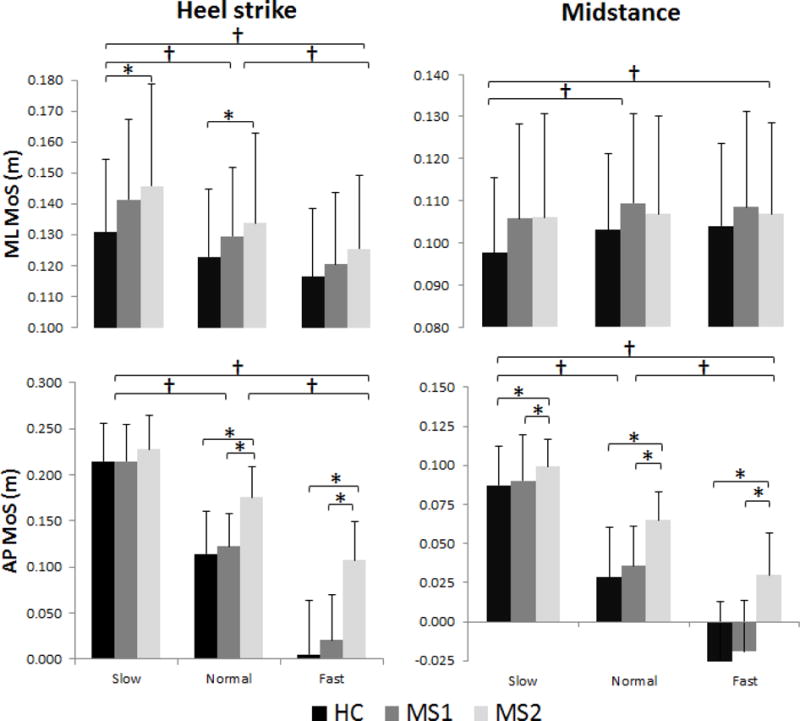
Comparison of AP and ML MoS across group and walking speed, at heel strike and mid stance. † represents significance difference between walking speed (p<0.001). * represents between group differences (p<0.05).
HC – healthy controls; MS1– MS subjects without clinical gait impairment; MS2– MS subjects with clinical gait impairment
3.2 ML Margin of Stability
There was a significant main effect of group for MoS at heel strike, but not at midstance (Table 2). At heel strike, MS2 had a higher MoS than HC (p=0.010) only. A significant main effect of speed was found at heel strike and at midstance. At heel strike, fast pace had a lower MoS than both preferred pace (p<0.001) and slow pace (p<0.001), and preferred pace had a lower MoS than slow pace (p<0.001). At midstance, slow pace had lower MoS than preferred pace (p<0.001) and lower MoS than fast pace (p=0.007). There was no significant interaction between group and walking speed in the ML direction (Figure 2).
3.3 Relationship with disability, falls history, and self-report gait & balance confidence
AP MoS at heel strike and midstance was positively correlated with number of falls (heel strike p=0.001; midstance p=0.030) and EDSS (heel strike p=0.008; midstance p=0.001). ML MoS at heel strike only was positively correlated with number of falls (p=0.027). No significant correlations between ABC and MoS were found (Table 3).
Table 3.
CoM velocity, step length, and step width compared across groups and walking conditions.
| CoM Velocity (m/s) mean (SD) |
Effect of Speed | Effect of Group | Interaction | |||
|---|---|---|---|---|---|---|
| Slow | Preferred | Fast |
F = 479.6 (p<0.001)* |
F = 36.9 (p<0.001)* |
F = 6.6 (p<0.001)* |
|
|
| ||||||
| HC | 0.943 (0.175) |
1.477 (0.126) |
1.944 (0.244) |
|||
| MS1 | 0.918 (0.225) |
1.421 (0.17) |
1.878 (0.21) |
|||
| MS2 | 0.736 (0.172) |
1.089 (0.16) |
1.409 (0.254) |
|||
|
| ||||||
|
Step Width (m) mean (SD) |
Effect of Speed | Effect of Group | Interaction | |||
|
| ||||||
| Slow | Preferred | Fast | F = 0.149 (p=0.862) |
F= 4.039 (p=0.023)* |
F = 1.855 (p=0.124) |
|
|
| ||||||
| HC | 0.080 (0.036) |
0.080 (0.032) |
0.073 (0.032) |
|||
| MS1 | 0.097 (0.028) |
0.099 (0.022) |
0.108 (0.020) |
|||
| MS2 | 0.099 (0.047) |
0.096 (0.039) |
0.096 (0.037) |
|||
|
| ||||||
|
Stride Length (m) mean (SD) |
Effect of Speed | Effect of Group | Interaction | |||
|
| ||||||
| Slow | Preferred | Fast |
F = 129.0 (p<0.001)* |
F = 17.0 (p<0.001)* |
F = 18.9 (p<0.001)* |
|
|
| ||||||
| HC | 1.224 (0.164) |
1.153 (0.141) |
1.677 (0.169) |
|||
| MS1 | 1.182 (0.14) |
1.474 (0.134) |
1.670 (0.170) |
|||
| MS2 | 1.151 (0.213) |
1.291 (0.191) |
1.23 (0.181) |
|||
HC – heathy controls; MS1– MS subjects without clinical gait impairment; MS2– MS subjects with clinical gait impairment.
Significant main effect (p < 0.05)
3.4 CoM velocity, Stride Length, Step Width
There was a significant main effect of group on CoM velocity, step width, and stride length (Table 4). MS2 walked significantly slower and with a shorter stride length, compared to MS1 (p<0.001) and HC (p<0.001). MS1 had a wider step width than HC (p=0.039). There was also a significant main effect of walking condition on stride length, but not on step width. Fast pace had a longer stride length than preferred pace and slow pace (p<0.001) and preferred pace had a longer stride length than slow pace (p<0.001) (Figure 3).
Table 4.
Correlation between AP and ML MoS at heel strike and midstance during preferred pace only and fall history, EDSS, and ABC scores. Pearson correlation (p-value).
| Number of Falls | EDSS | ABC | |
|---|---|---|---|
| AP MoS heel strike |
0.510* (0.001) |
0.411* (0.008) |
−0.010 (0.952) |
| AP MoS midstance |
0.344* (0.030) |
0.489* (0.001) |
0.024 (0.884) |
| ML MoS heel strike |
0.350* (0.027) |
0.277 (0.084) |
−0.165 (0.309) |
| ML MoS midstance | 0.107 (0.511) |
0.154 (0.344) |
−0.147 (0.364) |
significant correlationns (p < 0.05)
EDSS – Kurtzke’s expanded disability status scale
ABC – Activity Specific Balance Confidence questionnaire.
Figure 3.
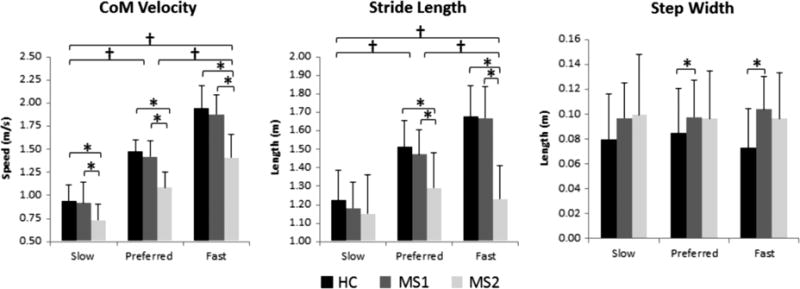
Differences between step length, step width, and CoM Velocity across groups and walking conditions. † represents significance difference between walking speed (p<0.001). * represents between group differences (p<0.05).
HC – healthy controls; MS1– MS subjects without clinical gait impairment; MS2– MS subjects with clinical gait impairment
4. Discussion
PwMS experience increased fall risk, but in order understand what gait and balance features contribute to increased falls, dynamic stability in PwMS must be better understood. The goal of this study was to compare MoS between PwMS and healthy control subjects under preferred, slow, and fast walking speeds, and to investigate the relationship between MoS and clinically important measures such as disability status and fall history. We hypothesized that PwMS would have different MoS than healthy controls, and that MoS would correlate with clinically important measures. The results of the study agree with our hypothesis since there were significant effect of group and of walking speed condition on MoS in both the AP and ML directions, and since MoS correlated with fall history and EDSS.
The present study found MS2 had a significantly higher AP MoS at heel strike and midstance, slower CoM speeds, and a shorter stride lengths, than both the MS1 (p<0.001) and HC (p<0.001). The MS2 walked slower than the MS1 in the present study, which was expected as PwMS were grouped based on their walking speed (Huisinga et al., 2014). EDSS was significantly correlated with AP MoS at heel strike and midstance which also indicates that disease severity effects MoS during gait. Slower walking leads to increased AP MoS, which is consistent with previous findings (Hak et al., 2013a). In general, PwMS increased their MoS for all walking speeds, not just the slow pace, compared to the control group. Maintaining a larger MoS may be a compensatory gait strategy to allow PwMS more response time to various external perturbations. PwMS have longer sensorimotor response times to perturbations (Cameron et al., 2008; Huisinga et al., 2014) which would increase response time needed to recover from a perturbation such as a trip. Walking speed and response time were the leading factors separating individuals who recovered from a laboratory induced trip, and those who fell (Pavol et al., 2001). In PwMS, increased AP MoS may be an adaptation to allow for increased stability since they are not able to sense and respond quickly to a perturbation during gait. Thus, encouraging PwMS to maintain a walking speed similar to controls regardless of their preferred pace may actually be a negative gait performance goal.
In the present study, MS1 had a wider step width and MS2 had a higher ML MoS at heel strike compared to HC. Slow pace had the highest ML MoS at heel strike (p<0.001), and the lowest ML MoS at midstance (p<0.001) across all groups. These result agree with previous work (Hof et al., 2007), which found that slow pace walking leads to a decrease in the minimum ML MoS (which occurs close to midstance). At heel strike, however, walking with an increase in step width leads to an increase in ML MoS (McAndrew Young and Dingwell, 2012). MS2 walked with a much slower speed which should decrease ML MoS at midstance, but they also walked with wide steps and ML MoS was similar to controls at midstance. This seems to indicate that walking with wider steps offsets the effect of slow walking induced changes to ML MoS at midstance. This finding agrees with previous work which found that like PwMS, post-stroke patients walked slower and with a wider step width compared to controls, and also did not have altered ML MoS at midstance (Hak et al., 2013b). The authors concluded that an increased step width is a strategy to deal with increased ML CoM sway seen during slow walking. Since movement strategies are thought to be independent between the AP and ML directions (O’Connor and Kuo, 2009), it is likely that PwMS slow their gait to increase their AP stability, as demonstrated by increased AP MoS at heel strike and midstance the tradeoff for this adaption is an increased step width to maintain a ML MoS. To test this idea, we performed post hoc Pearson correlations between walking speed and MoS and between step width and MoS. Walking speed was significantly correlated with AP MoS (heel strike r=−0.944, p<0.001; midstance r=−0.938, p<0.001) and with ML MoS (heel strike r=−0.410, p<0.001; midstance r = 0.069, p=0.451) while step width was not significantly correlated with AP MoS (heel strike: r=0. −0.025, p=0.787; midstance: r=−0.159, p=0.0.84) or ML MoS (heel strike: r=0.176, p=0.055; midstance: r=0.108, p=0.240). These results confirm the impact of walking speed on MoS in both directions, but they don’t indicate a specific relationship between step width and MoS. It may be necessary to evaluate this relationship further in a prospectively designed study which systematically alters step width and evaluates the effects on MoS.
Along with PwMS, other clinical populations, elderly individuals, and amputees all walk with slower preferred walking speeds, likely as a conservative gait strategy to improve stability (Boonstra et al., 1993; Chen et al., 2003; Dingwell and Cusumano, 2000; Ferrandez et al., 1990; Menz et al., 2003; Norlin and Odenrick, 1986). Conservative gait strategies, such as slower walking, shorter steps, and prolonged double support time, have been found to be reflective of a fear of falling, rather than fall occurrence (Maki, 1997) and have been suggested as mechanisms to minimize the forward CoM excursion beyond the stance foot base of support and to decrease the amount of time balancing on one foot (Maki, 1997). The ABC questionnaire, which reports confidence during daily activities, was not correlated with AP or ML MoS, which suggests that PwMS are not aware of their relative dynamic stability changes as it related to their perceived balance capabilities. However, both AP and ML MoS were significantly correlated with actual number of falls in the previous 6 months, indicating altered dynamic stability is associated with more falls in PwMS.
One limitation of our experimental procedure was the limited capture volume of the motion capture system since we were only able to capture 1–2 stance phases per trial. While walking on a treadmill verse over ground does induce known gait changes (Alton et al., 1998; Dingwell and Cusumano, 2000; Rosenblatt and Grabiner, 2010), repeating this analysis on a treadmill would allow for a longer trial with more steps and for us to regulate pace. However, all paces were self-selected so any fluctuation between speeds within each walking speed condition would be a reflection individual trial-to-trial variation present in any over-ground gait study.
This study examined MoS in PwMS and HC walking at their self-selected preferred, fast, and slow pace, to determine the difference in dynamic stability across groups. Study results indicate that slower walking speeds, shorter stride lengths, and wider step widths are conservative gait strategies that lead to altered MoS in both the AP and ML directions in PwMS. MoS was significantly correlated with clinical disability (EDSS) and with fall history. MoS is an effective measure to evaluate dynamic stability during gait. As this study only used retrospective fall occurrence in one pathological population, future work should extend these findings to prospective fall occurrence.
Acknowledgments
This work was supported by the National Multiple Sclerosis Society RG 4914A1/2 and the NIH National Center for Advancing Translational Science 1KL2TR00011
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflict of interest.
References
- Alton F, Baldey F, Caplan S, Morrissey MC. A kinematic comparison of overground and tredmil walking. Clinical biomechanics. 1998;13:434–440. doi: 10.1016/s0268-0033(98)00012-6. [DOI] [PubMed] [Google Scholar]
- Boonstra AM, Fidler V, Eisma WH. Walking speed of normal subjects and amputees: aspects and validity of gait analysis. Prosthetics and Orthotics International. 1993;17:78–82. doi: 10.3109/03093649309164360. [DOI] [PubMed] [Google Scholar]
- Cameron MH, Horak FB, Herndon RR, Bourdette D. Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. Somatosensory & motor research. 2008;25:113–122. doi: 10.1080/08990220802131127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JD, Ghushchyan V, Brett McQueen R, Cahoon-Metzger S, Livingston T, Vollmer T, Corboy J, Miravalle A, Schreiner T, Porter V, Nair K. Burden of multiple sclerosis on direct, indirect costs and quality of life: National US estimates. Multiple sclerosis and related disorders. 2014;3:227–236. doi: 10.1016/j.msard.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Chen CL, Chen HC, Tang SF, Wu CY, Cheng PT, Hong WH. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. American journal of physical medicine & rehabilitation/Association of Academic Physiatrists. 2003;82:925–935. doi: 10.1097/01.PHM.0000098040.13355.B5. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Cusumano JP. Nonlinear time series analysis of normal and pathological human walking. Chaos. 2000;10:848–863. doi: 10.1063/1.1324008. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Kang HG, Marin LC. The effects of sensory loss and walking speed on the orbital dynamic stability of human walking. Journal of biomechanics. 2007;40:1723–1730. doi: 10.1016/j.jbiomech.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Marin LC. Kinematic variability and local dynamic stability of upper body motions when walking at different speeds. Journal of biomechanics. 2006;39:444–452. doi: 10.1016/j.jbiomech.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Elble R, Thomas S, Higgins C, Colliver J. Stride-dependent changes in gait of older people. Journal of Neurology. 1991;238:1–5. doi: 10.1007/BF00319700. [DOI] [PubMed] [Google Scholar]
- Fellin RE, Rose WC, Royer TD, Davis IS. Comparison of methods for kinematic identification of footstrike and toe-off during overground and treadmill running. Journal of Sci Med Sport. 2010;13:646–650. doi: 10.1016/j.jsams.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandez AM, Pailhous J, Durup M. Slowness in elderly gait. Experimental aging research. 1990;16:79–89. doi: 10.1080/07340669008251531. [DOI] [PubMed] [Google Scholar]
- Goodman AD, Brown TR, Krupp LB, Schapiro RT, Schwid SR, Cohen R, Marinucci LN, Blight AR. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. The Lancet. 2009;373:732–738. doi: 10.1016/S0140-6736(09)60442-6. [DOI] [PubMed] [Google Scholar]
- Hak L, Houdijk H, Beek PJ, van Dieen JH. Steps to take to enhance gait stability: the effect of stride frequency, stride length, and walking speed on local dynamic stability and margins of stability. PloS one. 2013a;8:e82842. doi: 10.1371/journal.pone.0082842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hak L, Houdijk H, van der Wurff P, Prins MR, Mert A, Beek PJ, van Dieen JH. Stepping strategies used by post-stroke individuals to maintain margins of stability during walking. Clinical biomechanics. 2013b;28:1041–1048. doi: 10.1016/j.clinbiomech.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Hof AL, Gazendam MG, Sinke WE. The condition for dynamic stability. Journal of biomechanics. 2005;38:1–8. doi: 10.1016/j.jbiomech.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hof AL, van Bockel RM, Schoppen T, Postema K. Control of lateral balance in walking. Experimental findings in normal subjects and above-knee amputees. Gait & posture. 2007;25:250–258. doi: 10.1016/j.gaitpost.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Hof AL, Vermerris SM, Gjaltema WA. Balance responses to lateral perturbations in human treadmill walking. Journal of Exp Biol. 2010;213:2655–2664. doi: 10.1242/jeb.042572. [DOI] [PubMed] [Google Scholar]
- Holt KG, Hamill J, Andres RO. Predicting the minimal energy costs of human walking. Medicine and Science in Sports and Exercise. 1991;23:491–498. [PubMed] [Google Scholar]
- Huisinga JM, Mancini M, St George RJ, Horak FB. Accelerometry reveals differences in gait variability between patients with multiple sclerosis and healthy controls. Annals of biomedical engineering. 2013;41:1670–1679. doi: 10.1007/s10439-012-0697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisinga JM, St George RJ, Spain R, Overs S, Horak FB. Postural response latencies are related to balance control during standing and walking in patients with multiple sclerosis. Archives of physical medicine and rehabilitation. 2014;95:1390–1397. doi: 10.1016/j.apmr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipust JP, Huisinga JM, Filipi ML, Stergiou N. Gait variability measures reveal differences between multiple sclerosis patients and healthy controls. Motor control. 2012;16:229–244. doi: 10.1123/mcj.16.2.229. [DOI] [PubMed] [Google Scholar]
- Kang HG, Dingwell JB. Separating the effects of age and walking speed on gait variability. Gait & posture. 2008;27:572–577. doi: 10.1016/j.gaitpost.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Lee C, Farley C. Determinants of the center of mass trajectory in human walking and running. Expiremental Biology. 1998;201 doi: 10.1242/jeb.201.21.2935. [DOI] [PubMed] [Google Scholar]
- Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. Journal of American Geriatric Society. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- McAndrew Young PM, Dingwell JB. Voluntary changes in step width and step length during human walking affect dynamic margins of stability. Gait & posture. 2012;36:219–224. doi: 10.1016/j.gaitpost.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrew Young PM, Wilken JM, Dingwell JB. Dynamic margins of stability during human walking in destabilizing environments. Journal of biomechanics. 2012;45:1053–1059. doi: 10.1016/j.jbiomech.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrum C, Eysel-Gosepath K, Epro G, Meijer K, Savelberg HH, Bruggemann GP, Karamanidis K. Deficient recovery response and adaptive feedback potential in dynamic gait stability in unilateral peripheral vestibular disorder patients. Physiological reports. 2014;2:e1222. doi: 10.14814/phy2.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menz HB, Lord SR, Fitzpatrick RC. Age-related differences in walking stability. Age and Aging. 2003;32:137–142. doi: 10.1093/ageing/32.2.137. [DOI] [PubMed] [Google Scholar]
- Moe-Nilssen R, Helbostad J. Trunk accelerometry as a measure of balance control during quiet standing. Gait and Posture. 2002;16:60–68. doi: 10.1016/s0966-6362(01)00200-4. [DOI] [PubMed] [Google Scholar]
- Norlin R, Odenrick P. Development of gait in spastic children with cerebral palsy. Journal of Pediatric Orthopedics. 1986;6 doi: 10.1097/01241398-198611000-00006. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodrigues M, Weinshenker BG. Multiple Sclerosis. New England Journal of Medicine. 2000:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- O’Connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. Journal of neurophysiology. 2009;102:1411–1419. doi: 10.1152/jn.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai YC, Patton J. Center of mass velocity-position predictions for balance control. Journal of biomechanics. 1997;30:347–354. doi: 10.1016/s0021-9290(96)00165-0. [DOI] [PubMed] [Google Scholar]
- Pavol MJ, Owings TM, Foley KT, Grabiner MD. Mechanisms Leading to a Fall From an Induced Trip in Healthy Older Adults. Journal of Gerontology. 2001;56A:M428–M437. doi: 10.1093/gerona/56.7.m428. [DOI] [PubMed] [Google Scholar]
- Peterson EW, Ben Ari E, Asano M, Finlayson ML. Fall attributions among middle-aged and older adults with multiple sclerosis. Archives of physical medicine and rehabilitation. 2013;94:890–895. doi: 10.1016/j.apmr.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Rosenblatt NJ, Grabiner MD. Measures of frontal plane stability during treadmill and overground walking. Gait & posture. 2010;31:380–384. doi: 10.1016/j.gaitpost.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Sandroff BM, Motl RW. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait & posture. 2012;36:154–156. doi: 10.1016/j.gaitpost.2011.11.027. [DOI] [PubMed] [Google Scholar]
- Spain RI, St George RJ, Salarian A, Mancini M, Wagner JM, Horak FB, Bourdette D. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait & posture. 2012;35:573–578. doi: 10.1016/j.gaitpost.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle M. Three-dimensional motion of the center of gravity of the body during walking. Human Movement Sciences. 1997;16:347–355. [Google Scholar]


