Abstract
An insatiable appetite is a cardinal feature of Prader-Willi syndrome (PWS) with stomach rupturing as a reported consequence. Peptide YY, secreted by the intestine and released post prandially, inhibits appetite, while ghrelin, secreted by the stomach during mealtime hunger, stimulates appetite. Both peptide YY and ghrelin act at the brain level, particularly the hypothalamus. Recently, plasma ghrelin levels were reported to be elevated in children and adults with PWS but peptide YY levels have not been studied in this syndrome or ghrelin in infants with PWS. To further address the abnormal eating behavior in PWS, we obtained fasting plasma peptide YY and ghrelin levels in 12 infants and children with PWS ranging in age from 2.5 months to 13.3 years and compared them with values from normal populations reported in the literature. Plasma ghrelin levels in our patients with PWS were similar to those of other children with PWS and were significantly higher than those reported in obese children without PWS. Our infants with PWS had similar plasma ghrelin levels compared with our children with PWS but peptide YY levels in our children and infants with PWS were lower than reported in similarly aged individuals without PWS. In addition, we performed preliminary gene expression analysis of ghrelin and peptide YY and their receptors in patients with PWS using established lymphoblastoid cell lines but gene expression did not correlate with plasma ghrelin or peptide YY levels.
Keywords: Prader-Willi syndrome, ghrelin, peptide YY, infants, children
INTRODUCTION
Prader-Willi syndrome (PWS) is a complex genetic disorder characterized by infantile hypotonia, hypogonadism, small hands and feet, short stature, growth hormone deficiency, hyperphagia, early childhood obesity, mental deficiency, and a characteristic facial appearance1–3. A de novo paternally derived chromosome 15q11-q13 deletion is seen in about 70% of patients, while maternal disomy 15 (both chromosome 15s from the mother) is seen in about 25% of patients, and an imprinting center defect in the remaining patients2–4. PWS was the first reported example in humans of genetic imprinting or the differential expression of genetic information depending on the parent of origin. PWS is considered the most common genetic cause of marked obesity in humans and occurs in about one in 10,000 to 20,000 live births1.
The course and natural history of the disorder can be divided into two distinct stages. The first stage occurs during infancy and is characterized by central hypotonia, a weak cry, poor sucking reflex, sticky saliva, feeding difficulties, developmental delay, and hypogonadism. Failure to thrive is noted during this stage. The second stage occurs around 2 years of age and is characterized by continued developmental delay, onset of hyperphagia, and food foraging leading to obesity. Abnormal eating behavior, inability to vomit, obsessive food seeking and hoarding of food, and consumption of inedible items are characteristic of PWS. The severe hyperphagia-mediated obesity appears to result from a faulty satiety mechanism.
Food intake is regulated by the hypothalamus including the melanocortin and neuropeptide Y (NPY) systems in the arcuate nucleus5. The NPY Y2 receptor (Y2R) is thought to be an inhibitory presynaptic receptor and highly expressed in NPY neurons in the arcuate nucleus which is accessible to peripheral hormones6. Peptide YY is a Y2R agonist and released by the gastrointestinal tract7–8. Hence, eating behavior is directly influenced by gastrointestinal released peptides responding to the nutritional status and body composition of an individual and acting at the brain level9.
Ghrelin, a novel 28 amino acid peptide, primarily secreted by the stomach, was found to increase appetite, food intake and body weight and influences the release of growth hormone10–12. In addition, leptin, secreted by the adipose tissue, reflects the fat mass of an individual and plays a role in eating behavior13,14. Leptin and ghrelin both induce central effects mediated through the NPY receptor pathway in the hypothalamus and play a major role in energy balance15. Ghrelin levels are inversely correlated with body weight, are higher during starvation, and are increased in weight loss in humans11,12,16.
Peptide YY (PYY) is a 34 amino acid peptide secreted by the intestine, shares homology with pancreatic polypeptide and neuropeptide Y7, and is released postprandially in proportion to the caloric content of a meal. It remains elevated for several hours7,9. PYY causes intestinal constriction inhibiting jejunal and colonic motility17,18. Peripheral injection of PYY or intra-arcuate nucleus injection in the hypothalamus in rats inhibits food intake and reduces weight gain9. PYY also inhibits electrical activity of NPY nerve endings and activates adjacent pro-opiomelanocortin (POMC) neurons9. In humans, infusion of normal postprandial concentrations of PYY significantly decreases appetite and reduces food intake by 33% over a 24-hour period, ostensibly through the arcuate nucleus Y2R, to inhibit feeding9. Thus, PYY, ghrelin and leptin are primary candidates to investigate in patients with PWS, particularly during infancy and early childhood when eating behavior is markedly different during stage one (i.e., feeding difficulties and failure to thrive) compared with stage two (i.e., hyperphagia and rapid weight gain). However, genes for leptin, ghrelin, PYY or their receptors are not located on chromosome 15q11-q13.
Leptin levels have been examined previously in patients with PWS and found to reflect the body weight or fat mass, i.e., higher fat mass in patients with PWS showed higher leptin levels, as seen in obese controls19–21. Similarly, fasting plasma ghrelin levels were elevated (3- to 5-fold) in 18 adults with PWS compared with lean and obese control subjects12. The elevated levels of ghrelin were later confirmed in adults and children with PWS22,23. Ghrelin levels were also measured in cord blood from 90 full-term neonates without PWS15. However, to date no ghrelin study included infants with PWS or comparison of patients with PWS during stage one and stage two of clinical course development.
Herein, we report for the first time plasma PYY levels in children and infants and ghrelin in infants with PWS. We examine demographic, clinical and genetic subtype data and compare plasma ghrelin and PYY levels with similarly aged populations reported in the literature.
PATIENTS AND METHODS
Twelve children and infants with Prader-Willi syndrome (three females, nine males) were studied with an average age ± SD of 3.6 ± 4.1 years and age range of 2.5 months to 13.3 years (Table 1). PWS was confirmed by genetic testing (chromosome analysis with FISH and methylation PCR). Five patients had a 15q11-q13 deletion; six had maternal disomy 15; and one male had an imprinting defect determined by genetic analysis. Six patients with PWS were less than 2 years of age and identified during stage one or the failure to thrive period. Informed consent was approved through our local institutional review board and obtained from the parent or assent by the adolescent. Height and weight were obtained for each patient and body mass index (BMI) calculated (kg/m2). Five of our children with PWS were treated with growth hormone for a few weeks to 24 months duration.
TABLE 1.
Clinical, laboratory and genetic data for patients with Prader-Willi syndrome
| PWS patients | |
|---|---|
| n | 12 |
| Male | 9 |
| Female | 3 |
| Age (years) (mean ± SD) | 3.6 ± 4.1 |
| Deletion | 5 |
| UPD | 6 |
| Imprinting defect | 1 |
| BMI (mean ± SD) | 18.1 ± 3.6 |
| Plasma ghrelin (pg/ml) (mean ± SD) | 1152 ± 705 |
| Plasma PYY (pg/ml) (mean ± SD) | 51 ± 33 |
UPD = uniparental disomy 15.
Peripheral blood (overnight fasting) was collected in a refrigerated vacutainer tube containing aprotinin, a specific preservative for gastrointestinal protein provided by the reference laboratory. The plasma was immediately separated after centrifugation and stored at −70°C until used. Ghrelin and PYY were measured commercially within 2 weeks from the time of collection by Inter Science Institute (Englewood, CA) using radioimmunoassay and specific rabbit polyclonal antibodies directed against the individual peptides following established protocols7,16. All PWS samples were measured in duplicate and the results compared with control populations in the literature using similar methods and assays7,15,17,23,24.
Laboratory specificity was determined for ghrelin and PYY at 50% inhibition of binding level for these peptides compared with binding to other gastrointestinal peptides. Cross reactivity for both ghrelin and PYY was 100% for their respective assay and <0.01% for other measured gastrointestinal related peptides. Sensitivity determined as the least amount of ghrelin that could be distinguished from zero was 50 pg/ml, and for PYY 10 pg/ml. Based on three controls assayed in different runs in the laboratory setting, the intra-assay and interassay coefficients of variation were 8.4% and 9.8% for PYY, and 5.1% and 11.4% for ghrelin, respectively. The expected range established for the laboratory for fasting ghrelin was 520–700 pg/ml (for adults) and for fasting PYY 30–120 pg/ml (for adults). We similarly collected fasting plasma samples from adult control subjects which were sent to the laboratory for analysis. PYY and ghrelin levels for these adults were within the laboratory reference range for adults established by the laboratory (data not shown).
Statistical analyses included comparisons of group means by non-parametric Mann-Whitney U test and parametric t-tests and comparison of bivariate correlation coefficients.
RESULTS
Twelve children and infants with PWS (three females and nine males) were analyzed with a mean age ± SD of 3.6 ± 4.1 years and mean BMI ± SD of 18.1 ± 3.6 kg/m2. Four of the 12 patients with PWS had BMI scores greater than the 85th percentile or weight measures greater than the 95th percentile. The mean ghrelin ± SD was 1,152 ± 704 pg/ml and for PYY 51 ± 33 pg/ml for our patients with PWS. The mean ± SD ghrelin and PYY levels in our infants with PWS were 1417 ± 837 pg/ml and 68 ± 34 pg/ml, respectively, compared to 887 ± 473 pg/ml and 34 ± 25 pg/ml, respectively, for the six children with PWS older than 2 years. No significant difference was found in ghrelin or PYY levels in our patients with PWS with or without obesity (Mann-Whitney U test, p = 0.17; p = 0.23, respectively). No significant difference was found in the ghrelin or PYY levels in those patients with PWS younger or older than 2 years (Mann-Whitney U test, p = 0.15; p = 0.06, respectively). Five patients with PWS (including one female aged 20 months) were receiving growth hormone for a few weeks to 24 months. No significant difference was found in ghrelin or PYY levels in those children with PWS treated with growth hormone compared to those not receiving growth hormone (Mann-Whitney U test, p = 0.81; p = 0.16, respectively). There was also no significant difference in ghrelin or PYY levels in the patients with PWS with the 15q deletion or maternal disomy 15 (Mann-Whitney U test, p = 0.47; p = 0.20, respectively) (see Table 2).
TABLE 2.
Plasma ghrelin and peptide YY levels in patients with Prader-Willi syndrome
| n | Ghrelin (pg/ml) | Peptide YY (Pg/ml) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SD | p value* | Mean | SD | p value* | ||
| Male | 9 | 966 | 561 | 0.08 | 50 | 28 | 0.93 |
| Female | 3 | 1711 | 919 | 54 | 52 | ||
| GH therapy | 5 | 1149 | 591 | 0.81 | 34 | 21 | 0.16 |
| Without GH | 7 | 1154 | 823 | 63 | 35 | ||
| Deletion | 5 | 1371 | 883 | 0.47 | 68 | 31 | 0.20 |
| UPD | 6 | 907 | 566 | 43 | 30 | ||
| <2 years | 6 | 1417 | 837 | 0.15 | 68 | 32 | 0.06 |
| >2 years | 6 | 887 | 473 | 34 | 25 | ||
| Obese | 4 | 810 | 436 | 0.17 | 33 | 24 | 0.23 |
| Non-obese | 8 | 1323 | 773 | 60 | 34 | ||
GH = growth hormone; UPD = uniparental disomy 15.
p value based on Mann-Whitney U test.
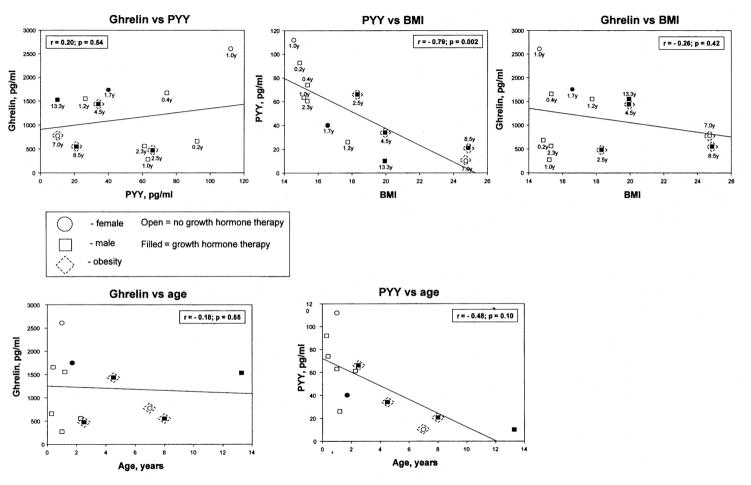
Correlation analysis was also undertaken comparing ghrelin with PYY, ghrelin with age, ghrelin with BMI, PYY with age, and PYY with BMI for the patients with PWS. Significant negative correlations were seen for PYY and BMI (r = −0.79; p = 0.002) for all patients with PWS (see Fig. 1). There were no significant correlations for the other parameters studied (Fig. 1).
Fig. 1.
Correlation analyses for plasma ghrelin with peptide YY (PYY), ghrelin with age, ghrelin with BMI, PYY with age, and PYY with BMI for patients with Prader-Willi syndrome. A significant negative correlation (p <0.01) was seen for PYY and BMI for the patients with Prader-Willi syndrome.
The average ± SD ghrelin level (1152 ± 704 pg/ml) in our children with PWS (age range of 2.5 months to 13.3 years, average age of 3.6 years) was compared with the average ± SD level (630 ± 246 pg/ml or 187 ± 88 pmol/liter) from 90 healthy full term newborns reported by Chanoine et al.15 A significant difference (t-test; p <0.001) was found in this comparison. In addition, ghrelin levels in our patients with PWS were compared with fasting levels in 20 normal controls (average ghrelin ± SD of 910 ± 344 pg/ml; average age ± SD of 8.7 ± 3.9 years; age range 4.4–17.0 years) and 17 obese controls (average ghrelin ± SD of 492 ± 253; average age ± SD of 10.6 ± 3.5 years; age range 5.1–17.4 years) reported by Haqq et al.23. No significant difference was found in the ghrelin levels in our patients with PWS compared to the normal controls but a significantly higher level was found in our patients with PWS compared to their reported obese controls (t-test; p <0.01) in agreement with their findings. Similarly, PYY levels in our 12 children and infants with PWS (average ± SD of 51 ± 33 pg/ml) were compared with fasting PYY levels (average ± SD of 131 ± 12 pg/ml or 32 ± 3 pmol/1) in 13 control newborns and infants reported by Adrian et al.24 with an average age of 9 months. The levels seen in our children and infants with PWS were significantly lower (t-test; p <0.001). In addition, the PYY levels for our six infants with PWS less than 2 years (average ± SD of 68 ± 32 pg/ml) and our PWS children greater than 2 years (average ± SD of 34 ± 25 pg/ml) were also significantly lower (t-test; p <0.001, for both infants and children with PWS) than in the 13 infants reported by Adrian et al.24.
DISCUSSION
Our fasting ghrelin levels were comparable to those reported previously for ghrelin and Prader-Willi syndrome (i.e., an elevated ghrelin level in patients with PWS compared with control populations in the literature). We examined for differences in PWS genetic subtype (deletion and uniparental disomy 15 [UPD]) but did not find significant differences in fasting PYY or ghrelin levels. In addition, no difference in ghrelin or PYY levels was found in males or females with PWS whether or not they were receiving growth hormone treatment. Although ghrelin is a potent growth hormone secretagogue15, growth hormone therapy in our children with PWS appeared not to impact on their plasma ghrelin levels compared to patients with PWS not receiving growth hormone. This observation is supported by the literature (i.e., ghrelin is not elevated in non-PWS patients with growth hormone deficiency receiving growth hormone25 or in our PWS individuals with obesity).
Our children with PWS were divided into two groups by age, i.e., >2 or <2 years old, approximately the demarcation between stage one and stage two of clinical course development for PWS, and no significant difference was found for either ghrelin or PYY levels. Our findings are in agreement with published reports on high ghrelin levels in adults and children with PWS. However, our report is the first to examine fasting plasma ghrelin levels in infants with PWS as young as 2.5 months, before the onset of obesity. The ghrelin levels were no different in individuals with PWS younger or older than 2 years but were elevated in both groups compared to reported levels in normal control children23. In addition, we measured plasma PYY levels for the first time in patients with PWS and found the levels to be lower in our children with PWS (average age 3.6 years) compared with PYY levels reported by Adrian et al.24 in control newborns and infants. While PYY inhibits and ghrelin stimulates eating, higher ghrelin levels should be reflected by lower PYY levels. This relationship was observed in our children and infants with PWS and reflects the insatiable appetite of individuals with this syndrome.
The genes for ghrelin and PYY and their receptors are not localized on chromosome 15 so it is unclear why ghrelin levels are high and PYY levels are low in PWS. However, lack of feedback inhibition from growth hormone and release of growth hormone from the brain may play a role in the elevated ghrelin levels and subsequently lower PYY levels in PWS. It will also be important to know whether the excess ghrelin levels are derived from the stomach or from other sources peripherally or centrally in the brain. Additionally, the ghrelin levels determined may not represent entirely active ghrelin but may reflect an inactive form of ghrelin-derived protein due to an alternative splicing or mutations of the ghrelin gene, as proposed by Haqq et al.23. Evidence does support altered or defective processing of precursor proteins, particularly vasopressin and polypeptide 7B2, in the hypothalamus in some patients with PWS26 including preproghrelin by prohormone convertase 2 into mature active ghrelin. If the measurable ghrelin level in PWS is mostly inactive hormone then one could speculate why higher levels of growth hormone are not observed in children with PWS. In addition, an unrelated defect in growth hormone secretion in children with PWS may not be affected by growth hormone secretagogues such as ghrelin. However, the lower PYY levels in our children and infants with PWS would indicate an active ghrelin protein with a normal interaction between ghrelin and PYY with the levels in the anticipated direction (i.e., high ghrelin and low PYY). Future studies are warranted to address the interaction of ghrelin, PYY, leptin and other appetite related hormones, their distribution, function and receptor status, peripherally and centrally.
Gene expression studies are underway to examine the level of expression of these apparently tightly regulated and interrelated hormones and their receptors to better understand their relationship in controlling eating behavior and obesity in PWS using quantitative RT-PCR of RNA isolated from actively growing lymphoblastoid cell lines27 from eight patients with PWS and five control individuals established at the time of blood collection for plasma ghrelin and PYY levels. The genes studied included ghrelin, PYY, and their recognized receptors (GHS-RIa, GHS-RIb, NPY2). Specifically, no significant correlation (positive or negative) was found between ghrelin gene expression and ghrelin or PYY levels (data not shown). Additional studies are underway to examine brain tissue collected at the time of autopsy from several individuals with PWS and control subjects in order to obtain gene expression data for these genes which are involved with eating behavior.
Acknowledgments
We thank Linda Heim and Deborah Moore for expert preparation of the manuscript. The research was partially funded by NIH grants (P01HD30329 and R01HD41672), Children’s Mercy Hospital Physician Scientist Award (GL01.4871) and The Hall Foundation (GL01.3095).
References
- 1.Butler MG. Prader-Willi: current understanding of cause and diagnosis. Am J Med Genet. 1990;35:319–332. doi: 10.1002/ajmg.1320350306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassidy SB. Prader-Willi syndrome. J Med Genet. 1997;34:917–923. doi: 10.1136/jmg.34.11.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler MG, Thompson T. Prader-Willi syndrome: clinical and genetic findings. Endocrinologist. 2000;10:3S–16S. doi: 10.1097/00019616-200010041-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholls RD, Knepper JL. Genome organization, function and imprinting in Prader-Willi and Angelman syndromes. Ann Rev Genomic Hum Genet. 2001;2:153–175. doi: 10.1146/annurev.genom.2.1.153. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 6.Broberger C, Landry M, Wong H, Walsh JN, Hökfelt T. Subtypes of Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology. 1997;66:393–408. doi: 10.1159/000127265. [DOI] [PubMed] [Google Scholar]
- 7.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessi HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen-Bjergaard U, Host U, Kelbaek H, Schifter S, Rehfeld JF, Faber J, Christensen NJ. Influence of meal composition on postprandial peripheral plasma concentrations of vasoactive peptides in man. Scand J Clin Lab Invest. 1996;56:497–503. doi: 10.3109/00365519609088805. [DOI] [PubMed] [Google Scholar]
- 9.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 10.Tschop M, Smiley D, Heiman M. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 11.Tschop M, Devanarayan V, Weyer C, Tataranni P, Ravussin E, Heiman M. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 12.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 13.Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt M. Plasma leptin levels and insulin relationships in obese and nonobese humans. Diabetes. 1996;45:695–698. doi: 10.2337/diab.45.5.695. [DOI] [PubMed] [Google Scholar]
- 14.Lahlou N, Landais P, De Boissieu D, Bougnères PF. Circulating leptin in normal children and during the dynamic phase of juvenile obesity: relation to body fatness, energy metabolism, caloric intake, and sexual dimorphism. Diabetes. 1997;46:989–993. doi: 10.2337/diab.46.6.989. [DOI] [PubMed] [Google Scholar]
- 15.Chanoine JP, Yeung LP, Wong AC, Birmingham CL. Immunoreactive ghrelin in human cord blood: relation to anthropometry, leptin and growth hormone. J Pediatr Gastroenterol Nutr. 2002;35:282–286. doi: 10.1097/00005176-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Shiiya T, Nakazzalo M, Mizuta M, Date Y, Mondal M, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretions. J Clin Endocrinol Metab. 2002;87:240–244. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- 17.Adrian TE, Bacarese-Hamilton AP, Savage K, Wolfe K, Besterman HS, Bloom SR. Plasma PYY concentrations in gastrointestinal diseases. Dig Dis Sci. 1984;29:35–39. [Google Scholar]
- 18.Berseth CL, Nordyke CK, Valdes MG, Furlow BL, Go VL. Responses of gastrointestinal peptides and motor activity to milk and water feedings in preterm and term infants. Pediatr Res. 1992;31:587–590. doi: 10.1203/00006450-199206000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Butler MG, Moore J, Morawiecki A, Nicolson M. Comparison of leptin protein levels in Prader-Willi syndrome. Am J Med Genet. 1998;75:7–12. [PMC free article] [PubMed] [Google Scholar]
- 20.Pietrobelli A, Allison DB, Faith MS, Beccaria L, Bosio L, Chiumello G, Campfield LA, Hymsfield SB. Prader-Willi syndrome: relationship of adiposity to plasma leptin levels. Obes Res. 1998;6:196–201. doi: 10.1002/j.1550-8528.1998.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 21.Meyers SE, Davis A, Whitman BY, Santiago JV, Landt M. Leptin concentrations in Prader-Willi syndrome before and after growth hormone replacement. Clin Endocrinol. 2000;52:101–105. doi: 10.1046/j.1365-2265.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 22.Delparigi A, Tschop M, Heiman ML, Salbe AD, Vozarova B, Sell SM, Bunt JC, Tataranni PA. High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader-Willi syndrome. J Clin Endocrinol Metab. 2002;87:5461–5464. doi: 10.1210/jc.2002-020871. [DOI] [PubMed] [Google Scholar]
- 23.Haqq AM, Farooqi IS, O’Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL, LaFranchi H, Purnell JQ. Serum ghrelin levels are inversely correlated with body mass index, age and insulin concentrations in normal children and are markedly increased in Prader-Willi syndome. J Clin Endocrinol Metab. 2003;88:174–178. doi: 10.1210/jc.2002-021052. [DOI] [PubMed] [Google Scholar]
- 24.Adrian TE, Smith HA, Calvert SA, Aynsley-Green A, Bloom SR. Elevated plasma peptide YY in human neonates and infants. Pediatr Res. 1986;20:1225–1227. doi: 10.1203/00006450-198612000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Janssen JA, van der Toorn FM, Hofland LJ, van Koetsveld P, Broglio F, Ghigo E, Lamberts SW, Jan van der Lely A. Systemic ghrelin levels in subjects with growth hormone deficiency are not modified by one year of growth hormone replacement therapy. Eur J Endocrinol. 2001;145:711–716. doi: 10.1530/eje.0.1450711. [DOI] [PubMed] [Google Scholar]
- 26.Gabreels S, Swaab D, Kleijn D, Seiden N, Van de Loo J, Van den Ven W, Martens G, Van Leeuwen F. Attenuation of the polypeptide 7B2, prohormone convertase PC2, and vasopressin in the hypothalmus of some Prader-Willi patients: indications for a processing defect. J Clin Endocrinol Metabol. 1998;83:591–599. doi: 10.1210/jcem.83.2.4542. [DOI] [PubMed] [Google Scholar]
- 27.Bittel DC, Kibiryeva N, Talebizadeh Z, Butler MG. Microarray analysis of gene/transcript expression in Prader-Willi syndrome: deletion versus UPD. J Med Genet. 2003;40:568–574. doi: 10.1136/jmg.40.8.568. [DOI] [PMC free article] [PubMed] [Google Scholar]